Abstract
Multiple myeloma patients deemed to not be candidates for high-dose therapy followed by stem cell rescue who nonetheless need chemotherapy have traditionally received an oral regimen combining melphalan and prednisone. With the advent of novel agents, however, such as immunomodulatory drugs and proteasome inhibitors that are active in the relapsed/refractory setting, there has been an impetus to incorporate these new options into front-line therapy. Several phase II studies have recently revealed that addition of either thalidomide, lenalidomide, or bortezomib to melphalan and prednisone increased the overall and complete response rates, albeit at the cost of some increased toxicity. Randomized phase III studies of melphalan and prednisone with thalidomide have already shown that, compared to melphalan and prednisone alone, the three-drug regimen prolonged time to progression and overall survival in this population, thereby defining a new standard of care. Moreover, our increasing knowledge of the molecular role that cytogenetic abnormalities play in the biology of multiple myeloma and our growing chemotherapeutic armamentarium are beginning to allow us to rationally select therapies based on these characteristics of each patient’s disease. Such a risk- and molecular-adapted strategy to the therapy of multiple myeloma promises to revolutionize and personalize our care of these patients and bring us closer to a cure for this disease.
Using the diagnostic criteria recommended by the International Working Group, patients with multiple myeloma are classified as having either asymptomatic or symptomatic (Table 1 ) disease.1 The latter population, generally considered candidates for a chemotherapy-based intervention, are then further divided into those who either are or are not eligible for high-dose chemotherapy followed by stem cell rescue.2 Criteria that are weighed to help make this second distinction have generally included age, performance status, and co-morbid medical conditions.2 There is some variability in these parameters and how they are applied, since studies examining stem cell transplantation have used different criteria. In regard to age, for example, which is the most objective of these measures, initial studies tended to enroll patients younger than 65 years of age,3 while more recent studies suggest that transplant is safe in at least some who are over the age of 70 (for example 4,5). On the other hand, data suggesting that patients with poor-risk chromosomal features have a short time to progression after autologous transplantation have led to suggestions that even younger patients with these abnormalities may not be candidates for standard approaches. Nonetheless, as little as two years ago, there was general agreement that the standard of care for patients who were not eligible for transplantation and yet needed chemotherapy was melphalan (Alkeran®) and prednisone (MP).2 This regimen had the advantages of ease of use with an oral, outpatient administration schedule, and was generally well tolerated.7 Moreover, while combination chemotherapy tended to induce a more rapid response, and a higher overall response rate, these differences did not translate into a survival advantage compared to that achieved with MP.7 Despite these findings, the low overall response rate with MP of 53.2% with few complete responders,7 and only modest improvements in 5-year survival for all patients with multiple myeloma over the years,8 clearly provided ample room for improvement. A recent explosion of knowledge about the pathobiology and pathogenetics of multiple myeloma, however, and our ever-increasing drug arsenal have now combined to produce tangible benefits for patients that are defining new standards of care. The chapter below will provide a review of some of these exciting developments and their molecular basis, and describe a potential future treatment algorithm for care of these patients.
Risk Stratification
In the past, patients diagnosed with multiple myeloma have been staged by a variety of systems, such as the Durie-Salmon method,9 to help in determining their risk and prognosis. A more accurate technique called the International Staging System (ISS) has recently been introduced after a multivariate analysis of parameters that were predictive of survival in over 11,000 patients worldwide.10 Three stages were defined based solely on results of the serum albumin and β2-microglobulin (Table 2 ) that identified groups with a median survival of 62 months for stage I, 44 months for stage II, and 29 months for stage III. As in many other such analyses, age was a poor prognostic factor, with patients who were 65 years of age or older having an inferior survival. When the impact of age on the ISS system was studied, it was found to be predictive of survival regardless of age when comparing patients who were younger than 65, and those who were 65 or older (Table 3 ). Also of note, ISS provided accurate prognostic data for patients who were undergoing either a high-dose chemotherapy regimen or a conventional-dose chemotherapy approach (Table 3 ). Thus, determining the ISS stage is an important tool to risk-stratify patients with multiple myeloma who are not candidates for stem cell transplantation.
The ISS stage represents a significant advance in predicting the prognosis of patients with multiple myeloma. However, it is likely that other new criteria will either be added to this method to further refine our ability to discriminate between patients with different risks, or that these criteria will eventually replace the ISS altogether. One example that has already been proposed is the use of the albumin, β2-microglobulin, and the tumor burden as measured by the number of plasma cells in the peripheral blood.11 Based on data demonstrating that the number of CD38+CD45− plasma cells per 50,000 circulating mono-nuclear cells is an independent prognostic variable, four risk groups were identified ranging in median overall survival from 13 months to 79+ months.
It is also becoming increasingly clear that cytogenetic abnormalities, detected either by routine karyotyping or by fluorescence in situ hybridization (FISH) analysis, play a major role in the prognosis of multiple myeloma. This has been most commonly defined in the population of patients who do go on to stem cell transplantation as an option. However, several studies have evaluated the role of chromosomal anomalies in patients receiving conventional chemotherapy, and generally confirmed that the same abnormalities confer a poor prognosis for outcome after therapy with such an approach as well. For example, among patients receiving a variety of predominantly melphalan-based regimens as initial therapy, deletions of 13q14 and 17p13 were associated by Konigsberg et al with a poor response to induction treatment, and a shorter median overall survival (OS), while 11q abnormalities were linked to a shorter median OS.12 Similarly, an analysis was performed by Fonseca et al of an Eastern Cooperative Oncology Group study where patients were destined to undergo induction with VBMCP (vincristine, BCNU, melphalan, cyclophosphamide, and prednisone) with or without interferon.13 Patients with a t(4;14) had a median OS of 26 months versus 45 months for controls, while −17p13 patients had a median OS of 23 months.14 Of note, in this study, −13q14 patients had an intermediate prognosis of 35 months, which led the authors to propose three cytogenetic risk groups with good, intermediate, and poor prognoses (Table 4 ).
