Abstract
Hemophilia A is classically caused by a congenital deficiency of factor VIII, but an acquired form due to inhibitors to factor VIII (FVIII) typically presents later in life. Patients who develop such acquired factor VIII inhibitors may present with catastrophic bleeding episodes, despite having no prior history of a bleeding disorder. Though the disorder is rare, it is known to cause significant morbidity and mortality. This review will focus on what is currently known about acquired hemophilia A, its pathogenesis, its associated etiologies, and its treatment.
Factor VIII and Acquired Inhibitors
Factor VIII functions as a cofactor to factor IXa in the tenase complex, and a deficiency of factor VIII thus reduces the generation of thrombin on the surface of activated platelets. Factor VIII is synthesized as a 330-kDa precursor protein with an A1-a1-A2-a2-B-a3-A3-C1-C2 domain structure.1 After proteolytic processing, FVIII associates with von Willebrand Factor (vWF) in heterodimers of a heavy (A1-a1-A2-a2) and a light (a3-A3-C1-C2) chain associated by a metal ion interaction. Most acquired FVIII inhibitors bind to the A2, A3 or C2 domains.2,3 Anti-C2 antibodies disrupt the binding of FVIII to phospholipid and vWF, while antibodies to A2 and A3 interfere with FVIII binding to factor X and factor IXa.
In congenital hemophilia A patients treated with factor VIII, alloantibodies to FVIII develop in 20–40% of patients. By contrast, acquired inhibitors/autoantibodies against FVIII in nonhemophiliacs occur in only about one case per million per year.4 In acquired hemophilia, autoantibodies are characteristically non-complement fixing, non-precipitating immunoglobulins from the IgG family that bind FVIII in a time- and temperature-dependent manner.5
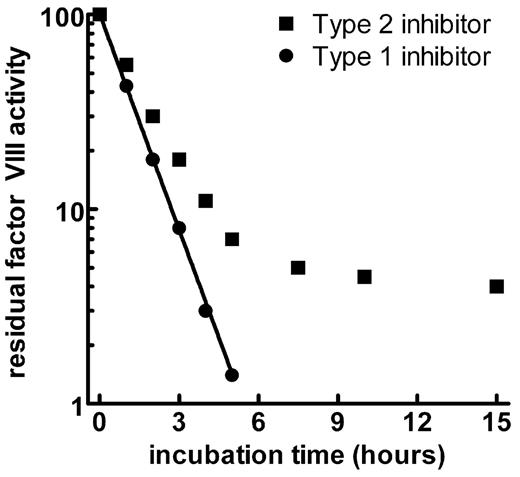
While most alloantibodies inactivate FVIII in direct proportion to their concentration (first-order kinetics), acquired inhibitors/autoantibodies show a non-linear inactivation pattern (type II or second-order kinetics). The kinetics plots in Figure 1 show a linear inactivation of FVIII by a type 1 alloantibody, with eventual complete inhibition of the FVIII activity. By contrast, the type 2 antibody shows a rapid initial inactivation phase followed by a slower phase of equilibrium where some factor VIII can usually be measured.6 Although the type 2 inhibitors usually do not totally inactivate factor VIII in vitro, the measurable factor activity offers little or no protection against hemorrhage in vivo.6,7
Clinical Features
Many features of acquired hemophilia set it apart from its congenital counterpart. First, acquired hemophilia has no known genetic inheritance pattern and is thus equally seen in males and females. Secondly, most acquired hemophilia occurs in adults, rather than in children. The median age at presentation is somewhere between 60 and 67 years, but a wide range of 2 through 89 years has been reported, including a small series of 6 children.4,8–12 Thirdly, the bleeding pattern can be quite different in these two sets of patients. Hemarthroses are the hallmark of congenital hemophilia, but they seldom occur in acquired hemophilia. Acquired hemophilia classically presents with purpura or soft tissue bleeding. Severe muscle bleeding, hematuria, epistaxis, gastrointestinal bleeding and even intracerebral bleeds are seen more frequently than are hemarthroses. No etiology is known for the difference in bleeding between acquired and congenital hemophilia, and no demonstrable platelet impairment has been found.13 Lastly, inhibitor development in congenital hemophilia no longer appears to increase mortality,14 whereas patients with acquired hemophilia continue to exhibit an increased mortality.11
