Abstract
Reduced-intensity conditioning (RIC) regimens were initially introduced to provide allogeneic stem cell transplantation (HCT), a potentially curative procedure for myeloid malignancies, for patients who were not considered eligible for conventional myeloablative HCT either because of advanced age or excessive comorbidities. A variety of RIC regimens have been studied. The exact definition of RIC remains arbitrary and generally depends upon the perceived toxicity of a given regimen rather than the actual dose of chemotherapy or radiotherapy administered. In several published series, RIC regimens have demonstrated a reduction in non-relapse mortality (NRM), thereby accomplishing the initial goal of expanding the patient population eligible for this potentially curative procedure. Most retrospective studies performed to date have shown a decrease in NRM and an increase in relapse-related mortality with the use of RIC as opposed to conventional myeloablative HCT in myeloid malignancies. This appears to be particularly true for patients who are in relapse at the time of HCT. In contrast, patients who are in remission at time of HCT appear to have a reduction in NRM without a subsequent increase in relapse-related mortality. There is interest in applying RIC to younger patients and to patients with fewer comorbidities as they may have a reduction in NRM without a concomitant increase in relapse. Prospective multicenter studies are needed to define the optimal conditioning regimen, which is likely dependent upon a variety of disease-specific and patient-specific factors.
Allogeneic hematopoietic cell transplantation (HCT) offers a curative option for patients with myeloid malignancies; however, a major limitation with conventional myeloablative HCT is conditioning toxicity and the ensuing non-relapse mortality (NRM). Reduced-intensity conditioning (RIC) regimens have been introduced in an attempt to lower NRM and to offer HCT to patients who otherwise would not be considered candidates because of age or comorbid conditions.1 Unlike conventional myeloablative conditioning, which relies upon high doses of chemotherapy or radiotherapy to eradicate malignant cells, RIC relies more upon the donor cell mediated graft-versus-leukemia (GVL) effects.2,3 Historically, conditioning intensity has been considered essential for preventing post-HCT relapse; therefore, a potential problem with RIC may be an increased risk of relapse. This is particularly true for diseases that are rapidly progressive or for patients with large tumor burdens at time of HCT. As GVL effects take time to develop, it is possible that the tempo of the underlying disease may outpace the control mediated by GVL effects. The ideal conditioning regimen is one with minimal morbidity, low NRM and no risk of relapse. Current research efforts are directed toward identifying the optimum conditioning regimens for specific populations which balance the competing side effects of NRM and relapse.
Development of RIC Regimens
The definition of RIC regimens remains arbitrary. One typically considers efficacy (eradication of the disease) and toxicity (particularly if this results in NRM) in assessments of intensity. A plethora of RIC regimens have recently been introduced, most as single-institution studies. Rather than an exhaustive review of these various regimens, we focus on the concepts that are driving the formulation of the RIC regimens, and summarize myeloid-specific studies in Table 1 .
Delivery of chemotherapy
New methods to deliver chemotherapy have been developed which have reduced associated toxicity. For example, intravenous (IV) busulfan (Bu) has been reported to reduce hepatotoxicity associated with HCT.4 Other developments include the use of alternative agents with a lower toxicity profile. For example, fludarabine (Flu) is increasingly used as an alternative to cyclophosphamide (Cy) in RIC regimens. Metabolites of Cy may contribute to morbidity and mortality following conventional myeloablative HCT.5 Furthermore, Flu, a purine analog, inhibits DNA repair and is a potent immunosuppressant that allows engraftment of donor stem cells with less conditioning toxicity.
Investigators at Dana Farber Cancer Institute reported results using a RIC regimen of Flu (120 mg/m2) and IV Bu (3.2 mg/kg) in 71 patients with myeloid and lymphoid malignancies who received HCT with HLA-matched related donors (MRD) (n = 30) or unrelated donors (URD) (n = 41).6 These results were retrospectively compared to results obtained with a conventional myeloablative regimen using total body irradiation (TBI 14 Gy) or oral Bu (16 mg/kg) with Cy (1800 mg/m2 × 2 days) in 81 patients followed by HCT with MRD (n = 52) or URD (n = 29). All patients were > 50 years of age. Overall survival (OS) was improved (P = 0.056) and NRM was reduced (P = 0.01) in patients who received RIC compared to conventional myeloablative conditioning. However, there was an increased risk of relapse with RIC (P = 0.052) resulting in no significant difference in relapse-free survival (RFS) between the two groups.