Although cytogenetic information clearly provides important prognostic information, one argument against its routine use outside of clinical trials has been that it did not impact on the choice of initial or subsequent therapy. One can make a cogent argument that this is no longer the case for patients who do have stem cell transplant as an option. For example, patients with a 4;14 translocation, which often results in overexpression of fibroblast growth factor receptor (FGFR)-3, have been reported to have an approximately 8-month median time to progression after an autologous stem cell transplant.6 This small benefit would suggest that nontransplant approaches might be preferred, or that other transplant approaches, such as a non-myeloablative allogeneic route, should be considered. Several agents are now being developed, however, that specifically target the FGFR-3 tyrosine kinase and have been shown to have encouraging activity in vitro.15,16 Such drugs are entering clinical trials in the relapsed/refractory setting and, if activity were documented, they would clearly be attractive candidates for incorporation into initial therapy. Though t(4;14) may represent a poor prognostic feature even in the absence of FGFR-3 expression,17 a regimen of MP with an FGFR-3 inhibitor could represent a rational, molecularly targeted approach to this patient subset. Another example of this type of approach may prove to be the use of the proteasome inhibitor bortezomib (VELCADE®), or a bortezomib-based regimen, in patients with deletions of chromosome 13. Originally, this agent was found to have activity against relapsed/refractory multiple myeloma in the phase I–III settings18–20 and, interestingly, subset analysis of the phase II study19 showed that patients with deletion (del) of 13 had the same response rate as controls. When results of the phase III trial20 were analyzed in this regard, it was found that patients with del 13 who received dexamethasone had an inferior survival compared with matched controls, as would be expected.21 Importantly, on the bortezomib arm, patients with del 13 did not have an inferior survival, suggesting that this agent was able to overcome the adverse effects of this abnormality. While the molecular basis for the poor prognosis of del 13 has not been definitively identified, one candidate is the retinoblastoma (pRb) tumor suppressor. Since pRb mutations cause cell cycle dysregulation due to a loss of control over the G1/S transition, it is possible that the accumulation of cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1 that occurs as a result of proteasome inhibition, may provide a block to cell cycle progression at G1/S that overcomes the impact of del 13. These hypotheses still need to be tested, and prospective clinical trials both in the relapsed/refractory and front-line settings focusing on del 13 patients and bortezomib need to be performed as well. However, the available data do provide an intriguing rationale for the use of, for example, MP with bortezomib in patients with del 13, and further highlight the future importance of routine cytogenetic and FISH studies in determining not just prognosis, but possibly the optimal choice of therapies.
Initial Therapy with MP-based Regimens
Though MP has been one of the standards of care for older patients, or those not eligible for stem cell transplantation, other options include dexamethasone alone,2 or even melphalan with dexamethasone (MD). One study that sheds important light on this was recently reported by the Intergroupe Francophone du Myélome (IFM), and randomized patients who were 65 to 75 years of age to receive MP, MD, dexamethasone alone, or dexamethasone with interferon.22 While none of these regimens induced a significant number of complete responses, patients receiving MD had a 70% overall response rate, defined as achieving at least a partial response, which was significantly higher than that seen with any of the other three regimens (Table 5 ). However, MD was also associated with a greater risk of severe toxicities, most notably severe pyogenic infections including pulmonary infections and septicemia, in agreement with prior such studies.23 Moreover, the higher response rate with MD did not translate into either a significantly better median time to progression or median overall survival. Thus, the authors concluded that MP remained the standard of care for induction therapy in this patient population and that it should be the starting point for the development of future combinations.
MP + Thalidomide
Using MP or an MP-type regimen as a backbone, a number of investigators have sought to add the immunomodulatory agent thalidomide (MPT). While this drug was first noted to have activity against relapsed/refractory disease,24 preclinical25 as well as clinical studies have documented its ability to add to the efficacy of other agents used against multiple myeloma, such as steroids, included as part of front-line therapies.26,27 Palumbo et al therefore enrolled 49 consecutive patients, ages 61–82, with newly diagnosed multiple myeloma, treated them with this three-drug oral combination (Table 6 ), and evaluated responses using the stringent criteria recommended by the European Group for Blood and Marrow Transplantation (EBMT)/International Bone Marrow Transplant Registry.28 Toxicities that reached a severity of grade 3 or 4 included hematologic complications in 22%, thromboembolic episodes involving either venous or arterial events in 20%, infections in 12%, including pneumonia and herpes zoster, neurologic complications in 8%, including paresthesias and coma, constipation in 6%, and cutaneous and cardiac events, seen in 2% each.29 This regimen did induce an overall response rate, defined as those patients who achieved at least a partial response, of 73%, including the 24% of patients who attained either a complete response (CR) or near-CR. Responses were seen rapidly, with more than 50% of patients reaching a PR within the first 2 months of therapy, and a median time to maximum response of 4 months, thus overcoming one of the disadvantages of MP with its relatively slow onset of action. Longer-term follow-up showed that the estimates for event-free and OS at 2 years were 64% and 91%, respectively.
A less dose-intensive approach to this concept was taken by Dimopoulos et al, who used MD as their backbone and also added thalidomide (MDT), but the latter was given for only 8 days every 5 weeks (Table 6 ) in 50 patients who were at least 75 years of age. This intermittent dosing schedule seemed to induce a decreased risk of some toxicities, such as grade 3 or 4 constipation, which was not seen at all with MDT, but other grade 3 or 4 events were comparable to those seen with MPT, including 22% of patients with neutropenia, 10% with thrombocytopenia, 9% with deep vein thrombosis, 4% with somnolence, and 2% each with tremor and ataxia.30 Responses again were very rapid, with a median time to 50% disease reduction of 2 months, and 72% of patients achieved at least a partial response, including 10% with a complete response. Median time to disease progression was found to be 21.2 months, while the median overall survival was 28.2 months.
