Abstract
Almost 80 years after Eli Moschcowitz published the first description of the disease, most patients with idiopathic thrombotic thrombocytopenic purpura (TTP) were found to have acquired autoantibody inhibitors of the ADAMTS13 metalloprotease. Plasma ADAMTS13 normally cleaves von Willebrand factor within nascent platelet-rich thrombi, and ADAMTS13 deficiency allows unchecked thrombus growth to cause microangiopathic hemolysis, thrombocytopenia, and tissue infarction. At present, ADAMTS13 deficiency with a high-titer inhibitor level appears to be associated with an increased risk of early death and subsequent relapse. Thus, acquired ADAMTS13 deficiency identifies a specific mechanism of TTP and is a potential biomarker of disease activity or risk. At present, two major clinical questions in the field may be summarized as follows. First, by emphasizing TTP caused by ADAMTS13 deficiency, are we in danger of neglecting other causes that should be treated with plasma exchange? Second, should we treat asymptomatic patients who have severe ADAMTS13 deficiency to prevent future disease, and if so, how?
The last few years have set the stage for a new approach to the management of thrombotic thrombocytopenic purpura (TTP). The criteria for idiopathic TTP have remained approximately constant, and plasma exchange is still the standard therapy. But the discovery of the ADAMTS13 metalloprotease has revolutionized our understanding of TTP and initiated a period of experimentation with regard to diagnosis and treatment. The management of TTP has been reviewed recently,1 and I will not discuss it comprehensively. Instead, I will try to integrate the new knowledge about ADAMTS13 into a model for the pathogenesis of TTP, relate it to our experience in caring for these patients, and suggest where we may look for further advances during the next few years.
I. Plasma Exchange for Idiopathic TTP
The modern era in the management of TTP began with a randomized trial, published in 1991, which established that plasma exchange is superior to plasma infusion for the treatment of TTP.2 During the decade before these results were reported, anecdotal experience indicated that plasma therapy could cure patients who otherwise had an expected survival of < 10%. By demonstrating that plasma exchange increased survival to > 80%, this key trial simultaneously established a standard of care and an enduring clinical definition of idiopathic TTP.2
The entry criteria for this study included microangiopathic hemolytic anemia (Coombs’ negative), thrombocytopenia (< 100,000/μL), and no alternative (secondary) explanation such as cancer, disseminated intravascular coagulation (DIC), or eclampsia. There was no requirement for neurological deficits, fever, or renal involvement. In fact, subjects with oliguria were excluded because they could not tolerate plasma infusion.2
Today we still diagnose idiopathic TTP using essentially the same criteria (Table 1 ): unexplained microangiopathic hemolytic anemia and thrombocytopenia. However, the spectrum of associated symptoms has changed. In contrast to case series from the era before plasma exchange, patients often do not have fever, significant renal impairment, or obvious neurological involvement at diagnosis.2 This shift toward earlier diagnosis follows naturally from the desire to provide plasma exchange therapy to every patient who may benefit. However, there has been little further improvement in prognosis. Approximately 20% of patients with idiopathic TTP still die during the first month of acute illness, and at least 30% experience one or more relapses within 2 years.3–5 Practically speaking, will we ever be able to predict who will respond to plasma exchange, or who will relapse? If so, can we offer effective alternative therapy?
II. ADAMTS13 Changed the Landscape
Before 1998, our ability to make such predictions was hampered by a fundamental lack of knowledge about the pathophysiology of TTP. In that year, adults with idiopathic TTP were reported to have acquired autoantibodies that inhibit a von Willebrand factor (VWF) cleaving protease, which is normally present in plasma.6,7 In 2001, the VWF cleaving protease was purified and cloned by several groups, shown to be a new member of the “a disintegrin and metalloprotease with thrombospondin type 1 repeats” family, and named ADAMTS13.8–12 Thanks to these discoveries, we now have a plausible model for the pathogenesis of TTP (Figure 1 ): autoantibodies inhibit the activity of ADAMTS13. The absence of ADAMTS13 allows VWF and platelets to accumulate unchecked in microvascular thrombi, which leads to platelet consumption, hemolysis, and microvascular occlusion. Plasma exchange is efficacious because it removes pathogenic autoantibodies and replenishes the missing ADAMTS13 protease, restoring the normal regulation of VWF-dependent platelet adhesion. As we will see, this is a reasonable model but it may not explain everything about idiopathic TTP.