Incorporation of novel agents
RIC regimens not only have reduced the standard doses of myeloablative chemotherapy and radiotherapy but also have incorporated new agents in an effort to reduce side effects. Alemtuzumab has been incorporated into RIC regimens to decrease the incidence of graft-versus-host disease (GVHD) and subsequently improve OS. Investigators at King’s College published results using Flu (150 mg/m2), oral Bu (8 mg/kg), and anti-CD52 antibody alemtuzumab (100 mg) in 62 patients with myelodysplastic syndrome (MDS), chronic myelomonocytic leukemia, or acute myeloid leukemia with multilineage dysplasia (tAML).7 Patients were 22–70 (median 53) years of age and received MRD (n = 24), HLA-matched (n = 25) or mismatched (n = 13) URD stem cells. The cumulative 100-day incidence of grade 3–4 GVHD was 9% for URD and 0% for MRD recipients. A majority (67%) of MRD recipients and 28% of URD recipients required donor lymphocyte infusions (DLI) for relapse of disease or decreasing donor chimerism. The projected 1-year OS (74%), NRM (15%), and RFS (62%) were encouraging and suggested that RIC regimens incorporating alemtuzumab should be explored further. Since this initial publication, several other centers have published results using RIC regimens incorporating alemtuzumab.8,9 Additional alternative agents including antithymocyte globulin (ATG),10 are being used in an effort to reduce GVHD and associated NRM. Longer follow-up on these regimens will be needed before making definitive conclusions, particularly regarding relapse rates. Ultimately, these regimens may prove to be particularly useful in patients allografted with unrelated donors.
Introduction of targeted therapy
RIC regimens have moved beyond reductions in conditioning intensity and toward delivering targeted therapy, thereby sparing extra-hematopoietic organs, such as the skin, liver, lungs and heart. The Fred Hutchinson Cancer Research Center (FHCRC) reported results using a regimen of Flu (90 mg/m2) with TBI 2 Gy and 131I-labeled anti-CD45 antibody in 33 patients with advanced MDS and refractory or relapsed AML.11 Patients received either MRD (n = 10) or URD (n = 23) PBSC. All patients achieved remission and complete donor engraftment by day 28. The day-100 NRM was 12% and RFS was 55% with a median follow-up of 9.5 months. Investigators at M.D. Anderson Cancer Center (MDACC) incorporated gemtuzumab ozogamicin (GO) into a RIC regimen containing Flu, 100–150 mg/m2, and melphalan, 140–180 mg/m2, (FM). GO is a humanized anti-CD33 monoclonal antibody conjugated to calicheamicin, which is effective in relapsed elderly AML with minimal toxicity.12 Fifty-two high-risk patients with MDS or AML were conditioned with FM-GO, and there was a significant improvement in OS compared to FM alone (P = 0.008).13 Further follow-up is needed to determine the ultimate advantage of RIC regimens that use targeted therapy, but the hope is that these regimens will result in decreased NRM without a subsequent increase in relapse.
Nonmyeloablative conditioning
A drastic reduction in conditioning intensity characterizes nonmyeloablative conditioning regimens. The Seattle Consortium, a collaborative HCT group including the FHCRC, City of Hope, Stanford University, University of Colorado, Baylor University, Oregon Health Sciences University, University of Utah and the University of Leipzig conducted preliminary clinical studies that have demonstrated the feasibility and safety of conditioning patients with low dose TBI (2 Gy) or Flu (90 mg/m2) and 2 Gy TBI (Flu/TBI).14,15 These studies demonstrated that nonmyeloablative conditioning using either related or unrelated donors could be used in an effective manner in patients who were considered ineligible for conventional myeloablative transplantation because of either advanced age or comorbidities.
Investigators at Stanford University reported results using 8 Gy total lymphoid irradiation (TLI) delivered over 10 doses with concurrent ATG 1.5 mg/kg/day for 4 days.16 Thirty-seven patients with acute leukemia (n = 13) and lymphoid (n = 24) malignancies received TLI followed by PBSC from MRD (n = 23) or URD (n = 14). Only 2 patients experienced acute GVHD, and potent antitumor effects were observed. Longer follow-up is needed before definitive statements can be made regarding RFS or OS; however, this regimen represents a potential dramatic reduction in NRM and warrants further investigative study in disease specific trials.
Advantages of Reduced Intensity Conditioning
The benefits of RIC most importantly include a reduction in NRM and morbidity. In a retrospective review of 193 patients who received nonmyeloablative conditioning, no cases of hepatic venoocclusive disease, a potentially life-threatening complication of HCT, were noted.17 Several analyses also provide evidence for decreased infectious complications following RIC HCT. In a retrospective analysis at the FHCRC, the incidence of fungal and bacterial infections in 56 patients after nonmyeloablative HCT was compared to 112 concurrent patients (matched for disease state, donor type, cytomegalovirus [CMV] risk group, age, and stem cell source) who received a conventional myeloablative HCT.18 Patients conditioned with a nonmyeloablative regimen had a shorter duration of neutropenia (median 0 vs. 9 days; P < 0.0001) and experienced significantly fewer episodes of bacteremia during the first 30 days after HCT (9% vs. 27%; P = 0.01). There was no difference in the incidence of fungal infections. Among patients considered at high risk for CMV reactivation, there was a trend to less CMV antigenemia and less CMV disease with nonmyeloablative conditioning.19 The cumulative incidence of idiopathic pneumonia syndrome was also significantly lower following nonmyeloablative conditioning (2.2% vs. 8.4%; P = 0.003).20 Besides a reduction in infectious complications, there was also a reduced incidence of acute GVHD with nonmyeloablative conditioning (P = 0.001).21
The advantages of RIC extend beyond a reduction in NRM. Among patients conditioned with the Flu/TBI regimen, 53% did not require hospitalization during HCT, and for hospitalized patients the median length of the original hospital stay was 8 days.14,22 Further, a retrospective analysis comparing transfusion requirements showed a reduction in the need for platelet (23% vs. 100%, P < 0.0001), and red blood cell transfusions (63% vs. 96%, P = 0.001) with nonmyeloablative vs. myeloablative HCT, respectively. Moreover, the number of platelet (median 0 vs. 24) and red blood cell (median 2 vs. 6) transfusions required for a given patient was significantly reduced with nonmyeloablative HCT (P = 0.0001).23 RIC offers a potential for significant reductions in cost and health care resources compared to conventional myeloablative HCT.