The encouraging results from the phase II study of MPT prompted Palumbo et al to proceed with a randomized phase III trial comparing MP with MPT as initial therapy in patients aged 60–85 years old.31 MPT was associated with a significantly increased risk of grade 3 or 4 thromboembolic complications, neurologic toxicities, infectious events, and gastrointestinal problems compared to MP. As a result of the thromboembolic complications, low-molecular-weight heparin prophylaxis was instituted during the trial in the form of enoxaparin (Table 6 ). This reduced the incidence of grade 3 or 4 events from 57% in the first 65 patients, to 39% in the next 64 patients (P = 0.042). Notably, despite this overall increased risk of adverse events, especially in the first cohort of patients enrolled and treated before enoxaparin was used, 20 patients died on the MPT arm (16%) compared with 27 (21%) in the MP group. Patients receiving MPT had a better overall response rate than those randomized to MP (Table 7 ), and the quality of the responses was better as well, with more complete, near-complete, and very good partial responses. Also, there was a lower incidence of both non-responsive disease, as well as progressive disease with MPT. Longer-term follow-up showed that 42 of 129 patients treated with MPT suffered progression, relapse or death (33%), compared with 62 of 126 (49%) on the MP arm. This translated into a 2-year event-free survival of 54% on MPT compared with 27% on MP (P = 0.0006). At 3 years, the OS rate was 80% for those on MPT versus 64% for those with MP alone, providing a clear indication that MPT needs to be considered as a standard of care for older patients with multiple myeloma.
A randomized phase III trial comparing MP to MPT has also been performed by the IFM,32 and incorporated a third arm with standard induction chemotherapy followed by mobilization and intermediate-dose melphalan supported by stem cell rescue. The latter was included in part because of data showing that this reduced-intensity approach, with melphalan at 100 mg/m2 (Mel 100), showed a survival advantage compared to MP in myeloma patients aged 50–70 years.33 In the final analysis of this three-arm study, MPT was associated with a higher risk of grade 3 or 4 neutropenia, infections, thrombocytopenia, thromboembolic complications, peripheral neuropathy, constipation, and cardiac events. However, MPT induced less anemia, neutropenia, thrombocytopenia, infections, and cardiac events than did intermediate-dose melphalan, and there were fewer toxic deaths and fewer deaths in the first 3 months of treatment overall in the MPT cohort. As was the case in the Italian study of MPT, the IFM trial found a higher overall response rate for MPT compared with MP (Table 8 ), and a better response quality as well, with more CRs and very good partial responses. Importantly, the progression-free survival (PFS) and OS were superior for MPT compared with MP (Table 8 ; P < 0.001 and P = 0.001, respectively). Also, MPT induced a longer PFS and OS than did the Mel 100 approach (P = 0.001 and P = 0.004, respectively). Together, these two randomized phase III studies strongly support the use of MPT as the current standard of care for most older patients with newly diagnosed multiple myeloma who require chemotherapy.
R-MP
Lenalidomide (Revlimid®) is a novel immunomodulatory agent that is more potent than thalidomide,25 and has shown activity both preclinically25 and clinically34 against multiple myeloma. This drug seems also to add to the efficacy of steroids in these model systems25,35 and to have a more favorable toxicity profile than thalidomide.35 While MPT provides a survival advantage compared with MP, its use is associated with a higher risk of adverse events, providing the impetus for substitution of lenalidomide for thalidomide in the MPT regimen. Results from a phase I/II study of MP with lenalidomide (R-MP) have recently been presented,36 and grade 3 or 4 hematologic toxicities included neutropenia in 66% of patients, requiring growth factor support in 58%, and with febrile neutropenia in 8%, thrombocytopenia in 34%, and anemia in 17%. Non-hematologic toxicities included skin reactions in 10%, infections in 5%, and thromboembolic complications in 5%, with pulmonary embolism in 2.4% and deep vein thrombosis in 2.4%, the latter all when patients were off aspirin. The overall response rate in this study using the EBMT criteria for all four patient cohorts after a median of seven cycles of therapy was 85.4%, including 17.1% of patients who achieved a CR and 24.4% who achieved a near-CR and there was also a trend for patients in the higher-dose cohorts to have a better response quality. Event-free survival at 16 months of follow-up in 53 patients was 87%, and in a retrospective analysis compared favorably with the 71% at 18 months seen for historical controls treated with MPT.
VMP
Proteasome inhibition with bortezomib is a rational approach to therapy of multiple myeloma both by itself, and in combination with other drugs, since this agent seems able to enhance chemosensitivity and overcome chemoresistance in both preclinical37–40 and clinical41,42 studies. Bortezomib was therefore incorporated into the MP regimen (VMP) by the Grupo Español de Multiple Myeloma (Table 6 ), who treated 60 patients with myeloma who were at least 65 years of age. Mateos et al found no dose-limiting toxicities in an initial phase I study of this regimen, and noted only grade 3 neutropenia and thrombocytopenia as the toxicities of highest grade.43 In a larger phase II component of this trial, grade 3 or 4 toxicities seen in at least 10% of patients included thrombocytopenia in 51%, neutropenia in 43%, neuropathy in 17%, diarrhea in 16%, and anemia in 10%. The overall response rate was 89% using the EBMT criteria, including 32% of patients with a CR and an additional 11% with a near-CR. Event-free and OS at 16 months of follow-up were 83% and 90%, respectively, which compared favorably with their historical controls for MP alone of 51% and 62%. Importantly, all 13 patients with pRb deletions achieved at least a PR, including 54% with either a CR or near-CR. Also, although the number of patients in these categories was small, the response rate seemed to be unaffected by immunoglobulin heavy chain gene rearrangements, such as t(4;14). Finally, fully half of those patients in CR were found to have achieved an immunophenotypic remission. These encouraging results form the basis for an ongoing international phase III trial that is comparing MP with VMP in a randomized fashion, and will also provide longer follow-up to establish the durability of VMP-induced responses.
