Abstract
Red cell exchange transfusions remain an effective but possibly underutilized therapy in the acute and chronic treatment of sickle cell disease. In sickle cell disease, increased blood viscosity can cause complications when the hemoglobin exceeds 10 g/dL even if this is due to simple transfusion. Red cell exchange can provide needed oxygen carrying capacity while reducing the overall viscosity of blood. Acute red cell exchange is useful in acute infarctive stroke, in acute chest and the multi-organ failure syndromes, the right upper quadrant syndrome, and possibly priapism. Neither simple or exchange transfusions are likely to hasten resolution of an acute pain episode.
Red cell exchange transfusion is an effective but perhaps underutilized therapy for both acute and chronic complications of sickle cell disease. In a red cell exchange, the patient’s red cells are removed and replaced by exogenous normal red cells. The exchange prevents the removed sickle cells from participating in new vaso-occlusive events, reduces hemolytic complications, and provides added oxygen carrying capacity while decreasing the blood viscosity.
The vast majority of transfusions for sickle cell disease are simple red cell transfusions, packed red cells administered intravenously. Simple transfusions are useful where added oxygen carrying capacity is needed. Unless accompanied by bleeding or rapid red cell destruction, both the hemoglobin concentration and the viscosity of the blood will increase.
Blood Viscosity andTransfusion
The viscosity of blood is a key concept in management of transfusion in sickle cell disease. Viscosity limits the flow of blood, providing the intrinsic resistance to blood flow in the presence of pressure. The classic Poiseuille equation for laminar flow of a fluid through a straight tube is:

A doubling of the viscosity (η) would cut the velocity in half. While this is clearly an oversimplification, since both nonlaminar flow and flow through curved or twisted vessels do occur in vivo, the viscosity effects under such circumstances tend to be even greater than the viscosity effects in a straight tube. For example, the flow of a liquid through a twisted tube is slower than through a straight tube and a doubling of viscosity may affect velocity even more. Turbulent or nonlaminar flow through a tube varies as the 5th rather than the 4th power of the radius, making turbulent flow slower and less likely in smaller vessels than larger vessels.
Blood viscosity increases with increasing hemoglobin level. Eventually, with increasing hemoglobin, viscosity limits blood flow and oxygen transport. Viscosity to varying degrees explains the complications of blood flow in erythrocytoses, but can be partly counteracted by an increase in plasma volume and an increase in cardiac output.1,2 Blood from an untransfused patient with sickle cell anemia has a significantly higher viscosity than normal blood at the same hemoglobin level.3 Deoxygenated sickle blood has nearly a 10-fold greater viscosity than oxygenated sickle blood at the same hemoglobin level, with the effect being greatest at low shear forces.3,4
Increased viscosity dramatically promotes the physiology of sickling. Looking at the Poiseuille equation above, flow will be slowest in vessels of small radius (capillaries, arterioles and venules) and under lower pressures (venules). Shear is also low in the venules. After sickle cells deliver their oxygen, the deoxygenated cells greatly increase the blood viscosity, slowing flow through the venules even more. Some compensation is provided by the increased cardiac output, and the expanded plasma volume in sickle cell disease,5 but the persistent anemia does not allow for compensation similar to the erythrocytoses.
Sickle cells do not sickle immediately upon deoxygenation. There is a delay time that greatly depends on the intracellular hemoglobin S concentration.6 This likely explains why the blood of sickle cell patients does not sickle continuously. With normal blood flow, red cells may be able to get back to the lung and reoxygenate before they sickle or at least get to veins so large that vaso-occlusion is less likely.