Overall, results with RIC have been characterized by the absence of severe myelosuppression and regimen related toxicities. Patients have little or no mucositis, diarrhea or hepatic VOD. In patients with normal peripheral blood cell counts pre-HCT, there was only mild myelosuppression, and most did not require platelet transfusions. Thus, if the intensity of conditioning is not the decisive factor in preventing relapse, then the concomitant reduction in NRM observed with RIC should offer a distinct advantage.
Impact of Comorbidities on the Selection of Conditioning Regimens
In addition to general physiological effects of aging, one basis for the increased morbidity and NRM observed with HCT in older patients are co-existing medical conditions. The FHCRC evaluated the relevance of the Charlson Comorbidity Index (CCI) scores in MRD and URD HCT with nonmyeloablative and myeloablative conditioning.24,25 The CCI predicted NRM and OS with both nonmyeloablative and myeloablative HCT regardless of donor type. Patients who received nonmyeloablative conditioning were older, had higher CCI scores, and experienced longer time from diagnosis to HCT. Yet, they had significantly lower NRM regardless of donor type than patients prepared with myeloablative regimens. More recently, the FHCRC published a modified index considering comorbidities that were most relevant for HCT (HCT-CI).26 Patients received either nonmyeloablative (n = 294) or conventional myeloablative (n = 761) conditioning followed by URD (42%) or MRD (58%) HCT. Using Cox proportional hazard models, the HCT-CI proved more sensitive in predicting survival and NRM than did the CCI. In this analysis, patients with MDS and AML and an HCT-CI score of ≥ 2 who received nonmyeloablative conditioning had a significantly lower NRM and significantly improved overall survival compared to similar patients who received conventional myeloablative conditioning. Comorbidities as assessed by HCT-CI may be helpful in determining the most appropriate conditioning intensity for any given patient.
Role of GVHD Following RIC Regimens
As mentioned in the introductory paragraph, RIC regimens are more reliant upon the donor cell mediated GVL effects. The FHCRC has retrospectively assessed outcomes in 322 patients with various hematologic malignancies following nonmyeloablative conditioning of Flu 90 mg/m2 and TBI 2 Gy and HLA-matched related (n = 192) or unrelated (n = 130) donors.2 Acute GVHD was associated with an increased risk of NRM, but had no impact on risk of relapse. However, patients who developed chronic extensive GVHD had a significantly decreased risk of relapse (P = 0.006) and improved RFS (P = 0.003) without an increased risk of NRM. These data suggest that while acute GVHD contributed only to NRM without controlling disease, the development of chronic GVHD may be associated with the GVL effect following RIC.
Studies in MDS and AML
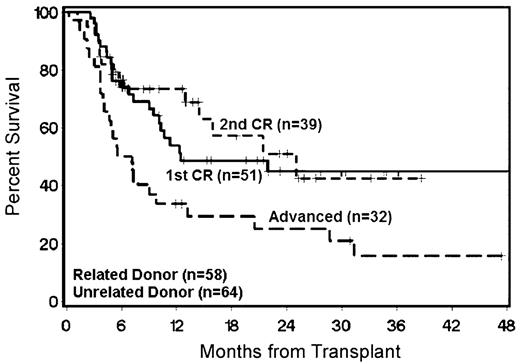
The Seattle Consortium has performed nonmyeloablative HCT in 122 patients with a diagnosis of AML using Flu/TBI.27 Patients were 17–74 (median 58) years of age, and 85% had de novo AML. Patients received MRD (n = 58), HLA-matched (n = 61) or mismatched URD (n = 3), PBSC (n = 116), or BM (n = 6). The indication for nonmyeloablative HCT was older age in the majority of patients (90%). Twelve percent of patients developed grade III–IV acute GVHD, and 36% developed chronic extensive GVHD. The day-100 NRM was 3%, and the 2-year NRM was 16%. The major causes of death unrelated to relapse were GVHD and infection. The 2-year OS was 48% and 2-year RFS was 44%. For patients with advanced disease at the time of HCT, the 2-year OS was 28% (Figure 1 ). Patients in first complete remission (CR1) transplanted from URD had a lower risk of relapse (16% vs. 50%, P = 0.005) and higher 2-year OS (63% vs. 44%, P = 0.13) than patients with MRD. This difference was presumably secondary to enhanced GVL effects mediated by URD stem cells. Among patients with second CR or advanced disease at HCT, there was no difference in outcome observed with respect to donor type. This observation suggests that GVL effects were not able to overcome the inherently high relapse risk in patients with more advanced disease. Nonmyeloablative HCT was successful even in patients of older age with advanced myeloid malignancies and significant comorbidities. The best results were seen in patients who were in CR at time of HCT, and for patients in CR1 unrelated donors were superior.