Regimens not Based on MP
Though MP forms the backbone of many of the novel regimens described above, some investigators have sought to replace components of this older standard of care. One example is a regimen with cyclophosphamide, interferon-α, and betamethasone, which has been tested in a phase II setting in patients 60–75 years of age.44 An overall response rate of 79% was noted in 22 patients, who had a median response duration of 14 months. Also, the bifunctional alkylating agent bendamustine has been substituted for melphalan in the MP regimen to form BP, and a randomized phase III study has shown that BP induced a more rapid response and a higher CR rate overall.45 Time to treatment failure was prolonged, as was remission duration, but so far this has not translated into an improved OS. Other investigators have looked to supplant MP with regimens not based on an alkylating agent, an example of which is one of the standards of care for younger patients, thalidomide with dexamethasone (thal/dex). Interim results of a phase III study comparing MP with thal/dex have so far shown that the latter induced a significantly greater incidence of thromboembolic complications, neuropsychiatric effects, and skin toxicities.46 Thal/dex did seem to induce a higher overall and CR rate, but more patients suffered progressive disease, indicating additional follow-up will be needed to determine which regimen provides a superior long-term outcome. Of note, however, two more mature single-arm studies of thal/dex have reported a briefer median time to progression and OS47,48 than has been seen with MPT, suggesting that thal/dex alone may not be optimal for nontransplant patients. Of greater interest may be the combination of thalidomide, pegylated liposomal doxorubicin, and dexamethasone (ThaDD; Table 6 ), which was studied in 50 patients over the age of 65.49 Grade 3 and 4 events seen in at least 10% of patients included infections, thromboembolic complications, neutropenia, and constipation. An overall response rate of 84% was reported, with 56% of patients achieving at least a very good PR. The activity of regimens incorporating thalidomide, pegylated liposomal doxorubicin, and dexamethasone is also strongly supported by studies including both untreated patients, as well as those with relapsed/refractory disease, showing comparable response rates.50 These combinations will require additional, longer-term follow-up, and in some cases larger and/or randomized phase III trials, to determine their proper place in our algorithm for treatment of nontransplant patients with multiple myeloma.
MaintenanceTherapy
In that chemotherapy by itself with our current regimens does not cure multiple myeloma, the concept of maintenance therapy with low doses of one or more active agents to prolong the remission duration and OS has been an attractive one. Many of these studies have been performed in the post-transplant setting with drugs such as interferons, steroids, and immunomodulatory agents. Few such studies, unfortunately, have looked specifically at older patients, and/or those who are not candidates for stem cell transplantation. A study by Alexanian et al did randomize patients treated initially with melphalan and intermittent, high-dose dexamethasone to receive maintenance with either α-interferon or dexamethasone.51 Both regimens resulted in similar median remission durations of 10 months, though patients who received interferon had a higher likelihood of responding to MD if this was reinstituted at the time of relapse, suggesting a possible benefit for interferon. However, when interferon-α-2b was studied as a maintenance regimen in a randomized fashion compared with observation alone in patients initially treated with MP, a benefit was not seen.52 While Schaar et al did find an improved PFS, there was no statistically significant benefit in OS, and one-third of patients discontinued interferon due to a drug-related toxicity.52 Compared with interferon, steroids offer the advantage of an oral, outpatient administration option, and have also been studied as a maintenance therapy. Berenson et al evaluated the use of prednisone at either 50 mg every other day or 10 mg every other day in patients who had responded to infusional vincristine, doxorubicin, and oral dexamethasone.53 With a median follow-up of 53 months, no difference was noted in PFS or OS from the start of induction therapy, but from the time of randomization to the two prednisone arms both PFS and OS were significantly prolonged on the higher dose steroid arm, suggesting a benefit for prednisone. In contrast, dexamethasone was studied as a maintenance therapy in older patients after induction with either MP or MD, and compared with observation alone by Shustik et al.54 PFS was prolonged significantly with dexamethasone, but steroid maintenance did not result in an enhanced OS, casting doubt on this approach. Several of the recently completed or currently ongoing studies have incorporated a maintenance phase as part of induction therapy for nontransplant patients (Table 6 ), including the use of thalidomide- or proteasome inhibitor–containing regimens. Unfortunately, none of these have included an observation-only comparison arm for the maintenance phase, which would be needed to verify the utility of this approach.
Conclusions
Melphalan and prednisone have for many years been the standard of care for initial therapy of patients who do not have stem cell transplant as a possible future therapeutic option (Figure 1 ). The question of whether a better regimen could be designed with the use of novel agents seems now to have been answered in the affirmative. For a large proportion of such patients, it now seems appropriate to consider MP with thalidomide to be the treatment of choice, since the latter has been documented to achieve a more rapid response, a higher response rate and quality and, most significantly, an improved OS. This regimen, and indeed any combination incorporating an immunomodulatory agent and either a steroid or anthracycline, or especially both, should be used with some type of prophylaxis against thromboembolic complications55,56 such as aspirin, warfarin, or a low molecular weight heparin, as reviewed in the subsequent chapter by Dr. Jeffrey Zonder. Bisphosphonates should be considered as well, per the guidelines recommended by the American Society of Clinical Oncology57 and as reviewed Dr. Bhoomi Mehrotra in this volume. In patients who have a poor performance status, compromised organ function, or some other comorbidity, however, such as a significant contraindication to anticoagulation, MP may still be appropriate, especially if they have a low disease burden. The success of MPT suggests the possibility that, since by some accounts over half of patients less than 65 are not transplanted,58 this regimen may be worthy of study in younger patients as well. If additional studies of R-MP prove to be encouraging from a toxicity perspective and show a similar or better efficacy profile, then R-MP may prove to be the ultimate standard of care for all of these patient populations. In this regard, it is notable that an intergroup study comparing MPT with R-MP is currently being planned. Other regimens such as VMP and ThaDD appear attractive as well, and bear watching and further investigation, and the results of the randomized study comparing MP with VMP are eagerly anticipated.
The use of maintenance therapy after induction with MP or another regimen remains an open question. For the patient population under consideration here, there has been a paucity of trials for this indication, and in those that have been performed there is no definitive evidence of a survival advantage. It is clear, therefore, that despite the fact that age remains a significant barrier for accrual onto clinical trials for cancer patients,59 a concerted effort needs to be made to enroll patients onto the large, well-designed studies that will be needed to answer this question, as well as the one posed subsequently. Several recently completed and current studies do incorporate a maintenance regimen that follows induction therapy, but unfortunately do not do so in a randomized fashion. This has the danger of leading to the acceptance of maintenance therapy as a standard of care without proof that this expensive, toxicity-prone, and inconvenient approach provides a survival benefit, which should be the goal of such therapy. PFS may not be an appropriate endpoint for such studies, since patients whose disease recurs or progresses after maintenance therapy may have disease that is no longer responsive to that agent. If maintenance therefore removes one treatment option from the relapsed/refractory setting, patients may ultimately have been done a disservice, and, at least at this time, maintenance therapy should be undertaken only in the context of a clinical trial.