Slower flow can cause cells to exceed the delay time and initiate red cell sickling in smaller vessels such as venules. Slow flow also provides additional opportunities for cell–cell interactions, cell adhesion to endothelium, activation of coagulation systems, and other time dependent processes that may facilitate the piling up of cells that often causes vaso-occlusion.7,8 Flow velocity varying with the fourth power of the radius makes such events most likely in the smaller venules, where sickling and vaso-occlusion is well documented.9
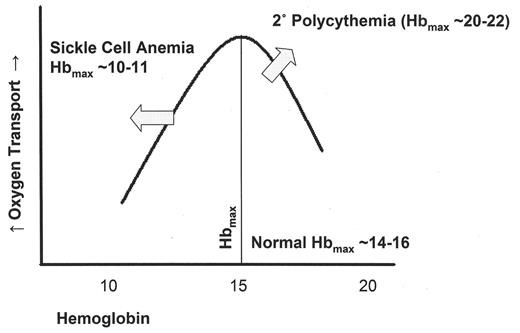
Transfusing normal red blood cells to patients with sickle cell disease increases the viscosity, although not as much as if the same amount of sickle red blood cells were added. Figure 1 shows a diagram of oxygen transport (or venous oxygen tension) vs. hemoglobin levels. As the hemoglobin increases there is an initial increase in oxygen transport, but as viscosity effects take hold, transport decreases. In patients with erythrocytoses, such as the secondary polycythemias, cardiac output and plasma volume can increase along with the red cell volume. This shifts the curve up and to the right, generating Hbmax values as high as 20–22 g/dL.1,2 The curve for normals generally has a Hbmax for oxygen transport at 14–16 g/dL.2 The viscosity effects in sickle cell anemia shift the curve dramatically to the left resulting in a Hbmax of 10–11 g/dL.3 The effect is greatest with more than 60% sickle red cells and decreases but is not eliminated even when more than 40% of the cells are normal red cells.
In acute situations where the amounts of blood needed to either reduce the % HbS or provide oxygen carrying capacity will cause the hemoglobin to exceed 10 g/dL, simple red cell transfusion risks viscosity-related complications. Red cell exchange can administer the blood without the corresponding increase in viscosity. In situations where the hemoglobin is already above 10 (which it is at baseline hemoglobin for many patients with compound heterozygosity for sickle and hemoglobin C (SCD-SC), viscosity can be lowered, and flow improved, as normal red cells are substituted for sickle red cells.
Red cell exchange can be accomplished by bleeding and infusing red cells manually or more efficiently through the use of an apheresis machine which spins blood to separate the plasma from cells. The machine then can replace the patient’s red cells on a mL for mL basis with normal cells, minimizing fluid shifts and blood pressure variations. Table 1 shows one recipe for manual exchange. A third type of red cell exchange is the partial manual exchange where a fixed volume of cells is removed and a greater number of packed red cells is administered.
Acute Red Cell Exchange
Red cell exchange is most useful in situations where it is important not just to provide oxygen carrying capacity but to decrease immediate complications of sickle cell disease. Replacement of sickle cells by normal cells can help prevent further vaso-occlusion, although pre-existing vaso-occlusion may not be reversed. Red cell exchange also rapidly decreases the rate of hemolysis which can decrease liver processing of bilirubin, damage to renal tubular cells and the scavenging of nitric oxide by free hemoglobin released from sickle cells.10
The importance of early intervention in stroke is nicely described in Dr. Orah Platt’s paper in this volume on the central nervous system in sickle cell disease. Exchange transfusion is a recommended part of the initial treatment of vaso-occlusive stroke in children.11 It is performed shortly after a non-contrast CT documents an infarctive rather than hemorrhagic stroke. Additional studies, such as MRI/MRA, should await the completion of the red cell exchange. The suggested goal of the exchange is less than 30% HbS and a hemoglobin close to but not greater than 10. While there are few data, red cell exchange may also be useful in limiting the effects of stroke in adults.
Acute chest syndrome (ACS) was defined by the National Acute Chest Study Group12 as a new infiltrate consistent with consolidation and at least segmental in size, accompanied by at least one of: chest pain, a temperature over 38.5°C, tachypnea, wheezing, or cough. This broad, inclusive definition allowed collection of invaluable data on the causes and associations of ACS. All classical episodes of pneumonia are included in this definition. Some physicians make a distinction between “just” pneumonia and the presumably more severe ACS. Lumping the two together emphasizes the potential severity of all cases where airspace disease may be accompanied by hypoxia related to vasoocclusion in the pulmonary vessels.