Despite the above studies, the long-term benefits of RIC regimens in comparison to conventional myeloablative regimens remain undetermined. Several retrospective analyses comparing RIC regimens to conventional myeloablative regimens have been published. Most of these studies included patients with MDS and AML, and, frequently, there was no distinction made between de novo and secondary AML within these cohorts or between primary and secondary MDS.
Conventional myeloablative versus RIC
The European Blood and Marrow Transplantation group (EBMT) reported a retrospective analysis of 722 patients ≥ 50 years of age with AML who underwent conventional myeloablative conditioning (n = 407) or RIC (n = 315) followed by MRD HCT.28 Cytogenetics, FAB classification, WBC, and disease status at the time of HCT were not statistically different between the two cohorts. The conditioning regimens analyzed included a variety of agents with variable dosing. RIC regimens included Flu with Bu (≤ 8 mg/kg) (53%) or TBI (< 3 Gy) (24%); whereas, patients conditioned with conventional myeloablative regimens received TBI ≥ 10 Gy or Bu > 8 mg/kg. The retrospective nature of this analysis and the variety of regimens used makes direct comparisons difficult. Notable findings included a significant reduction in acute and chronic GVHD and NRM (18% vs. 32%, P < 0.001) with RIC versus conventional myeloablative HCT; however, the relapse rate was significantly higher (41% vs. 24%, P < 0.0001). Subsequently there was no overall difference in 2-year RFS/OS between RIC and conventional myeloablative HCT (40%/47% vs. 44%/46%, respectively).
The EBMT reported a similar retrospective analysis in 836 patients with MDS and AML who received either conventional myeloablative conditioning (n = 621) or RIC (n = 215) followed by MRD HCT.29 Similar to the previous study reported by the EBMT, both RIC and conventional myeloablative conditioning regimens included a variety of agents with variable dosing. However, in this analysis, conventional myeloablative regimens were defined as containing Cy with TBI ≥ 8 Gy or Bu 16 mg/kg delivered through oral or IV route. RIC regimens may have contained Flu with TBI 2–4 Gy, Bu 8–10 mg/kg, or other alkylating agents with or without alemtuzumab or ATG. Patients who received RIC regimens were significantly older, had significantly more advanced disease status, and were more likely to have received PBSC as compared to patients who received a conventional myeloablative HCT. There were no significant differences in regards to IPSS risk and cytogenetic risk between the two cohorts, but this information was not available in many patients. The 3-year NRM was significantly reduced with RIC, but at the expense of a significantly higher 3-year relapse rate. Ultimately, there were no significant differences in 3-year RFS or OS between RIC and conventional myeloablative conditioning (33% vs. 39%, respectively; P = 0.9).
Both of these analyses highlight the potential advantages and disadvantages of RIC regimens, chiefly reduced NRM and increased relapse rates, respectively. Critiques of these analyses include the variety of conditioning regimens, the lack of a uniform GVHD prophylaxis, differences in risk factors between the cohorts, and the inclusion of only MRD. As stated earlier, RIC regimens are more dependent upon GVL effects than conventional myeloablative HCT; therefore, the use of unrelated donors could theoretically have reduced the increased relapse rates observed with RIC. Nonetheless, both of these analyses serve as a foundation for future controlled prospective studies to evaluate RIC and conventional myeloablative HCT.
RIC versus nonmyeloablative conditioning
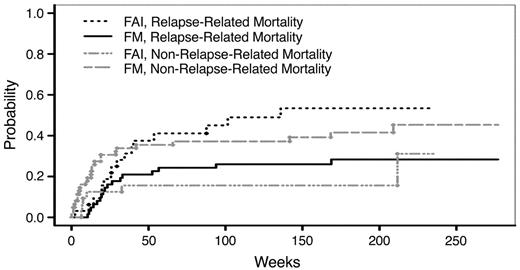
At MDACC, 94 patients with MDS or AML were conditioned with a reduced intensity regimen of Flu (100–150 mg/m2) and melphalan (140 or 180 mg/m2) (FM) (n = 62) or a nonmyeloablative regimen of Flu (120 mg/m2), cytarabine (4 g/m2), and idarubicin (36 mg/m2) (FAI) (n = 32).30 Patients who received FM were 22–75 (average 54) years of age, compared to FAI patients who were 27–74 (average 61) years of age (P = 0.001). Patients conditioned with FAI were more likely to receive BM (84% vs. 42%) as a source of stem cells (P < 0.001) and solely from MRD; whereas, more patients conditioned with FM received PBSC from URD (n = 29) rather than MRD (n = 25) or HLA-mismatched related donors (n = 8). Despite being significantly younger, patients conditioned with FM had significantly higher NRM when compared to FAI conditioned patients (39% vs. 16%, P = 0.036); this was counterbalanced by a significantly lower relapse-related mortality in the FM regimen compared to the FAI regimen (26% vs. 53%, P = 0.029) (Figure 2 ). With a median follow up of 40 months, the actuarial 3-year survival rate was not significantly different between the two cohorts (30% after FAI and 35% after FM, P = 0.79). It is conceivable that the increased use of BM and the absence of unrelated donors in the FAI cohort could account for the higher relapse-related mortality. Nonmyeloablative conditioning resulted in lower NRM, but at the expense of increased relapse rates when compared to RIC in this analysis. Therefore, in a selected patient population who are either at increased risk for NRM or at lower risk for relapse, nonmyeloablative HCT may be preferable.