Due to its frequent confusion with other life-threatening conditions such as DIC and its occurrence in a more elderly population, uncontrolled bleeding from acquired hemophilia can lead to severe morbidity and even mortality prior to its correct diagnosis and resultant treatment. Three large studies suggest a mortality rate of 8–22%, with lower mortality rates reported in the more recent studies, which used improved hemostatic agents.9–11 Most patients present with severe bleeding; one series of 215 patients reported a rate of hemorrhage or bleeding requiring transfusion of 87%.9 Life-threatening hemorrhage is more commonly seen in the first several weeks of presentation but can occur at any time if treatment is not initiated.4
Morbidity can arise from many different complications. Muscle bleeds can be severe enough to lead to compartment syndrome and tissue death. Exploratory surgery or arterial punctures for angiograms can lead to severe blood loss and uncontrollable hemorrhage if proper treatment is not provided.4
Disease associations
Though the majority of patients with acquired hemophilia are labeled idiopathic, multiple series and case reports have suggested possible associated illnesses for up to 50% of cases. The most common associated illnesses are autoimmune, with the two largest series showing an autoimmune association in 17–18%.9,10 This subset of patients is most commonly diagnosed with systemic lupus erythematosus, rheumatoid arthritis or Sjögren’s syndrome.9,15 Another frequently reported comorbidity in case-series and case reports is cancer or precancerous states including both solid tumors and lymphoproliferative malignancies.9,16–18 In addition, skin disorders (such as pemphigus and epidermolysis bullosa), drugs (including penicillin and interferon), infections and even chronic graft-versus-host disease have been reported.9
The postpartum state is one of the more frequent settings in which acquired hemophilia may occur.16,19–21 and acquired hemophilia should be considered early in the evaluation of cases of abnormal bleeding in the postpartum setting. Most commonly the inhibitor develops after parturition but sometimes can be found during labor, leading to severe blood loss and even hysterectomy. When the inhibitor does develop during pregnancy, there is a risk of transplacental transfer of the antibody and neonatal hemorrhage.22 The prognosis is good in postpartum acquired hemophilia, with favorable outcomes reported in up to 97% of patients.23
Diagnosis
Diagnosis of acquired hemophilia requires both clinical acumen and sophisticated laboratory evaluation. When a patient presents with the aforementioned clinical picture, an assessment of the degree and time course of onset of bleeding must be rapidly undertaken. Historical features pointing to any of the underlying conditions associated with acquired FVIII inhibitors should be elucidated.
The most common screening laboratory abnormality seen is an isolated prolonged aPTT, with a normal PT, thrombin time and platelet count. This is an important point as this bleeding syndrome can be easily confused with disseminated intravascular coagulation, which would normally present with thrombocytopenia and a prolonged PT and aPTT, as well as a low fibrinogen and elevated fibrin degradation products. It is also important to rule out non-specific inhibitors (i.e., lupus anticoagulant or heparin) that could prolong the aPTT.
