Abstract
The antiphospholipid syndrome (APS) is an autoimmune thrombophilic condition that is marked by the presence of antibodies that recognize phospholipid-binding proteins. The clinical manifestations of APS include vascular thrombosis and pregnancy complications, especially recurrent spontaneous miscarriages. This article provides an update on diagnostic and therapeutic approaches to this disorder.
Introduction
The antiphospholipid syndrome (APS) is an autoimmune thrombophilic condition that is marked by the presence in blood of antibodies that recognize phospholipid-binding proteins. The clinical manifestations of APS include vascular thrombosis and pregnancy complications, especially recurrent spontaneous miscarriages. This section describes some of the recent developments in understanding the mechanism of the syndrome and provides an update on diagnostic and therapeutic approaches to this disorder. The reader is referred to a recent chapter1 for a more comprehensive review.
β2-glycoprotein I
The major antigen recognized by antiphospholipid (aPL) autoantibodies is β2-glycoprotein I (β2GPI), also known as apolipoprotein H, a member of the complement control protein, or short consensus repeat (SCR), superfamily. The protein has a fishhook shape and binds to anionic phospholipid bilayers through cationic and hydrophobic aminoacids in the fifth of its 5 SCR domains, near the carboxy-terminus.2 Recent evidence has indicated that a subset of aPL antibodies associated with increased risk of thrombosis and embolism recognize an epitope in domain I of β2GPI that consists of Gly40-Arg43.3,4 It has been suggested that antibody-mediated dimerization and pentamerization of β2GPI increase the affinity/aviditity of antibody-β2GPI immune complexes for phospholipid and that this increase may be responsible for the pathogenic effects of aPL antibodies.4
The mechanism of thrombosis in APS and the role of β2GPI in the process are not yet established. For a recent review of current ideas on the pathogenesis of APS, see Giannakopoulos et al.5 Among the proposed mechanisms are several possibilities:
Anti-β2GPI complexes may interfere with endogenous anticoagulant mechanisms such as crystallization of annexin A5 anticoagulant shield,6 fibrinolysis triggered via annexin A27 and mediated via plasmin,8 the proteins C and S mechanism,9 tissue factor pathway inhibitor,10 and others.
Anti-β2GPI complexes may trigger signaling events on cells such as blood leukocytes, endothelium, platelets, and trophoblasts that may lead to the expression of prothrombotic and proadhesive phenotypes.11
Anti-β2GPI antibodies may activate complement and trigger an inflammatory reaction on the vascular and/or trophoblast surface.12
Diagnosis of APS
Criteria
Criteria for defining the syndrome for research purposes have been published13 and are listed in Table 1 . Patients are considered to have the secondary form of APS if they have concurrent systemic lupus erythematosus (SLE) and primary APS in its absence (Table 2 ). Note that this list is broader than the tests required for the research definition above. The multicentered Euro-Phospholipid project, which analyzed data from 1000 unselected patients who met the criteria for APS, found that 53% of the cohort had primary APS, and 41% had APS with SLE or lupus-like conditions.14
Overview of laboratory tests
The laboratory tests that are frequently used to diagnose this condition are shown in Table 3 . In retrospect, the first test that identified this condition was the biologic false-positive (BFP) syphilis test, which actually reported auto-antibody recognition of phospholipid-binding proteins, primarily β2GPI (in contrast to true-positive syphilis tests in which antibodies recognize phospholipid directly). The BFP syphilis test was first refined into a quantitative anticardiolipin immunoassay by Harris and coworkers.15 Subsequent refinements included immunoassays that used other phospholipids, such as phosphatidylserine, and immunoassays that detected antibodies against the putative antigens, primarily β2GPI, themselves.