III. Clinical Correlations of ADAMTS13 Deficiency
III.A. Specificity and sensitivity for idiopathic TTP
Subsequent studies have begun to demonstrate the potential and also the limitations of ADAMTS13 testing. Modest decreases in plasma ADAMTS13 activity occur in a variety of acute and chronic illness, but rarely are the values < 25% of normal. ADAMTS13 is synthesized mainly in the liver, and severe ADAMTS13 deficiency (< 5%) has occurred with hepatic failure from various causes. Severe ADAMTS13 deficiency appears to be more frequent in sepsis-induced DIC. In a retrospective study of 109 patients, ADAMTS13 levels were < 5% in 17 patients and < 20% in 51 patients.13
In contrast, studies of patients with thrombotic microangiopathy have confirmed the specificity of severe ADAMTS13 deficiency (undetectable, or < 5%) for idiopathic TTP, although the sensitivity remains controversial. Severe ADAMTS13 deficiency rarely occurs in “secondary” TTP associated with cancer, hematopoietic stem cell or solid organ transplantation, preeclampsia, systemic infections, drug toxicity, or other predisposing conditions. Also, severe ADAMTS13 deficiency is almost unheard of in diarrhea-associated hemolytic uremic syndrome caused by Shiga toxin–producing E. coli (D+HUS) or other thrombotic microangiopathy accompanied by oliguric renal failure (“atypical” HUS). The Oklahoma TTP-HUS registry provides representative data: none of 92 patients with these varieties of secondary TTP or D+HUS had severe ADAMTS13 deficiency.4 Similarly, a major referral laboratory in Switzerland reported that 3 of 188 subjects (1.6%) with secondary TTP and none of 130 subjects with HUS had severe ADAMTS13 deficiency.14
On the other hand, when patients with idiopathic TTP are stratified by plasma ADAMTS13 activity level, the incidence of severe ADAMTS13 deficiency (< 5% of normal pooled plasma) has varied from 33%4 to 100%,6,7,15 with intermediate values of 52% to 94% in other studies.3,14,16–18 Some of this variation probably reflects differences in case definitions for idiopathic TTP or assay methodology. In any case, some patients diagnosed with idiopathic TTP do not have severe ADAMTS13 deficiency, at least in vitro. Whether the short-term prognosis differs for idiopathic TTP with or without ADAMTS13 deficiency is not yet clear. One study suggests that the initial response to plasma exchange is similar for the two groups.4
As an aside, the recent progress in understanding familial and idiopathic TTP has been matched by similar advances regarding atypical HUS. In a study of 156 patients, mutations in regulators of the alternative complement pathway (factor H, factor I, or MCP) were identified in 74 of them.19 Thus, the clinical distinction between TTP (mild renal insufficiency) and HUS (severe renal insufficiency) often correlates with distinct pathophysiologic mechanisms. Because the features of idiopathic TTP and atypical HUS can overlap, laboratory testing for ADAMTS13 and complement function could be important, because familial TTP caused by ADAMTS13 deficiency responds to simple plasma infusion, whereas atypical HUS responds poorly if at all to intensive plasma exchange and has a much worse long term prognosis.19
III.B. Exceptional causes of autoimmune TTP
In general, secondary TTP (Table 1 ) is not associated with severe ADAMTS13 deficiency and rarely responds to plasma exchange, but there are a few interesting exceptions.
Autoimmune diseases of various kinds have been described in association with severe ADAMTS13 deficiency and thrombotic microangiopathy that is indistinguishable from “idiopathic” TTP.20–22 Conversely, patients with idiopathic TTP and severe ADAMTS13 deficiency (< 5%) often have manifestations of systemic lupus erythematosus or other autoimmune diseases, such as antinuclear antibodies, polyarthritis, malar rash, extramembranous glomerulonephritis, discoid lupus, or autoimmune thyroiditis.23 Many autoimmune diseases can cause hemolytic anemia, thrombocytopenia and organ dysfunction by several mechanisms, and ADAMTS13 testing may facilitate the recognition of idiopathic TTP in these complex cases.