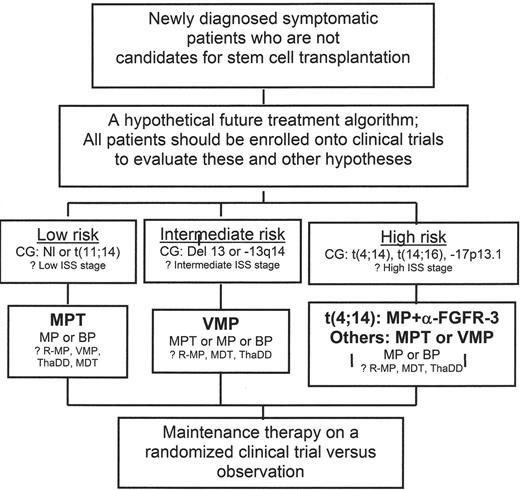
A final important issue is whether one regimen will be devised that is active for all patients, or whether a risk- or cytogenetics-adapted approach will prove optimal. Unfortunately, most of the studies reported to date have not prospectively stratified patients based on these approaches, making a firm conclusion difficult. However, there is an increasing realization that multiple myeloma can be sub-classified into several groups of genetically heterogeneous diseases, and that these differences impact on the biology of the disease.60 It seems likely that a cytogenetically adapted strategy will represent the most rational, molecularly targeted approach to myeloma therapy. Based on what we know at this time, one possible future treatment algorithm is presented in Figure 2 . Patients with low-risk multiple myeloma, including those with normal cytogenetics or a t(11;14), would at this time be candidates for primary therapy with MPT, with MP or BP representing reasonable alternatives if MPT were felt to be contraindicated. Those with lesions of chromosome 13, including del 13 or −13q14, could benefit most from VMP therapy, with MPT, MP, or BP serving as suitable alternatives if a proteasome inhibitor-based regimen were not appropriate. In the high-risk category, patients with t(4;14) and overexpression of FGFR-3 might benefit most from an MP regimen containing an inhibitor of this receptor tyrosine kinase, though in the absence of any data in this regard MPT would seem the standard, with VMP of interest as well. No clinical data are currently available to extrapolate therapy for those with t(14;16) or −17p13.1, and so MPT would also seem most appropriate for these patients at this time. However, in that both lesions result in dysregulation of the G1/S transition, the ability of VMP to induce accumulation of cyclin-dependent kinase inhibitors that would act at this point in the cell cycle suggests that this might be a molecularly rational approach. Hopefully, ongoing and future trials will evaluate the validity of these hypotheses and treatment algorithms, and bring us closer to a personalized approach to multiple myeloma that is likely to optimize the outcome of patients afflicted with this disease.
Myeloma-related organ or tissue impairment.
| Criterion . | Diagnostic Parameters . |
|---|---|
| Hypercalcemia | Serum calcium > 0.25 mmol/L above the upper limit of normal, or > 2.75 mmol/L (> 11.0 mg/dL) |
| Renal insufficiency | Creatinine >173 mmol/L (> 1.9 mg/dL) |
| Anemia | Hemoglobin 2 g/dL below the lower limit of normal, or hemoglobin <10 g/dL |
| Bony lesions | Lytic bony lesions, or osteoporosis with compression fractures (possibly requiring magnetic resonance or computed tomography as part of the evaluation) |
| Other associated findings | Symptomatic hyperviscosity, amyloidosis, recurring bacterial infections (> 2 episodes in 12 months) |
| Criterion . | Diagnostic Parameters . |
|---|---|
| Hypercalcemia | Serum calcium > 0.25 mmol/L above the upper limit of normal, or > 2.75 mmol/L (> 11.0 mg/dL) |
| Renal insufficiency | Creatinine >173 mmol/L (> 1.9 mg/dL) |
| Anemia | Hemoglobin 2 g/dL below the lower limit of normal, or hemoglobin <10 g/dL |
| Bony lesions | Lytic bony lesions, or osteoporosis with compression fractures (possibly requiring magnetic resonance or computed tomography as part of the evaluation) |
| Other associated findings | Symptomatic hyperviscosity, amyloidosis, recurring bacterial infections (> 2 episodes in 12 months) |
The International Staging System for multiple myeloma.10
| Stage . | Criteria . | Median Overall Survival . |
|---|---|---|
| I | Serum β2-microglobulin < 3.5 mg/L AND Serum albumin ≥ 3.5 g/dL | 62 months |
| II | Neither stage I or stage III | 44 months |
| III | Serum β2-microglobulin ≥ 5.5 mg/L | 29 months |
| Stage . | Criteria . | Median Overall Survival . |
|---|---|---|
| I | Serum β2-microglobulin < 3.5 mg/L AND Serum albumin ≥ 3.5 g/dL | 62 months |
| II | Neither stage I or stage III | 44 months |
| III | Serum β2-microglobulin ≥ 5.5 mg/L | 29 months |
Age, chemotherapy, and the international staging system for multiple myeloma.10
| Stage . | Median Overall Survival Age < 65 years . | Median Overall Survival Age ≥ 65 years . |
|---|---|---|
| I | 69 months | 47 months |
| II | 50 months | 37 months |
| III | 33 months | 24 months |
| Stage . | Median Overall Survival Age < 65 years . | Median Overall Survival Age ≥ 65 years . |
|---|---|---|
| I | 69 months | 47 months |
| II | 50 months | 37 months |
| III | 33 months | 24 months |
| Stage . | Median Overall Survival High-Dose Chemotherapy . | Median Overall Survival Conventional-Dose Chemotherapy . |
|---|---|---|
| I | 111 months | 55 months |
| II | 66 months | 40 months |
| III | 45 months | 25 months |
| Stage . | Median Overall Survival High-Dose Chemotherapy . | Median Overall Survival Conventional-Dose Chemotherapy . |
|---|---|---|
| I | 111 months | 55 months |
| II | 66 months | 40 months |
| III | 45 months | 25 months |
One possible cytogenetics-based prognostic grouping.14