The most common way to further delineate the cause of the prolonged aPTT and distinguish acquired hemophilia from other disease states is to perform a mixing study. Mixture of the patient’s plasma with normal control plasma will correct the test value to the normal range in the presence of a factor deficiency but not in the presence of an acquired inhibitor. Additionally, if one suspects the presence of an acquired inhibitor to FVIII, the mixture should be incubated at 37°C for at least 1 hour, since the inhibition is dependent on time and temperature. A weak inhibitor may require 1 or 2 hours of incubation compared to only minutes for a strong inhibitor.24
Once an inhibitor is suspected as the cause of a prolonged aPTT, further testing should be done to eliminate the two most common causes: heparin contamination and the lupus anticoagulant. If heparin is present in the plasma, a reptilase time will be normal, with a prolonged thrombin time. Additionally, either protamine or heparinase will neutralize the prolongation of the aPTT, and the anti-Xa activity in the plasma will be positive. Exclusion of the lupus anticoagulant can be more difficult. Lupus anticoagulants are nonspecific inhibitors that can prolong both the aPTT and the PT, depending on the sensitivity of the reagents to perturbations in the bound phospholipids. The assays measuring activities of multiple clotting factors may show an inhibitory pattern, suggesting the presence of a nonspecific inhibitor. More specific assays, such as the dilute phospholipids aPTT, dilute Russell viper venom time or kaolin clotting time, may be positive. It is critical that the addition of exogenous phospholipid to the assay should correct the prolonged value, confirming the presence of a lupus anticoagulant. In general, acquired FVIII inhibitors do not exhibit phospholipid dependence. Weak FVIII inhibitors may require dilution of the sample to show specificity against FVIII, as compared to other clotting factors.
After a mixing study confirms the presence of an inhibitor and other nonspecific inhibitors have been ruled out, the FVIII activity should be measured. If the FVIII activity is low, the titer of the FVIII antibody should be ascertained. The strength of an inhibitor can quantified by using the Bethesda assay, which measures residual FVIII activity after incubation of normal plasma with serial dilutions of patient plasma for 2 hours at 37°C.25 The inhibitor titer in Bethesda units represents the reciprocal of the dilution of the patient’s plasma that leads to 50% inhibition in the assay described. The Nijmegen modification adds buffer to the Bethesda assay, minimizing shifts in pH and thus permitting more accurate determination of low titer inhibitors.26 Due to their type 2 kinetics, autoantibodies can form complexes with factor VIII that may show some residual FVIII activity, causing the exact determination of a titer to be difficult.7 While the exact inhibitor titer in acquired hemophilia may have less biologic meaning, the titers can be followed during treatment as a measure of efficacy.
Clinical management
As mentioned above, acquired hemophilia can be difficult to diagnose and causes significant morbidity and mortality. It may thus be prudent to refer these patients to specialized centers with laboratory and clinical expertise in hemostasis, as well as the necessary pharmacy and blood bank support to manage these complex patients.
Due to the advanced age of many patients who present with acquired hemophilia, it is important to do a thorough history and physical to identify any possible comorbid states or medications that might have influenced the development of an inhibitor. Frequently, treatment of the underlying disorder or removal of an offending drug can aid in removal of the inhibitor.
Treatment of acquired hemophilia requires a two-pronged approach aimed at: 1) initial treatment of bleeding and its complications and 2) removal and/or eradication of the inhibitor. Notably, none of the agents discussed in this section have licensed indications in the United States for treatment of acquired hemophilia. See Table 1 for a suggested treatment algorithm.
Initial Treatment of Bleeding
Agents that increase FVIII levels
If one can successfully raise the plasma FVIII levels to 30–50% in a patient with acquired hemophilia, hemostasis can generally be achieved. This may be achieved with either 1-deamino-8-d-arginine vasopressin (DDAVP) or infusions of FVIII (either human or porcine).
1-deamino-8-d-arginine vasopressin:
DDAVP (desmopressin) can provide a transient rise in factor VIII levels, enough for therapeutic purposes in patients with type 1 von Willebrand’s disease and most patients with mild hemophilia A. Patients with acquired hemophilia A who have very low titer inhibitors (< 3 BU) may have sufficient FVIII released to neutralize their autoantibodies. In most patients with acquired FVIIII inhibitors, however, DDAVP treatment alone is insufficient to provide hemostasis.