Pregnancy-associated thrombotic microangiopathy has many causes that can be difficult to diagnose correctly. The features of preeclampsia or HELLP syndrome may overlap those of idiopathic TTP, but these patients do not have ADAMTS13 deficiency.24 However, pregnancy can trigger TTP in women who do have congenital or acquired ADAMTS13 deficiency.25 ADAMTS13 testing may be useful for discriminating among the varieties of thrombotic microangiopathy during pregnancy and postpartum.
Ticlopidine causes thrombotic microangiopathy with a frequency of 1 case per 1600 to 5000 patients treated, usually after 2 to 12 weeks of drug use, usually by inducing the formation of autoantibodies to ADAMTS13.26 The mechanism of autoantibody induction is unknown. Ticlopidine-associated autoimmune TTP responds to plasma exchange, which reduces the mortality from 60% without treatment to 14–20% with treatment.27 TTP is much less common with clopidogrel, a related thienopyridine antagonist of platelet ADP receptor signaling,28 and in many cases has not been associated with autoantibodies to ADAMTS13.
III.C. Prognostic significance
In three studies that included both idiopathic and secondary TTP (excluding D+HUS), patients with severe ADAMTS13 deficiency had a good response rate to plasma exchange (89–100%) and low mortality (8–19%), whereas patients without ADAMTS13 deficiency had a lower response rate (54–82%) and higher mortality (18–56%).3–5 Unfortunately, knowledge of the ADAMTS13 level added little because most of the differences in response rate and mortality were captured by the clinical distinction between idiopathic and secondary TTP.3,4
Focusing specifically on idiopathic TTP, however, ADAMTS13 assays may provide useful prognostic information. In the Oklahoma TTP-HUS registry, 6 of 14 patients (43%) with severe ADAMTS13 deficiency later relapsed, compared to 2 of 25 patients (8%) without severe ADAMTS13 deficiency.4 In studies of idiopathic TTP with severe ADAMTS13 deficiency, patients with detectable inhibitors had a delayed response to plasma exchange18,29 and an increased risk of relapse.3,17 Deaths occurred only in patients with detectable inhibitors, for whom the death rate was 17% to 25%.3,17,18 Although relatively few patients have been studied to date, the results suggest that severe ADAMTS13 deficiency with a significant inhibitor titer at diagnosis confers an increased risk of early death and relapsing disease.
III.D. A biomarker of disease
For most patients, a complete response to plasma exchange is accompanied by normalization of ADAMTS13 activity and disappearance of ADAMTS13 inhibitors, if present.3,17 Interestingly, one-fourth to one-third of responders has persistent total ADAMTS13 deficiency, and many such patients also have unchanged or increased inhibitor titers during remission.3,17 Why does their disease remit in the first place, and what is their risk of relapse?
The resolution of thrombotic microangiopathy without improvement in ADAMTS13 deficiency also occurs in familial TTP caused by ADAMTS13 mutations. Some subjects with congenital ADAMTS13 deficiency require continuous prophylactic treatment with plasma to prevent thrombotic microangiopathy, whereas others have long disease-free intervals that may last years without treatment, but develop acute thrombotic microangiopathy in association with infections, surgery, pregnancy, or other stress. A very few adults with total ADAMTS13 deficiency have never had thrombotic microangiopathy at all.30 Thus, inflammatory stress exacerbates TTP in congenital ADAMTS deficiency, and the stress of surgery31 or pregnancy25 does the same in acquired idiopathic TTP. In both familial and acquired idiopathic TTP, the resolution of stress may restore a condition of compensated microvascular thrombosis that is insufficient to cause overt disease, thereby accounting for remissions with plasma therapy despite persistent ADAMTS13 deficiency, as well as relapses with later episodes of inflammatory stress.
Nearly all patients with relapsing idiopathic TTP have ADAMTS13 deficiency at the time of relapse,3,17 although it is difficult to predict when or whether a relapse will occur. However, relapsing TTP is a catastrophic and sometimes fatal illness that strikes at least 30% of patients within two years of surviving an acute episode of TTP. It is worth considering whether the endpoint of treatment should remain the achievement of complete clinical response, or whether the goal should include the eradication of autoantibody inhibitors and restoration of a normal ADAMTS13 level.