| Risk Group . | Cytogenetics . | Median Overall Survival . |
|---|---|---|
| Poor | t(4;14) t(14;16) −17p13 | 24.7 months |
| Intermediate | −13q14 | 42.3 months |
| Good | All others | 50.5 months |
| Risk Group . | Cytogenetics . | Median Overall Survival . |
|---|---|---|
| Poor | t(4;14) t(14;16) −17p13 | 24.7 months |
| Intermediate | −13q14 | 42.3 months |
| Good | All others | 50.5 months |
Melphalan- and dexamethasone-based regimens as initial therapy for multiple myeloma.22
| . | MP (n= 109) . | MD (n= 110) . | Dex (n= 109) . | Dex + IFN (n= 101) . |
|---|---|---|---|---|
| Abbreviations: Dex, dexamethasone; IFN, interferon; MD, melphalan and dexamethasone; mos., months; MP, melphalan and prednisone; n, number of patients in each cohort | ||||
| Quality of initial response | ||||
| At least a partial response | 41% | 70% | 40% | 42% |
| Complete response | 1% | 3% | 1% | 1% |
| Long-term outcome measures | ||||
| Median progression-free survival | 21.1 mos. | 22.9 mos. | 12.2 mos. | 15.2 mos. |
| Median overall survival | 34.0 mos. | 39.6 mos. | 33.4 mos. | 32.0 mos. |
| Toxicities | ||||
| Deaths due to progression | 1 (1%) | 0 | 3 (3%) | 9 (9%) |
| Severe pyogenic infection | 12 (11%) | 22 (20%) | 14 (13%) | 11 (11%) |
| Any severe toxicity | 20 (18%) | 36 (33%) | 34 (31%) | 31 (31%) |
| . | MP (n= 109) . | MD (n= 110) . | Dex (n= 109) . | Dex + IFN (n= 101) . |
|---|---|---|---|---|
| Abbreviations: Dex, dexamethasone; IFN, interferon; MD, melphalan and dexamethasone; mos., months; MP, melphalan and prednisone; n, number of patients in each cohort | ||||
| Quality of initial response | ||||
| At least a partial response | 41% | 70% | 40% | 42% |
| Complete response | 1% | 3% | 1% | 1% |
| Long-term outcome measures | ||||
| Median progression-free survival | 21.1 mos. | 22.9 mos. | 12.2 mos. | 15.2 mos. |
| Median overall survival | 34.0 mos. | 39.6 mos. | 33.4 mos. | 32.0 mos. |
| Toxicities | ||||
| Deaths due to progression | 1 (1%) | 0 | 3 (3%) | 9 (9%) |
| Severe pyogenic infection | 12 (11%) | 22 (20%) | 14 (13%) | 11 (11%) |
| Any severe toxicity | 20 (18%) | 36 (33%) | 34 (31%) | 31 (31%) |
Novel induction regimens for non-transplant myeloma patients.
| Regimen . | Dosing and Schedule . | Reference . |
|---|---|---|
| Abbreviations: MDT, melphalan and dexamethasone with thalidomide; MP, melphalan and prednisone; MPT, melphalan and prednisone with thalidomide; R-MP, melphalan and prednisone with lenalidomide; ThaDD, thalidomide with pegylated liposomal doxorubicin and dexamethasone; VMP, melphalan and prednisone with bortezomib | ||
| MDT | Induction: Melphalan 8 mg/m2 orally on days 1–4 + Dexamethasone 12 mg/m2 orally on days 1–4 and 17–20 + Thalidomide 300 mg orally on days 1–4 and 17–20; Given on an every 5 week schedule for 3 cycles | 30 |
| Maintenance: Melphalan 8 mg/m2 orally on days 1–4 + Dexamethasone 12 mg/m2 orally on days 1–4 + Thalidomide 300 mg orally on days 1–4; Given every 5 weeks for 9 cycles | ||
| MPT | Italian Multiple Myeloma Network Phase II Study | 29 |
| Induction: Melphalan 4 mg/m2 orally on days 1–7 + Prednisone 40 mg/m2 orally on days 1–7 + Thalidomide 100 mg orally each day; Given on a monthly schedule for 6 months | ||
| Maintenance: Thalidomide 100 mg orally each day until progression | ||
| Supportive care: At physician’s discretion | ||
| Italian Multiple Myeloma Network Phase III Study | 31 | |
| Induction: Same as in phase II study above | ||
| Maintenance: Same as in phase II study above | ||
| Supportive care: Enoxaparin 40 mg subcutaneously each day during the first four cycles; Other agents at physician’s discretion | ||
| Intergroupe Francophone du Myélome Phase III Study | 32 | |
| Induction: 12 courses of standard MP at 6-week intervals with thalidomide at up to 400 mg daily | ||
| Maintenance: None; thalidomide was stopped at the end of therapy with MP | ||
| Supportive care: At physician’s discretion | ||
| R-MP | Induction: Melphalan 0.18 or 0.25 mg/kg orally on days 1–4 + Prednisone 2 mg/kg orally on days 1–4 + Lenalidomide 5 or 10 mg orally for 21 days; Given on an every 4–6 week schedule | 36 |
| Supportive care: Ciprofloxacin and aspirin | ||
| VMP | Induction: Melphalan 9 mg/m2 orally on days 1–4 + Prednisone 60 mg/m2 orally on days 1–4 + Bortezomib 1.0 or 1.3 mg/m2 intravenously days 1, 4, 8, 11, 22, 25, 29, and 32; Given on an every 6 week schedule for four cycles | 43 |
| Maintenance: Melphalan 9 mg/m2 orally on days 1–4 + Prednisone 60 mg/m2 orally on days 1–4 + Bortezomib 1.0 or 1.3 mg/m2 intravenously days 1, 8, 15, and 22; Given on an every 5 week schedule for five cycles | ||
| Supportive care: Intravenous bisphosphonates every 4 weeks; other measures at physician’s discretion | ||
| ThaDD | Induction: Thalidomide 100 mg orally each evening + pegylated liposomal doxorubicin 40 mg/m2 intravenously on day 1 + dexamethasone 40 mg orally on days 1–4 and 9–12, every 28 days | 49 |
| Supportive care: Warfarin 1.25 mg orally each day + vitamin B6 + ciprofloxacin 250 mg orally twice daily + intravenous zoledronate + erythropoietic and hypoglycemic agents as needed | ||