A second avenue of diagnostic testing for these antibodies involves their detection through tests that, paradoxically, report interference with phospholipid-dependent coagulation reactions, referred to as lupus anticoagulant (LAC) tests. These include modified forms of the partial thromboplastin time (PTT), the dilute Russell Viper venom time (dRVVT), kaolin clotting time, dilute prothrombin time (dPT) (also known as the tissue thromboplastin inhibition test), and the textarin ecatrin test. High-affinity (avidity) phospholipid-binding antibody-cofactor-phospholipid complexes are the likely basis for the LAC phenomenon. Both of the approaches—immunoassay and LAC testing—may be considered to be empirically derived surrogate tests for the syndrome. The sensitivities and specificities of the tests are variable, and a single negative test cannot rule out the diagnosis in a patient. It is generally recommended that a panel of tests be done to attempt to exclude the diagnosis. Patients who test positive for all three of the major assays—positive LAC, elevated anticardiolipin antibodies and elevated anti-β2GPI antibodies (referred to as “triple positivity”), are at markedly increased risk for thrombosis16,17 and for pregnancy complications.18 In a series of Italian obstetric patients with APS, “triple-positivity” and/or previous history for thromboembolism was associated with markedly increased risk of thromboembolism (odds ratio [OR] = 57.5, 95% confidence interval [CI] 2.7–1160) along with increased risk of subsequent pregnancy losses (OR = 34.4, 95% CI 3.5–335.1).18
These tests are not useful for screening asymptomatic general medical or obstetrical populations. Testing should be restricted to patients suspected of having APS because of their clinical presentations and to patients who have SLE, a group in which the presence of aPL antibodies has been correlated with increased risk of thrombosis. Patients with APS constitute a minority of individuals who have detectable aPL antibodies (Table 2 ). The prevalence of aCL and LAC in normal healthy populations has been reported to range between 1.0% and 5.6% and between 1.0% and 3.6%, respectively.19–21 The prevalence of elevated aPL antibodies may also increase with age, as suggested by a study of 89 healthy women, with a mean age of 86 (range, 75 to 102 years), of whom 64% had elevated anticardiolipin (aCL) antibody levels and 32% had elevated β2GPI antibodies.22
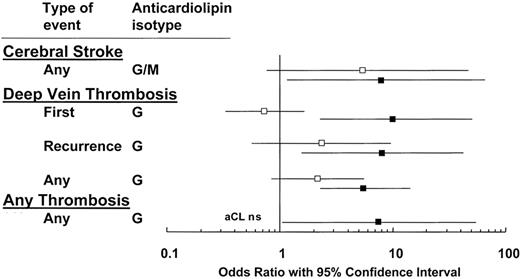
A positive LAC appears to be more specific for APS than an elevated aCL. In a meta-analysis of the risk of VTE in patients with aPL antibodies, excluding patients with SLE, the mean OR for LAC was 11.0.23 A systemic literature review found that, of five studies, all reported significant associations between LAC and thrombosis, with OR ranging from 5.7 to 9.4 (Figure 1 ).24 A positive LAC is consistently associated with increased risks for arterial and venous events and is more predictive than ELISA-based assays.
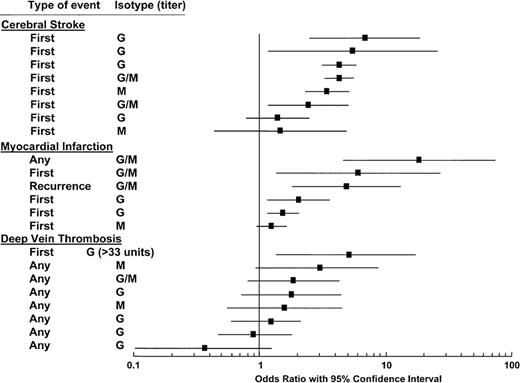
aCL are not as strong a risk factor for thrombosis as LAC. In a systemic literature review, only 15 of 28 studies showed significant associations between aCL and thrombosis.24 The statistical significance varied depending on the type of thrombosis; aCL were associated with cerebral stroke and myocardial infarction more so than with DVT (Figure 2 ). In all the studies, there was a correlation between higher antibody titers and increased OR for thrombosis. However, the reader should be aware that there are also negative data regarding the value for aCL testing for VTE. A prospective nested case-control study of 317 patients with elevated aCL and 655 control patients within the Longitudinal Investigation of Thromboembolism Etiology (LITE) cohort of 20,000 disease-free participants found no association between elevated titers of aCL and VTE.25 The ORs comparing the IgG and IgM aCLs above the 95th percentile and within quintiles were not statistically significant.