Nonmyeloablative versus conventional myeloablative conditioning
The FHCRC reported a retrospective analysis of 150 patients ≥ 40 years of age with MDS or tAML who received a myeloablative regimen of oral Bu (16 mg/kg) and Cy (120 mg/kg) (n = 112), or a nonmyeloablative regimen, with 2 Gy TBI (n = 2) or Flu/TBI (n = 36).31 Patients were treated with a nonmyeloablative regimen because of age or comorbid illnesses. They were 40–73 (median 62) years of age, compared to 40–65 (median 52) years in the myeloablative cohort (P < 0.001). Nonmyeloablative patients also had significantly higher HCT-CI scores than myeloablative patients (P = 0.01). A larger proportion of nonmyeloablative patients had tAML (53% vs. 31%, P = 0.06) and had higher-risk disease by IPSS (53% vs. 30%, P = 0.004) as compared to patients in the myeloablative cohort. Stem cell sources were MRD (59% and 65%) or URD (41% and 35%) in the myeloablative and nonmyeloablative cohorts, respectively. Despite a higher risk of NRM among the nonmyeloablative cohort, there was no significant difference in 3-year NRM between the nonmyeloablative (41%) and the myeloablative cohort (34%, P = 0.94). The 3-year OS (48% vs. 27%, P = 0.56) and RFS (44% vs. 28%, P = 0.6) were not significantly different between myeloablative versus nonmyeloablative conditioning, respectively. Given the low intensity of conditioning and the relatively higher risk of relapse in the nonmyeloablative cohort, it was of particular interest that relapse rates did not differ significantly between the two cohorts. The major limitation with this analysis was that almost all patients who received nonmyeloablative conditioning had achieved a reduction in BM myeloblasts to < 5% following pre-HCT induction chemotherapy. This may have resulted in a selection bias favoring the nonmyeloablative cohort; however, there were other factors such as advanced age, higher IPSS categories, and higher HCT-CI scores that negatively biased the nonmyeloablative cohort. Despite these limitations, nonmyeloablative HCT resulted in similar OS and RFS as compared to conventional myeloablative HCT.
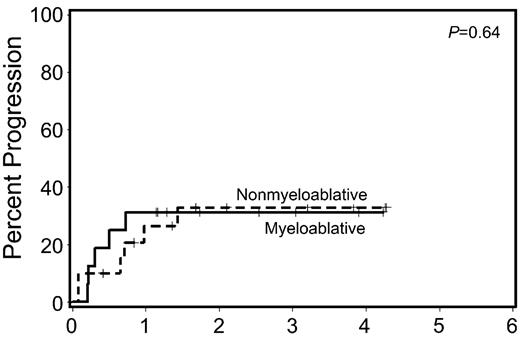
Available data suggested that patients who received induction chemotherapy before nonmyeloablative HCT have superior engraftment and survival when compared to patients who did not. For that reason, a subset analysis of the FHCRC study examined patients who entered HCT with < 5% BM myeloblasts following pre-HCT chemotherapy. OS (P = 0.84) and RFS (P = 0.93) after HCT among patients with < 5% BM myeloblasts following induction chemotherapy were similar following myeloablative and nonmyeloablative HCT (Figure 3 ). Investigators at Chaim Sheba Medical Center reported analogous results in 112 consecutive patients with MDS or AML. Significant differences were found in 2-year OS (50% vs. 47%) between a myeloablative regimen of IV Bu (12.8 mg/kg) with either Flu or Cy versus a RIC regimen of Flu (150–160 mg/m2) with IV Bu (6.4 mg/kg) followed by MRD (n = 59), HLA-matched (n = 45), or mismatched URD (n = 8) HCT.32 Patients who received RIC were more likely to be older, to be in CR at HCT, and have an URD. For patients in CR at time of HCT, there was no difference in 2-year OS (~50%) with RIC or conventional myeloablative HCT. However, patients with active disease at time of HCT had a significantly improved 2-year OS (45% vs. 0%, P = 0.01) with conventional myeloablative HCT versus RIC. Allogeneic GVL effects may be more important than the intensity of HCT conditioning in preventing relapse in patients with MDS or AML who are in CR at the time of HCT. This observation raises the question of whether nonmyeloablative HCT as a consolidation strategy should be considered in all patients with MDS or AML who achieve complete responses with pre-HCT induction chemotherapy.