Factor VIII concentrates:
Infusions of human factor VIII in patients with low-titer inhibitors (< 5 BU) may provide hemostasis.27 Doses of FVIII larger than those in congenital hemophilia are required, and massive doses are occasionally necessary and may not always be effective.4 The dose of FVIII required to achieve hemostatic levels may be empiric, and factor VIII activity levels should be carefully monitored though the course of therapy. FVIII treatment may be rendered more effective if combined with plasmapheresis and immunomodulatory medications.28
Historically, porcine factor VIII has been given with good effect. Most of the autoantibodies in acquired hemophiliacs have minimal inhibitory activity against porcine FVIII, leading to its successful use in these patients.27,29,30 A Bethesda assay detecting the inhibitory activity of the patient’s antibody to porcine VIII was used to determine those patients likely to respond to porcine FVIII. Unfortunately, porcine FVIII is no longer commercially available, but a recombinant, porcine, B domain–deleted FVIII molecule (OBI-1, Octagen) has been shown to neutralize antibodies to human FVIII in vitro and is currently in phase II clinical trials.31
FVIII bypassing agents
If a patient has severe bleeding and an inhibitor titer greater than 5 BU, it is more prudent to begin therapy with an agent that bypasses FVIII, either an activated prothrombin complex concentrate (aPCC) or recombinant factor VIIa (rVIIa). Both of these agents have been used in congenital hemophilic inhibitors, and their use has been extrapolated to patients with acquired FVIII inhibitors. Monitoring the efficacy of these agents relies on clinical acumen alone, since following standard measures of coagulation such as the PT or aPTT is not useful. More sophisticated whole blood–clotting assays may provide more data on the hemostatic efficacy of these agents.32,33 Notably, no studies have been done comparing aPCCs to rVIIa in acquired hemophilia, and no single agent is considered to be the standard of care for providing hemostasis in acquired hemophilia. Therefore, product selection is at the discretion of the provider, keeping in mind the location of bleeding, the severity of bleeding, and any comorbidities.
Activated prothrombin complex concentrates:
Currently in the United States, the only aPCC available is FEIBAε. FEIBA is a plasma-derived concentrate containing activated clotting factors that has undergone a single-viral inactivation with dry heat vapor treatment.11 A retrospective study of 34 patients with acquired hemophilia showed an overall complete response rate of 86% with a dosing regimen of FEIBA at 75 units/kg every 8–12 hours with a median number of 10 doses to control a severe bleed.34 Currently, no assay is available to monitor response to FEIBA, making duration of treatment dependent on clinical judgment. A maximum dose of 200 units/kg within a 24-hour period should not be exceeded, due to a risk of thromboembolism (including myocardial infarction).13 Large doses of aPCCs may trigger an anamnestic rise in inhibitor titer, as these products may contain some FVIII.4
Recombinant factor VIIa:
rVIIa was initially developed for use in patients with congenital hemophilia with inhibitors, but it has also been used in acquired hemophilia. rVIIa binds to the surface of activated platelets, where it supports thrombin generation, thus bypassing the need for FVIII.35 Since rVIIa is made from cultured mammalian cells and is free from human protein, it does not have the potential to transmit human pathogens. Early studies using rFVIIa as a second-line agent for the treatment of acquired hemophilia showed a complete response rate in 75% of bleeding episodes and a partial response in an additional 17%.11 Typical dosing is 90–120 μg/kg every 3 hours until bleeding is stopped. As with FEIBA, laboratory monitoring is difficult and efficacy is determined clinically. Of note, case reports of arterial thrombosis have been published, and one report was specific to a patient with acquired hemophilia.36 At present, it is difficult to quantify the risk of rFVIIa based on case reports or by the extrapolation of the above data.
Inhibitor Elimination
Inhibitor removal
It is possible to use extracorporeal means to remove the autoantibodies to FVIII with either plasmapheresis or immunoadsorption with staphylococcal protein A.37 This modality may be useful in bleeding or presurgical patients with high titer inhibitors who have failed to respond to bypassing agents. Following pheresis or immunoadsorption, these patients should be treated with FVIII replacement to achieve hemostasis.