IV. Rituximab for Idiopathic TTP
The discovery that idiopathic TTP usually is an autoimmune disease has encouraged the routine use of immunosuppressive agents. For example, the efficacy of corticosteroids has not been demonstrated conclusively, but many of us combine prednisone with plasma exchange, expecting that it may reduce autoantibody production. Even so, many patients do not respond satisfactorily, and during the past few years immunosuppression with rituximab has become popular as salvage therapy for refractory or relapsing TTP. Rituximab is a humanized monoclonal antibody against CD20, which is expressed on B cells, and it rapidly clears B cells from the circulation. Most antibody-producing plasma cells live only a few days, and destruction of B cells can prevent the replenishment of these pathological plasma cells. Several case reports and small series suggest that rituximab induces complete responses in the majority of patients with TTP refractory to plasma exchange, corticosteroids, and other treatments such as vincristine or splenectomy. Responses to rituximab correlate with disappearance of ADAMTS13 inhibitors and a rise of the ADAMTS13 level into the normal range.32,33
Success with refractory disease suggests that rituximab could be useful for newly diagnosed patients who achieve remission but are at high risk of relapse.33 Assessing the efficacy of rituximab in this setting may be difficult because the majority of patients do not experience relapses, and the utility of ADAMTS13 data for predicting the risk of relapse is still uncertain. These issues will be addressed in a proposed randomized trial of plasma exchange, with or without rituximab, as primary therapy for idiopathic TTP.34
V. Autoimmune ADAMTS13 Deficiency and Thrombosis Without TTP
In principle, ADAMTS13 deficiency might cause devastating thrombosis in a critical organ such as the brain or heart, without enough microvascular thrombosis to cause microangiopathic hemolysis and thrombocytopenia. In fact, 3 patients have been described who presented with acute cerebrovascular events but normal platelet counts, normal or trivially elevated LDH, and no schistocytes. Because these patients had a prior history of TTP, ADAMTS13 activity was checked and found to be low, with a detectable inhibitor, and they responded to plasma exchange or rituximab.35,36 Such cases raise the frightening possibility that ADAMTS13 deficiency may cause stroke or other thrombosis in patients without any history of TTP. If this proves to occur at a significant frequency, then ADAMTS13 testing would be appropriate for patients with acute thrombotic events who have no obvious risk factors, especially if accompanied by thrombocytopenia.
VI. The Evolving Approach to Thrombotic Microangiopathy
Recent discoveries have provided new insight into the mechanism of idiopathic TTP but have had a relatively modest impact on the clinical approach to diagnosing and treating thrombotic microangiopathy. This may change as the results of ongoing clinical research become available.
VI.A. Clinical strategy for today
Empiric plasma exchange for all idiopathic TTP.
For now, plasma exchange remains the primary treatment for idiopathic TTP, reducing the acute mortality from > 90% to < 20%. Therefore, any patient who reasonably may have idiopathic TTP should receive plasma exchange as soon as possible, typically at 1.0 or 1.5 volumes of plasma per day. Prednisone can be included at 1 or 2 mg/kg per day, in divided doses. The criteria for selecting these patients include microangiopathic hemolytic anemia, thrombocytopenia, and the absence of an obvious alternative explanation such as cancer, sepsis, disseminated intravascular coagulation, tissue transplantation, certain drugs, and recent bloody diarrhea. Significant renal insufficiency at presentation is substantially more common in HUS but is not a sufficient reason to withhold plasma exchange because it does occur sometimes in idiopathic TTP that is caused by ADAMTS13 deficiency. However, a rapidly rising creatinine, especially with oliguria, should prompt an aggressive search for secondary causes of TTP, for infection by Shiga toxin–producing organisms, and in some cases for complement regulatory defects.
ADAMTS13 testing and difficult decisions.
If rapid testing is available, a finding of severe ADAMTS13 deficiency can help to justify the continuation of plasma exchange when the clinical presentation is not typical of idiopathic TTP, especially when thrombotic microangiopathy occurs in association with preexisting autoimmune disease or during pregnancy. Also, patients with other potential causes of secondary TTP do rarely acquire antibodies to ADAMTS13. Appropriate testing can identify this minority with coincidental TTP caused by ADAMTS13 deficiency that will, in fact, respond to plasma exchange.