Reduced Intensity Conditioning in CML
In contrast to MDS and AML, few papers evaluating RIC regimens in chronic myelogenous leukemia (CML) have been published. This is not because of a presumed lack of efficacy of RIC, but rather because of the effectiveness of tyrosine kinase inhibitors. Based on our knowledge of the sensitivity of CML to GVL effects, one would surmise that RIC regimens might be particularly effective for CML.
The Seattle Consortium published results with a nonmyeloablative regimen of Flu/TBI or 2 Gy TBI in 24 patients (median age 58 years) with CML-chronic phase 1 (CP1) (n = 14), second chronic phase (CP2) (n = 4) or accelerated phase (AP) (n = 6).33 All patients received PBSC from MRD. Patients were not eligible for conventional myeloablative HCT because of age, comorbid illness, or failed preceding autologous or allogeneic HCT. Five patients (21%) died from NRM, and the 100-day NRM was 4%. The 2-year estimates of OS were 70% for patients in CP1 and 56% for patients beyond CP1. Results from the Seattle Consortium using URD were not as initially encouraging because of high rates of graft rejection (45%).34 This analysis included patients who received BM and patients treated with low postgrafting immunosuppression who have been associated with poor engraftment following nonmyeloablative HCT. Investigators at Hadassah-Hebrew University Hospital published results in a younger cohort of patients (median age 35 years) using a RIC regimen of Flu, 180 mg/m2, oral Bu, 8 mg/kg, and ATG, 20–40 mg/kg, in 24 patients with CML-CP1.35 Nineteen patients received MRD and 5 received URD PBSC. Six patients had mixed chimerism and 3 required therapy with DLI. The 100-day NRM was 0% and there were only 3 mortalities in the series, all secondary to GVHD. The estimated 5-year OS and RFS was 85%. At a median of 42 months from HCT, all patients had a complete molecular response. The EBMT has reported results on 186 patients with CML (median age 50 years) who received RIC.36 Patients with all stages of CML were included: CP1 (n = 118), CP2 (n = 26), AP (n = 30), and blast crisis (n = 12). Various RIC regimens were included as long as the Bu dose was ≤ 8 mg/kg and the TBI dose was ≤ 6 Gy. Patients received PBSC (n = 133) or BM (n = 53) from MRD (n = 125), HLA-mismatched related (n = 8), HLA-matched (n = 47) and mismatched URD (n = 5), and unspecified (n = 1). The 100-day NRM was 6.1%, and the 2-year NRM was 23.3%. The 3-year OS and RFS was 58% and 37%, respectively. At least 40% of patients attained a complete molecular response and at least 62% a complete cytogenetic response. All of these studies indicate that RIC is a viable strategy in patients with CML; however, longer follow-up is necessary to determine the ultimate utility of RIC for CML, particularly in the era of tyrosine kinase inhibitors.
Summary and Conclusions
RIC regimens provide a potentially curative option for patients who were previously considered ineligible for HCT. Current research is focused on optimizing conditioning intensity for disease- and patient-specific parameters. Studies to date have confirmed a lower NRM with RIC, but at the expense of higher relapse rates. For patients with advanced age or high comorbidity scores, RIC may be preferable to conventional myeloablative conditioning because of a reduction in NRM. For patients with advanced disease or rapidly proliferating malignancies, conditioning intensity appears to be important in preventing post-HCT relapse. However, for patients in CR there may be no advantage in using conventional myeloablative regimens. Preliminary data suggest a potential benefit with RIC in defined patients with myeloid malignancies. However, no disease-specific prospective trials comparing results with RIC and conventional myeloablative HCT have been published. The Seattle Consortium currently has a prospective randomized trial evaluating nonmyeloablative HCT with Flu/TBI vs. conventional myeloablative HCT with 12–16 mg/kg of Bu with Flu or Cy for patients with MDS or AML in CR at the time of HCT. Other current trials include a TBI dose escalation study in patients with early-stage MDS and an ongoing study evaluating the utility of 131I anti-CD45 regimens in patients with advanced MDS or AML at time of HCT. The optimal conditioning regimen depends upon a variety of patient- and disease-specific characteristics, such as HCT-CI scores, patient age, disease type and disease status at time of HCT, as well as donor characteristics such as the use of URD. Disease-specific multi-institutional prospective studies are needed to evaluate the optimal RIC regimens.
Reduced-intensity conditioning (RIC) regimens in myeloid malignancies.