Immunosuppression
Attempting inhibitor eradication and deciding clinical efficacy may not be straightforward, given the wide spectrum of inhibitor duration. Inhibitors may remit spontaneously (especially the inhibitors that develop post partum and those associated with drug therapy) but may also persist for months to years.9,38 Unfortunately, most of the reported literature consists of case reports or small series without standardized reporting, making the true incidence of spontaneous remission difficult to assess. Nonetheless, most published guidelines and treatment algorithms recommend attempting inhibitor eradication as an early measure, since treatment may be prolonged and bleeding complications can prove catastrophic.39–41
Prednisolone at 1 mg/kg/day results in inhibitor abolition in approximately 30% of patients.9,42 The addition of cyclophosphamide at 50 to 100 mg/day can increase the response rate to 60–70%.9,43 Along with cyclophosphamide, other agents that have been used are azathioprine, vincristine, mycophenolate mofetil, and 2-chlorodeoxyadenosine.9,44–46 Most patients show response in 3 to 6 weeks, but some may have a prolonged response time of months.4 Also, relapse is not uncommon when immunosuppression is stopped or reduced, necessitating re-institution of steroid therapy, with prolonged exposure and resultant side effects.13 Plasmapheresis has been combined with immunosuppression but its overall contribution is unclear.47,48
Immunoglobulin
Infusion of intravenous immunoglobulin (IVIG) has also been used as therapy. One study of 16 patients using a total dosing of 2 g/kg over 2 or 5 days showed a response rate of 30%.49 Maximum response time was 40 days, and patients with lower initial Bethesda titers showed a better response. Of note, some of these patients were receiving immunoglobulin as second-line therapy, making it a reasonable option for those patients who do not initially respond to immunosuppression.
Cyclosporine
Cyclosporine A has also been used alone or with prednisolone as salvage therapy, but it is especially effective in patients with underlying systemic lupus erythematosis.50–53 Serum levels must be closely monitored to avoid toxicities and side effects. In successful cases, cyclosporine can usually be discontinued after a year of therapy without recurrence.4
Targeted/biologic therapy
In the last several years, anti-CD20 monoclonal antibody (rituximab) has been used in the management of various autoimmune diseases, as well as lymphomas. “Off label” use of rituximab has been studied in acquired hemophilia, showing promising results of durable remission.34,54–60 The usual dose is 375 mg/m2 each week for 4 weeks. Most responses are seen within the first 2 weeks of therapy, and the general consensus is that it should be considered in patients who are resistant to first-line therapy or cannot tolerate standard immunosuppressive therapy.13 Some groups propose that rituximab should be included as first-line therapy in combination with prednisone for patients with an inhibitor titer greater than 5 but less than 30 and in addition to prednisone and cyclophosphamide for those patients with a titer greater than 30.57
Conclusion
Acquired hemophilia has a significant mortality rate of 8–22% and can be easily missed, with sometimes disastrous results. Prompt recognition is critical, since early therapy directed toward achieving hemostasis and inhibitor eradication can be life-saving. Larger randomized clinical trials are required to better evaluate the newer therapies for this rare but devastating illness.
How we treat acquired hemophilia. These recommendations are based on clinical experience, rather than on randomized clinical trials.
|
|
Kinetics of type 1 and type 2 inhibitors against factor VIII.
Type 1 inhibitors develop in patients with congenital hemophilia A and are generally alloantibodies that show complete neutralization of FVIII activity. Acquired inhibitors to FVIII show type 2 kinetics, with a rapid neutralization phase, followed by an equilibrium in which residual FVIII activity can be detected in vitro.
Kinetics of type 1 and type 2 inhibitors against factor VIII.
Type 1 inhibitors develop in patients with congenital hemophilia A and are generally alloantibodies that show complete neutralization of FVIII activity. Acquired inhibitors to FVIII show type 2 kinetics, with a rapid neutralization phase, followed by an equilibrium in which residual FVIII activity can be detected in vitro.
University of North Carolina School of Medicine, Department of Medicine, Division of Hematology/Oncology, Chapel Hill, NC