ADAMTS13 as a biomarker in relapsing TTP.
Relapsing idiopathic TTP almost always is caused by persistent or recurrent autoimmune ADAMTS13 deficiency, and immunosuppression can prevent subsequent relapses by restoring normal ADAMTS13 activity. Therefore, testing can identify refractory or relapsing patients with severe ADAMTS13 deficiency, often with detectable inhibitors, who may benefit from immunosuppression. ADAMTS activity and inhibitor titer also serve as biomarkers to monitor the response to treatment.
VI.B. Questions for tomorrow
Routine ADAMTS 13 assays.
The possibility of using ADAMTS13 data to manage thrombotic microangiopathy obviously is premised on the widespread availability of ADAMTS13 assays that are rapid, robust, and feasible for most clinical laboratories. Several such assays are under development. As one example, a potentially suitable fluorogenic ADAMTS13 substrate has been described.37 Assays using this substrate can be adapted to measure ADAMTS13 inhibitors, but more sensitive inhibitor assays still are needed. Some pathogenic antibodies appear to promote the clearance of ADAMTS13 from blood without inhibiting its activity,38 and assays for these non-neutralizing antibodies and for ADAMTS13 antigen39 could be useful as well.
Primary versus secondary immunosuppression.
The successful treatment of relapsing TTP with rituximab suggests that combining rituximab with plasma exchange could improve the long-term prognosis of newly diagnosed idiopathic TTP, especially if high-risk patients could be identified. Whether ADAMTS13 data will be useful for selecting patients for immediate immunosuppressive treatment is unknown. Whether such treatment will actually reduce the rate of future relapses also is unknown. A related issue is whether asymptomatic patients with persistent ADAMTS13 deficiency should be treated while in remission to prevent future relapses. These questions will need to be answered by clinical trials.34
Idiopathic TTP without ADAMTS13 deficiency.
The ability to routinely measure ADAMTS13 level will enable closer study of patients with idiopathic TTP despite having normal ADAMTS13 activity. Either their thrombotic microangiopathy is caused by defective ADAMTS13 function that cannot be detected with our current in vitro assays, or it is caused by another mechanism that needs to be characterized.
Thrombosis without idiopathic TTP.
The clinical uses of ADAMTS13 assays may prove to extend beyond the evaluation of patients with idiopathic TTP. Patients with severe autoimmune ADAMTS13 deficiency can present with acute neurological deficits, in the absence of overt thrombotic microangiopathy. This phenomenon could be investigated as a potential cause of stroke in persons who lack traditional risk factors and also have no history of TTP. Finally, non-immune ADAMTS13 deficiency occurs in some patients with liver failure or sepsis-induced DIC, and has been proposed to contribute to microvascular thrombosis and renal injury. If prospective studies confirm a proposed relationship between ADAMTS13 deficiency and tissue injury in these settings,13 then replacement therapy to increase ADAMTS13 levels could be evaluated.
Classification and characteristics of thrombotic microangiopathy syndromes.