| Study Group . | Regimen* . | Disease Category . | Median Age (yrs) . | No. of Patients . | Donor Type . | Stem Cell Source . | Acute GVHD III–IV . | Chronic GVHD . | OS . | RFS . | NRM . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbreviations: ~, Estimated from figure; NA, not available; MRD, HLA-matched related donor; MMRD, HLA-mismatched related donor; URD, unrelated donor; BM, bone marrow stem cells; PBSC, peripheral blood stem cells; Flu, fludarabine; TBI, total body irriadiation; ATG, anti-thymocyte globulin; | |||||||||||
| *Regimens: 131I + Flu/TBI = 131I-anti-CD45 Antibody + Flu/TBI FAI = Flu 120 mg/m2 + cytarabine 1 g/m2 + idarubicin 36 mg/m2 FB + ATG = Flu 150 mg/m2 + Busulfan 8 mg/kg + ATG 20–40 mg/kg FB2 = Flu 150–160 mg/m2 + iv Busulfan 6.4 mg/kg FBC = Flu 150 mg/m2 + Busulfan 8 mg/kg + Alemtuzumab 100 mg Flu/TBI = Flu 90 mg/m2 + TBI 2 Gy FM = Flu 25–30 mg/m2 + melphalan 140 or 180 mg/m2 FMC = Flu 150 mg/m2 + melphalan 140 mg/m2 + Alemtuzumab 100 mg GO + FM = Gemtuzumab ozogamicin + FM | |||||||||||
| **TBI < 3 Gy or Bu ≤ 8 mg/kg | |||||||||||
| †TBI 2–4 Gy or Bu 8–10 mg/kg | |||||||||||
| ‡TBI ≤ 6 Gy or Bu ≤ 8 mg/kg | |||||||||||
| MDACC30 | FAI | AML + MDS | 61 | 32 | 81% MRD; 19% MMRD | 84% BM; 16% PBSC | 11% | 27% | 30% | 19% | ~18% (1-year) |
| MDACC30 | FM | AML + MDS | 54 | 62 | 40% MRD; 47% URD, 13% MMRD | 42% BM; 58% PBSC | 19% | 39% | 35% | 32% | ~21% (1-year) |
| FHCRC11 | 131I + Flu/TBI | AML + MDS | 61 | 33 | 30% MRD; 70% URD | 100% PBSC | NA | NA | NA | 55% | 12% (day 100) |
| MDACC13 | GO + FM | AML + MDS | NA | 52 | NA | NA | NA | NA | 40% | 30% | NA |
| FHCRC31 | Flu/TBI | AML + MDS | 62 | 38 | 68% MRD; 32% URD | 95% PBSC; 5% BM | 22% | 55% | 27% | 28% | 22% (1-year) |
| King’s College7 | FBC | AML + MDS | 56–52 | 62 | 39% MRD; 61% URD | 66% PBSC; 34% BM | 9% | ~20% | 74% | 62% | 15% (1-year) |
| Queen Elizabeth Hospital8 | FMC | AML + MDS | 52 | 76 | 46% MRD; 54% URD | 68% PBSC; 32% BM | 0% | NA | 41% | 37% | 19% (1-year) |
| University of Chicago9 | FMC | AML + MDS | 52 | 52 | 44% MRD; 49% URD | NA | 10% | 18% | 48% | 38% | 33% (1-year) |
| Seattle Consortium27 | Flu/TBI | AML | 58 | 122 | 48% MRD; 52% URD | 95% PBSC; 5% BM | 12% | 36% | 48% | 44% | 14% (1-year) |
| Chaim Sheba Med. Center32 | FB2 | AML + MDS | 57 | 41 | 34% MRD; 66% URD | NA | 8% | 31% | 47% | 43% | 8% (cumulative) |
| EBMT28 | TBI or Bu** | AML | 57 | 315 | 100% MRD | 90% PBSC; 10% BM | 8% | 48% | 47% | 40% | 18% (2-years) |
| EBMT29 | TBI or Bu† | AML + MDS | 56 | 215 | 100% MRD | 87% PBSC; 13% BM | 15% | 45% | 41% | 33% | 20% (1-year) |
| Seattle Consortium33 | Flu/TBI | CML | 58 | 24 | 100% MRD | 100% PBSC | 12% | 32% | 54% | 54% | 21% (cumulative) |
| Hadassah-Hebrew Univ.35 | FB + ATG | CML | 35 | 24 | 79% MRD; 21% URD | 100% PBSC | 29% | 55% | 85% | 85% | 0% (day 100) |
| EBMT36 | TBI or Bu‡ | CML | 50 | 186 | 67% MRD; 25% URD | 72% PBSC; 28% BM | 32% | 43% | 58% | 37% | 23.3% (2-year) |
| Study Group . | Regimen* . | Disease Category . | Median Age (yrs) . | No. of Patients . | Donor Type . | Stem Cell Source . | Acute GVHD III–IV . | Chronic GVHD . | OS . | RFS . | NRM . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbreviations: ~, Estimated from figure; NA, not available; MRD, HLA-matched related donor; MMRD, HLA-mismatched related donor; URD, unrelated donor; BM, bone marrow stem cells; PBSC, peripheral blood stem cells; Flu, fludarabine; TBI, total body irriadiation; ATG, anti-thymocyte globulin; | |||||||||||
| *Regimens: 131I + Flu/TBI = 131I-anti-CD45 Antibody + Flu/TBI FAI = Flu 120 mg/m2 + cytarabine 1 g/m2 + idarubicin 36 mg/m2 FB + ATG = Flu 150 mg/m2 + Busulfan 8 mg/kg + ATG 20–40 mg/kg FB2 = Flu 150–160 mg/m2 + iv Busulfan 6.4 mg/kg FBC = Flu 150 mg/m2 + Busulfan 8 mg/kg + Alemtuzumab 100 mg Flu/TBI = Flu 90 mg/m2 + TBI 2 Gy FM = Flu 25–30 mg/m2 + melphalan 140 or 180 mg/m2 FMC = Flu 150 mg/m2 + melphalan 140 mg/m2 + Alemtuzumab 100 mg GO + FM = Gemtuzumab ozogamicin + FM | |||||||||||