| Regimen . | Dosing and Schedule . | Reference . |
|---|---|---|
| Abbreviations: MDT, melphalan and dexamethasone with thalidomide; MP, melphalan and prednisone; MPT, melphalan and prednisone with thalidomide; R-MP, melphalan and prednisone with lenalidomide; ThaDD, thalidomide with pegylated liposomal doxorubicin and dexamethasone; VMP, melphalan and prednisone with bortezomib | ||
| MDT | Induction: Melphalan 8 mg/m2 orally on days 1–4 + Dexamethasone 12 mg/m2 orally on days 1–4 and 17–20 + Thalidomide 300 mg orally on days 1–4 and 17–20; Given on an every 5 week schedule for 3 cycles | 30 |
| Maintenance: Melphalan 8 mg/m2 orally on days 1–4 + Dexamethasone 12 mg/m2 orally on days 1–4 + Thalidomide 300 mg orally on days 1–4; Given every 5 weeks for 9 cycles | ||
| MPT | Italian Multiple Myeloma Network Phase II Study | 29 |
| Induction: Melphalan 4 mg/m2 orally on days 1–7 + Prednisone 40 mg/m2 orally on days 1–7 + Thalidomide 100 mg orally each day; Given on a monthly schedule for 6 months | ||
| Maintenance: Thalidomide 100 mg orally each day until progression | ||
| Supportive care: At physician’s discretion | ||
| Italian Multiple Myeloma Network Phase III Study | 31 | |
| Induction: Same as in phase II study above | ||
| Maintenance: Same as in phase II study above | ||
| Supportive care: Enoxaparin 40 mg subcutaneously each day during the first four cycles; Other agents at physician’s discretion | ||
| Intergroupe Francophone du Myélome Phase III Study | 32 | |
| Induction: 12 courses of standard MP at 6-week intervals with thalidomide at up to 400 mg daily | ||
| Maintenance: None; thalidomide was stopped at the end of therapy with MP | ||
| Supportive care: At physician’s discretion | ||
| R-MP | Induction: Melphalan 0.18 or 0.25 mg/kg orally on days 1–4 + Prednisone 2 mg/kg orally on days 1–4 + Lenalidomide 5 or 10 mg orally for 21 days; Given on an every 4–6 week schedule | 36 |
| Supportive care: Ciprofloxacin and aspirin | ||
| VMP | Induction: Melphalan 9 mg/m2 orally on days 1–4 + Prednisone 60 mg/m2 orally on days 1–4 + Bortezomib 1.0 or 1.3 mg/m2 intravenously days 1, 4, 8, 11, 22, 25, 29, and 32; Given on an every 6 week schedule for four cycles | 43 |
| Maintenance: Melphalan 9 mg/m2 orally on days 1–4 + Prednisone 60 mg/m2 orally on days 1–4 + Bortezomib 1.0 or 1.3 mg/m2 intravenously days 1, 8, 15, and 22; Given on an every 5 week schedule for five cycles | ||
| Supportive care: Intravenous bisphosphonates every 4 weeks; other measures at physician’s discretion | ||
| ThaDD | Induction: Thalidomide 100 mg orally each evening + pegylated liposomal doxorubicin 40 mg/m2 intravenously on day 1 + dexamethasone 40 mg orally on days 1–4 and 9–12, every 28 days | 49 |
| Supportive care: Warfarin 1.25 mg orally each day + vitamin B6 + ciprofloxacin 250 mg orally twice daily + intravenous zoledronate + erythropoietic and hypoglycemic agents as needed | ||
MP or MPT as initial therapy for multiple myeloma.31
| Response Category . | MPT (n= 129) . | MP (n= 126) . |
|---|---|---|
| Abbreviations: MP, melphalan and prednisone; MPT, melphalan and prednisone with thalidomide; n, number of patients in each cohort | ||
| Overall response rate (at least a partial response) | 98 (76.0%) | 60 (47.6%) |
| Complete response | 20 (15.5%) | 3 (2.4%) |
| Partial response | 78 (60.4%) | 57 (45.2%) |
| Near-complete response | 16 (12.4%) | 6 (4.8%) |
| Very good partial response | 11 (8.5%) | 6 (4.8%) |
| Partial response | 51 (39.5%) | 45 (35.7%) |
| Minimal response | 7 (5.4%) | 21 (16.7%) |
| No response | 7 (5.4%) | 19 (15.1%) |
| Progressive disease | 10 (7.8%) | 21 (16.7%) |
| Not available | 7 (5.4%) | 5 (4.0%)‘ |
| Response Category . | MPT (n= 129) . | MP (n= 126) . |
|---|---|---|
| Abbreviations: MP, melphalan and prednisone; MPT, melphalan and prednisone with thalidomide; n, number of patients in each cohort | ||
| Overall response rate (at least a partial response) | 98 (76.0%) | 60 (47.6%) |
| Complete response | 20 (15.5%) | 3 (2.4%) |
| Partial response | 78 (60.4%) | 57 (45.2%) |
| Near-complete response | 16 (12.4%) | 6 (4.8%) |
| Very good partial response | 11 (8.5%) | 6 (4.8%) |
| Partial response | 51 (39.5%) | 45 (35.7%) |
| Minimal response | 7 (5.4%) | 21 (16.7%) |
| No response | 7 (5.4%) | 19 (15.1%) |
| Progressive disease | 10 (7.8%) | 21 (16.7%) |
| Not available | 7 (5.4%) | 5 (4.0%)‘ |
MP, MPT, or intermediate-dose melphalan for multiple myeloma.32
| Response Quality . | MP (n= 196) . | MPT (n= 125) . | Mel 100 (n= 126) . |
|---|---|---|---|
| Abbreviations: Mel 100, patients received two cycles of infusional vincristine, doxorubicin, and oral dexamethasone, followed by stem cell mobilization and up to two autologous stem cell transplants with melphalan as a preparative regimen at 100 mg/m2; mos., months; MP, melphalan and prednisone; MPT, melphalan and prednisone with thalidomide; n, number of patients in each cohort | |||
| Complete response | 2% | 16% | 17% |
| At least a very good partial response | 8% | 50% | 43% |
| At least a partial response | 40% | 81% | 73% |
| Response durability | |||