| Category . | Clinical Features* . | Mechanism . | Treatment . |
|---|---|---|---|
| *Clinical features listed are in addition to microangiopathic hemolytic anemia and thrombocytopenia. | |||
| **Conditions associated with autoimmune ADAMTS13 deficiency and idiopathic TTP include certain autoimmune disorders, pregnancy, and ticlopidine. | |||
| Idiopathic TTP | Coombs negative, without DIC or other conditions associated with secondary TTP. Severe renal failure is uncommon. Specific conditions** that may coexist with idiopathic TTP are discussed in the text. | Autoimmune ADAMTS13 deficiency in a majority of patients. | > 80% response to plasma exchange. May benefit from immunosuppression. |
| Secondary TTP | Associated conditions include cancer, infection, hematopoietic stem cell transplantation, solid organ transplantation, chemotherapy, certain drugs. | Mechanisms are mostly unknown. ADAMTS13 deficiency is rare. | With few exceptions, responses to plasma exchange are unlikely. Treatment and prognosis are dictated by the specific associated conditions. |
| Diarrhea-associated HUS (D+HUS) | Acute renal failure, often oliguric, preceded by bloody diarrhea. | Endothelial damage by Shiga toxin–producing E. coli. ADAMTS13 deficiency is rare. | No demonstrated efficacy of plasma exchange. |
| Atypical HUS | Acute renal failure, often oliguric, without a prior diarrheal illness. | Complement regulatory protein defects in at least 50% of patients; ADAMTS13 deficiency is rare. | No demonstrated efficacy of plasma exchange, except possibly for factor H deficiency. |
| Category . | Clinical Features* . | Mechanism . | Treatment . |
|---|---|---|---|
| *Clinical features listed are in addition to microangiopathic hemolytic anemia and thrombocytopenia. | |||
| **Conditions associated with autoimmune ADAMTS13 deficiency and idiopathic TTP include certain autoimmune disorders, pregnancy, and ticlopidine. | |||
| Idiopathic TTP | Coombs negative, without DIC or other conditions associated with secondary TTP. Severe renal failure is uncommon. Specific conditions** that may coexist with idiopathic TTP are discussed in the text. | Autoimmune ADAMTS13 deficiency in a majority of patients. | > 80% response to plasma exchange. May benefit from immunosuppression. |
| Secondary TTP | Associated conditions include cancer, infection, hematopoietic stem cell transplantation, solid organ transplantation, chemotherapy, certain drugs. | Mechanisms are mostly unknown. ADAMTS13 deficiency is rare. | With few exceptions, responses to plasma exchange are unlikely. Treatment and prognosis are dictated by the specific associated conditions. |
| Diarrhea-associated HUS (D+HUS) | Acute renal failure, often oliguric, preceded by bloody diarrhea. | Endothelial damage by Shiga toxin–producing E. coli. ADAMTS13 deficiency is rare. | No demonstrated efficacy of plasma exchange. |
| Atypical HUS | Acute renal failure, often oliguric, without a prior diarrheal illness. | Complement regulatory protein defects in at least 50% of patients; ADAMTS13 deficiency is rare. | No demonstrated efficacy of plasma exchange, except possibly for factor H deficiency. |
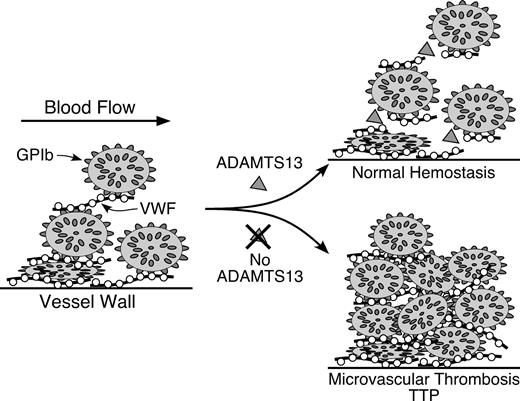
Pathogenesis of idiopathic thrombotic thrombocytopenic purpura (TTP) caused by ADAMTS13 deficiency.
Multimeric von Willebrand factor (VWF) adheres to endothelial cells or to connective tissue exposed in the vessel wall. Platelets adhere to the VWF through platelet membrane glycoprotein GPIb. In flowing blood, VWF in the platelet-rich thrombus is stretched and cleaved by the metalloprotease ADAMTS13, limiting thrombus growth. If ADAMTS13 is absent, VWF-dependent platelet accumulation continues, eventually causing microvascular thrombosis and TTP.
Pathogenesis of idiopathic thrombotic thrombocytopenic purpura (TTP) caused by ADAMTS13 deficiency.
Multimeric von Willebrand factor (VWF) adheres to endothelial cells or to connective tissue exposed in the vessel wall. Platelets adhere to the VWF through platelet membrane glycoprotein GPIb. In flowing blood, VWF in the platelet-rich thrombus is stretched and cleaved by the metalloprotease ADAMTS13, limiting thrombus growth. If ADAMTS13 is absent, VWF-dependent platelet accumulation continues, eventually causing microvascular thrombosis and TTP.
Departments of Medicine and Biochemistry and Molecular Biophysics, and Howard Hughes Medical Institute, Washington University School of Medicine, St. Louis, MO