| **TBI < 3 Gy or Bu ≤ 8 mg/kg | |||||||||||
| †TBI 2–4 Gy or Bu 8–10 mg/kg | |||||||||||
| ‡TBI ≤ 6 Gy or Bu ≤ 8 mg/kg | |||||||||||
| MDACC30 | FAI | AML + MDS | 61 | 32 | 81% MRD; 19% MMRD | 84% BM; 16% PBSC | 11% | 27% | 30% | 19% | ~18% (1-year) |
| MDACC30 | FM | AML + MDS | 54 | 62 | 40% MRD; 47% URD, 13% MMRD | 42% BM; 58% PBSC | 19% | 39% | 35% | 32% | ~21% (1-year) |
| FHCRC11 | 131I + Flu/TBI | AML + MDS | 61 | 33 | 30% MRD; 70% URD | 100% PBSC | NA | NA | NA | 55% | 12% (day 100) |
| MDACC13 | GO + FM | AML + MDS | NA | 52 | NA | NA | NA | NA | 40% | 30% | NA |
| FHCRC31 | Flu/TBI | AML + MDS | 62 | 38 | 68% MRD; 32% URD | 95% PBSC; 5% BM | 22% | 55% | 27% | 28% | 22% (1-year) |
| King’s College7 | FBC | AML + MDS | 56–52 | 62 | 39% MRD; 61% URD | 66% PBSC; 34% BM | 9% | ~20% | 74% | 62% | 15% (1-year) |
| Queen Elizabeth Hospital8 | FMC | AML + MDS | 52 | 76 | 46% MRD; 54% URD | 68% PBSC; 32% BM | 0% | NA | 41% | 37% | 19% (1-year) |
| University of Chicago9 | FMC | AML + MDS | 52 | 52 | 44% MRD; 49% URD | NA | 10% | 18% | 48% | 38% | 33% (1-year) |
| Seattle Consortium27 | Flu/TBI | AML | 58 | 122 | 48% MRD; 52% URD | 95% PBSC; 5% BM | 12% | 36% | 48% | 44% | 14% (1-year) |
| Chaim Sheba Med. Center32 | FB2 | AML + MDS | 57 | 41 | 34% MRD; 66% URD | NA | 8% | 31% | 47% | 43% | 8% (cumulative) |
| EBMT28 | TBI or Bu** | AML | 57 | 315 | 100% MRD | 90% PBSC; 10% BM | 8% | 48% | 47% | 40% | 18% (2-years) |
| EBMT29 | TBI or Bu† | AML + MDS | 56 | 215 | 100% MRD | 87% PBSC; 13% BM | 15% | 45% | 41% | 33% | 20% (1-year) |
| Seattle Consortium33 | Flu/TBI | CML | 58 | 24 | 100% MRD | 100% PBSC | 12% | 32% | 54% | 54% | 21% (cumulative) |
| Hadassah-Hebrew Univ.35 | FB + ATG | CML | 35 | 24 | 79% MRD; 21% URD | 100% PBSC | 29% | 55% | 85% | 85% | 0% (day 100) |
| EBMT36 | TBI or Bu‡ | CML | 50 | 186 | 67% MRD; 25% URD | 72% PBSC; 28% BM | 32% | 43% | 58% | 37% | 23.3% (2-year) |
Overall survival in 122 acute myeloid leukemia (AML) patients following nonmyeloablative hematopoietic cell transplantation (HCT) according to disease status at time of HCT.
Overall survival in 122 acute myeloid leukemia (AML) patients following nonmyeloablative hematopoietic cell transplantation (HCT) according to disease status at time of HCT.
Cumulative incidence of relapse-related mortality and non-relapse mortality for FM- versus FAI-conditioned patients.
Abbreviations: FM, fludarabine, melphalan; FAI, fludarabine, cytarabine, idarubicin.
This figure was originally published in Blood.
Cumulative incidence of relapse-related mortality and non-relapse mortality for FM- versus FAI-conditioned patients.
Abbreviations: FM, fludarabine, melphalan; FAI, fludarabine, cytarabine, idarubicin.
This figure was originally published in Blood.
Relapse rates among patients with <5% marrow myeloblasts at time of hematopoietic cell transplantation (HCT) following pre-HCT induction chemotherapy.
Figure originally published in Leukemia.
Relapse rates among patients with <5% marrow myeloblasts at time of hematopoietic cell transplantation (HCT) following pre-HCT induction chemotherapy.
Figure originally published in Leukemia.
Fred Hutchinson Cancer Research Center and the University of Washington School of Medicine, Seattle, WA, USA.
This work was supported by grants CA78902, CA18029, HL 082941, CA 087948 and HL36444 of the National Institutes of Health, Bethesda MD.
Acknowledgments: The authors would like to thank Bonnie Larson, Helen Crawford and Sue Carbonneau for help with manuscript preparation.