In the ACS, either simple or exchange transfusion successfully and rapidly increased oxygenation.12 No clear difference was found between red cell exchange and simple transfusion, but this was not a randomized trial and such differences could have been missed. In more severe cases, including hypoxia not controllable with oxygen therapy, patients requiring ventilator therapy, multilobar processes, and especially after a simple red cell transfusion has not improved the patient, red cell exchange should be considered. The goal is similar to that for acute stroke with a HbS under 30% (for patients with SCD-SS) while keeping the Hb less than 10. This goal generally requires some form of red cell exchange. Simple transfusion alone would only achieve these numbers if the original Hb were close to 3 (3 g/dL of HbS plus 7 g/dL of HbA for a total of 10 g/dL of Hb at 30% HbS). Some practitioners initiate red cell exchange even earlier based on some of the risks defined in this study.12
Multiorgan failure syndrome (MOFS) usually begins as a severe pain episode with dramatic hemolysis but goes on to involve multiple organs generally including the central nervous system, liver and kidney.13 It often occurs in sickle syndromes with relatively high baseline hemoglobin concentrations suggesting viscosity as a major contributor. Red cell exchange is nearly always essential for effective treatment of MOFS.
The liver can be affected by acute severe cholestasis, also called the right upper quadrant syndrome, often with bilirubins over 50 and high ALT levels consistent with destruction of liver cells. Again, exchange transfusion is likely the best option but data is lacking.14,15 This needs to be distinguished from the more common “hepatic crisis” which generally resolves on its own without exchange. In “hepatic crisis,” right upper quadrant pain may still be present but the bilirubin is generally less than 20 and very high ALT levels are absent.
Concern over exchange transfusion for treatment of priapism was raised by one case report16 and one small series17 where the incidence of neurologic catastrophe was astoundingly high. In these cases, the Hb was increased to 12 or more by partial exchange transfusions. A recent abstract reported treating 8 patients with sickle cell anemia (and some compound heterozygotes) with apheresis red cell exchange transfusion for priapism.18 The Hb was kept below 10 g/dL and there were no neurologic events, arguing that the partial exchange results may have been viscosity based and that apheresis red cell exchange does not seem to be exceptionally risky. Unfortunately, there are no available randomized trials evaluating the efficacy of even simple transfusion, much less red cell exchange for priapism.
The Preoperative Transfusion Sickle Cell Study Group showed that for the most common surgical procedures, simple transfusion to a Hb of 10 was equivalent to exchange transfusion for patients with sickle cell anemia in prevention of postsurgical complications vs. no transfusion.19 Those receiving simple transfusion had fewer transfusion related complications. Patients with initial hemoglobins greater than 10 (such as in many patients with SCD-SC) should receive exchange transfusion for major surgeries such cholecystectomy to decrease the incidence of postoperative complications such as ACS.15,20 Many clinicians still use exchange transfusion for surgeries they consider more risky, such as open heart surgery, retinal surgery, or joint replacement surgery, especially in older patients and for those with significant comorbidities. These procedures and populations were not well represented in the preoperative transfusion study.
Red cell exchange has been anecdotally reported as useful in the treatment of parasitemia of the blood with malaria21 in patients without sickle cell disease. Despite the protection of the heterozygote from mortality of (especially cerebral) malaria, the patient with a sickle syndrome is subject to greater mortality and morbidity from these organisms. Red cell exchange might help reduce both parasitemia and sickling complications and buy time for antimalarials to work.
In acute red cell exchange for an untransfused patient, we have found it advantageous to provide 1.5 apheresis red cell volumes in order to better ensure reaching a value of less than 30% HbS for SCD-SS or 15% HbS in the case of compound heterozygotes such as SCD-SC. For patients with persistent illness, it is wise to follow the % HbS or % HbA sequentially as patients will make more sickle cells and may need repeat exchange transfusion. A delayed transfusion reaction can also drop the % HbA quite rapidly.