| Progression-free survival | 17.1 mos. | 27.6 mos. | 19.4 mos. |
| Overall survival | 32.2 mos. | 53.6 mos. | 38.6 mos. |
| Response Quality . | MP (n= 196) . | MPT (n= 125) . | Mel 100 (n= 126) . |
|---|---|---|---|
| Abbreviations: Mel 100, patients received two cycles of infusional vincristine, doxorubicin, and oral dexamethasone, followed by stem cell mobilization and up to two autologous stem cell transplants with melphalan as a preparative regimen at 100 mg/m2; mos., months; MP, melphalan and prednisone; MPT, melphalan and prednisone with thalidomide; n, number of patients in each cohort | |||
| Complete response | 2% | 16% | 17% |
| At least a very good partial response | 8% | 50% | 43% |
| At least a partial response | 40% | 81% | 73% |
| Response durability | |||
| Progression-free survival | 17.1 mos. | 27.6 mos. | 19.4 mos. |
| Overall survival | 32.2 mos. | 53.6 mos. | 38.6 mos. |
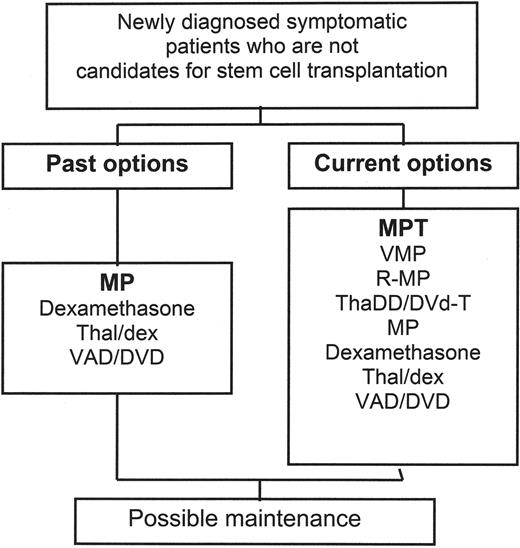
Past and current treatment algorithms for patients with multiple myeloma.
The recent standard treatment algorithm for patients who are not candidates for stem cell transplantation included MP as the standard of care, though other regimens could be used as well, including dexamethasone alone, thalidomide and dexamethasone, and infusional vincristine, doxorubicin, and dexamethasone, or its equivalent with pegylated liposomal doxorubicin. Results from two randomized phase III studies now indicate that MPT provides a survival advantage over MP, suggesting that this should be the current standard of care for patients who can tolerate this therapy.
Abbreviations: MP, melphalan and prednisone; MPT, melphalan and prednisone with thalidomide; R-MP, melphalan and prednisone with lenalidomide; ThaDD, thalidomide with pegylated liposomal doxorubicin and dexamethasone; thal/dex, thalidomide with dexamethasone; VAD/DVD dexamethasone, vincristine, adriamycin; DVd-T, pegylated liposomal doxorubicin/vincristine/reduced-dose dexamethasone, thalidomide
Past and current treatment algorithms for patients with multiple myeloma.
The recent standard treatment algorithm for patients who are not candidates for stem cell transplantation included MP as the standard of care, though other regimens could be used as well, including dexamethasone alone, thalidomide and dexamethasone, and infusional vincristine, doxorubicin, and dexamethasone, or its equivalent with pegylated liposomal doxorubicin. Results from two randomized phase III studies now indicate that MPT provides a survival advantage over MP, suggesting that this should be the current standard of care for patients who can tolerate this therapy.
Abbreviations: MP, melphalan and prednisone; MPT, melphalan and prednisone with thalidomide; R-MP, melphalan and prednisone with lenalidomide; ThaDD, thalidomide with pegylated liposomal doxorubicin and dexamethasone; thal/dex, thalidomide with dexamethasone; VAD/DVD dexamethasone, vincristine, adriamycin; DVd-T, pegylated liposomal doxorubicin/vincristine/reduced-dose dexamethasone, thalidomide
A hypothetical future treatment algorithm for patients with multiple myeloma.
A proposed treatment algorithm using novel agents, which is described in greater detail in the text. This algorithm includes regimens for which there is current support, such as MPT, as well as others that still require validation in a prospective fashion.
Abbreviations: BP, bendamustine and prednisone; FGFR, fibroblast growth factor receptor; ISS, International Staging System; MDT, melphalan and dexamethasone with thalidomide; MP, melphalan and prednisone; MPT, melphalan and prednisone with thalidomide; R-MP, melphalan and prednisone with lenalidomide; ThaDD, thalidomide with pegylated liposomal doxorubicin and dexamethasone; VMP, melphalan and prednisone with bortezomib
A hypothetical future treatment algorithm for patients with multiple myeloma.
A proposed treatment algorithm using novel agents, which is described in greater detail in the text. This algorithm includes regimens for which there is current support, such as MPT, as well as others that still require validation in a prospective fashion.
Abbreviations: BP, bendamustine and prednisone; FGFR, fibroblast growth factor receptor; ISS, International Staging System; MDT, melphalan and dexamethasone with thalidomide; MP, melphalan and prednisone; MPT, melphalan and prednisone with thalidomide; R-MP, melphalan and prednisone with lenalidomide; ThaDD, thalidomide with pegylated liposomal doxorubicin and dexamethasone; VMP, melphalan and prednisone with bortezomib
The University of North Carolina at Chapel Hill School of Medicine; the Department of Medicine, Division of Hematology/Oncology; the Lineberger Comprehensive Cancer Center; and the Department of Pharmacology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina
RZO, a Leukemia & Lymphoma Society Scholar in Clinical Research, and a Jefferson-Pilot Fellow in Academic Medicine, would also like to acknowledge grant support from the Leukemia & Lymphoma Society (6096-07), the Multiple Myeloma Research Foundation, and the National Cancer Institute (RO1 CA102278)