Chronic Maintenance of Low S Levels
Red cell exchange may also be useful when there is a need for chronic maintenance of low Hb S levels. There are multiple indications for keeping HbS low, the best documented of which are primary and secondary stroke prevention (see Dr. Orah Platt’s paper in this volume). Other indications with less supporting data include elements of the triad of pulmonary hypertension, priapism and leg ulcers associated with red cell hemolysis,10 chronic hypoxia not controllable with oxygen therapy, repeated episodes of ACS or MOFS, or multiple severe pain episodes interfering with major life goals. Exchange (or simple transfusion) for an acute pain episode is not likely to hasten resolution and should be avoided.15
Prophylactic transfusion in pregnancy was not found to be helpful in a randomized trial of simple transfusion.22 Automated red cell exchange has been used with good results, but the study was not randomized and we cannot determine its true efficacy.23 Red cell exchange may be useful in patients with a dramatic increase in pain episodes during pregnancy, or possibly in those with preeclampsia.
Once the decision is reached to limit %HbS for chronic therapeutic reasons, the clinician and patient have a choice of chronic simple transfusion, partial manual exchange or full exchange transfusion. It is possible to keep HbS low with repeated simple transfusion in most patients. For the unusual patient with a brisk hematopoietic drive not suppressed by transfusion, we have found that either hydroxyurea with simple transfusion, or exchange transfusion can help.
A major advantage of full or apheresis exchange transfusion has been its iron neutrality, since the removed HbS has just as much iron as the administered HbA. Several investigators have documented stable iron levels in patients on red cell exchange transfusion,24–27 while most partial exchange programs still result in significant iron loading. Adherence to chelation therapy has been notoriously poor in SCD, particularly in teenagers and older patients. An analysis of barriers to chelation therapy and new approaches for adherence are currently under study.28 Whether adherence to recently approved oral iron chelation will prove sufficient to allow simple or partial exchange transfusion without iron loading remains to be seen.
Apheresis red cell exchange can avoid the dramatic increases in intravascular volume which occur in simple transfusions and can be helpful in patients with cardiac disease or other conditions where fluid overload is a problem.29
Amounts to Exchange
Red cell volume calculations are generally done by the apheresis service for automated exchanges but by the physician for manual exchange. A reasonable assumption is a total blood volume (TBV) of 70 cc per kg, but children may run closer to 85 cc per kg.30 The red cell volume is then easily calculated as total blood volume times the hematocrit. For an effective red cell exchange starting with an untransfused patient, plan on 1.5 red cell volumes administered during the exchange. This is because some of the administered red cells will be removed later in the procedure as the exchange continues. In an automated or apheresis exchange, removal of recently administered normal cells may be increased dramatically if the draw port is distal to the return port either by confusion of the ports on a single apheresis catheter or if blood is returned to a connected vein on the same arm. The draw site generally needs at least a 19G steel backeye needle, while the return can be as small as a 21G, so it may be tempting to place a draw line higher up in the arm (i.e., near the antecubital area) and to use a distal vein near the hand for the return. In general opposite arms should be used for draw and return. It is also important to realize that the apheresis machines deal with intravascular volume and are not set up to serve as dialysis machines. Once a patient is effectively exchanged, chronic exchange can usually be done every 4 weeks with 1 red cell volume with good results for most patients. Some patients will require adjustment to a larger volume (1 or 2 extra units but generally not more than 1.5 red cell volumes), as the post-HbA level will be too low. Some will require a shorter time interval since their lifespan of transfused cells seems shorter.
Cautions
Each apheresis machine has a certain volume of blood that the machine uses for processing. If this volume will be more than 15% of the patient’s total blood volume or if the patient is sensitive to fluid shifts, a blood prime (filling the machine or return line with exogenous blood) may be useful.29 For patients more than 20% below their usual hemoglobin, either a simple transfusion to increase the hemoglobin or a blood prime helps avoid vascular compromise.
Dehydration is prevalent in patients requiring red cell exchange.31 Patients with acute sickling pain may not have kept up with their constant fluid requirement or patients may be placed NPO after midnight for the surgical insertion of an apheresis line. Restricting food after midnight, but allowing water until a few hours before the procedure is a more reasonable alternative. If the patient must be strictly NPO, admission for overnight hydration is reasonable.32 It is often advisable to hold morning blood pressure medications, particularly diuretics and ACE inhibitors, until after the procedure.33 The greater number of units transfused confers a risk of citrate toxicity,29,33 which, if recognized, is easily treated with calcium. All standard risks of transfusion therapy remain present.
Vascular access
In our unit, about one-half of adult sickle cell patients have peripheral veins adequate for apheresis red cell exchange.34 This is yet another reason to work diligently to preserve IV access in patients with SCD, including oral rehydration31 and pain management subcutaneously, orally, or by the oral-transmucosal route to avoid IVs when possible.
Fewer than 10% of our chronic exchange patients have an indwelling line, port or graft, the vast majority for renal dialysis. For those without adequate venous access, we have interventional radiology place temporary femoral apheresis catheters which are removed in the apheresis unit at the end of the procedure. This is well accepted by our patients and we have had only one catheter failure34 and no significant complications from now over 1000 placements in sickle cell patients, some monthly for more than 8 years.
Most venous access devices do not have the ability to support the flow rates needed by apheresis machines and the experience with permanent access devices in sickle cell disease is poor.31,35,36 Vortex ports37,38 and double or dual ports39 have been found acceptable but the experiences are generally small and more data are badly needed on both safety and efficacy.
Manual red cell exchange (modified from ref. 11).
| For pediatric patients, use smaller comparable volumes (such as 5–10 cc/kg for bleeds and saline and calculate red cell volume based on 1- to 1.25-fold the amount of blood removed in bleeds). |
| If patient has a starting hemoglobin close to or more than 10, this protocol may result in significantly higher hemoglobin after fluid equilibration post exchange. The red cell volume withdrawn in the two 500 cc bleeds is less than the red cells administered by the two units of blood (by the amount the patient’s hemoglobin is less than normal). You may wish to consider a 500 mL bleed at the end or alternate infusing 1 unit instead of 2 units in the second (and fourth if needed) cycle of 3 steps. While this difference is even greater for those with lower hemoglobins, such patients are less likely to exceed a hemoglobin of 10 g/dL by the end of the procedure. |
|
| For pediatric patients, use smaller comparable volumes (such as 5–10 cc/kg for bleeds and saline and calculate red cell volume based on 1- to 1.25-fold the amount of blood removed in bleeds). |
| If patient has a starting hemoglobin close to or more than 10, this protocol may result in significantly higher hemoglobin after fluid equilibration post exchange. The red cell volume withdrawn in the two 500 cc bleeds is less than the red cells administered by the two units of blood (by the amount the patient’s hemoglobin is less than normal). You may wish to consider a 500 mL bleed at the end or alternate infusing 1 unit instead of 2 units in the second (and fourth if needed) cycle of 3 steps. While this difference is even greater for those with lower hemoglobins, such patients are less likely to exceed a hemoglobin of 10 g/dL by the end of the procedure. |
|
Oxygen transport versus hemoglobin.
Curve adapted from references 1–3.
Oxygen transport increases with increasing hemoglobin until viscosity effects reduce flow and transport. The point at which oxygen transport is maximum is indicated as Hbmax. In erythrocytoses such as secondary polycythemias, there is both increased red cell volume and increased total blood volume. Increased cardiac output allows the curve to move up and to the right, dramatically increasing oxygen transport along with the Hbmax. In sickle cell anemia, the decreased hemoglobin and higher blood viscosity due to the presence of sickle cells shift the curve sharply to the left, decreasing the Hbmax. Oxygen transport can remain at normal rather than decreased levels due to increased cardiac output.
Oxygen transport versus hemoglobin.
Curve adapted from references 1–3.
Oxygen transport increases with increasing hemoglobin until viscosity effects reduce flow and transport. The point at which oxygen transport is maximum is indicated as Hbmax. In erythrocytoses such as secondary polycythemias, there is both increased red cell volume and increased total blood volume. Increased cardiac output allows the curve to move up and to the right, dramatically increasing oxygen transport along with the Hbmax. In sickle cell anemia, the decreased hemoglobin and higher blood viscosity due to the presence of sickle cells shift the curve sharply to the left, decreasing the Hbmax. Oxygen transport can remain at normal rather than decreased levels due to increased cardiac output.
The American Society for Apheresis web site at http://www.apheresis.org/ provides a good online resource for questions on apheresis red cell exchange.