Results for aCL and anti-β2GPI immunoassays can vary widely among clinical laboratories. In a multilaboratory quality assurance program, high interlaboratory variation in numerical results were found in 12 serum samples when testing for aCL IgG, aCL IgM, and IgG β2GPI. In semiquantitative reporting, a complete consensus was obtained in only 4 of 36 occasions (11%). General consensus (where more than 90% of participating laboratories agreed that a given serum sample gave a result of either positive or negative) was only seen on 13 of 36 samples.26
As mentioned above, a subpopulation of β2GPI antibodies that recognize an epitope of β2GPI consisting of Gly40-Arg43 has been highly correlated with thrombosis and with LAC positivity. In a study of 198 patients with a variety of autoimmune disorders, including SLE, APS, and “lupus-like” disease, patients with detectable antibodies having Gly40-Arg43 specificity correlated highly with LAC activity and with a history of thrombosis (OR 18.9 vs 1.1).27 These findings were followed up with a retrospective study that showed an OR for thrombosis of 42.3 for patients with β2GPI-dependent lupus anticoagulant activity compared with 1.6 for those with lupus anticoagulant activity independent of β2GPI antibodies.4 Whether such assays that identify specific aPL epitopic targets may provide increased specificity in clinical testing for APS is currently being investigated.
The data regarding testing for aPL antibodies in stroke are not consistent. In a nested case-controlled study of men in the Physicians’ Health Study, composed of men ages 40 to 84 years, the IgG aCL in 61 participants with ischemic stroke were not significantly different from those of controls (P > .2), and there was no evidence of increased risk for stroke in those with higher aPL antibody levels.28 The Antiphospholipid Antibodies and Stroke Study (APASS) group recently completed the first prospective study of the role of aPL antibodies in recurrent ischemic stroke in collaboration with the Warfarin versus Aspirin Recurrent Stroke Study (WARSS).29 WARSS was a randomized, blinded study that compared the risk of recurrent ischemic strokes in patients assigned to treatment with either aspirin (325 mg/day) or warfarin (target international normalized ratio [INR] 2.2, range 1.4–2.8). The APASS trial included 890 patients on warfarin and 882 patients on aspirin and found no increase in rate of recurrent thrombotic events associated with baseline antiphospholipid status as measured at the time of the initial stroke. Since this study began prior to the formalization of clinical criteria for APS diagnosis, it did not require confirmatory retesting of aPL antibodies, which may account for the surprisingly high proportion of participants with aPL antibody positivity (41%). Interestingly, the 7% of patients with baseline positivity for both LAC and aCL did tend to have a higher rate of recurrence than patients who were negative for both antibodies (32% vs 24%; RR 1.36, 95% CI 0.97–1.92; P = .07).
In an earlier prospective case-controlled study, aPL antibodies were found to be an independent stroke risk factor in all groups, causing a fourfold increase in the risk for ischemic stroke.30 Overall, the available data suggest that testing for aCL and LAC appears to be warranted for patients who have transient ischemic attacks or strokes at a young age, for those who have these events in the absence of other risk factors such as cigarette smoking, hypertension, and diabetes mellitus, and for patients who also have other features of the APS. However, indiscriminate testing of the patient population with cerebrovascular events is not indicated.31
Summary of recommendations for diagnostic testing
At the present time, testing for aPL antibodies should usually be restricted to patients who have had thrombosis, embolism, or pregnancy complications that may be attributable to APS, and to patients with SLE even if they have not have any of the above manifestations. A panel of tests should always be done when APS is suspected since individual tests may yield false negatives. Persistence of the abnormal test should be confirmed after 12 weeks.
Treatment of Thrombosis in APS
The intensity of anticoagulation that should be used to treat patients diagnosed with APS has recently been systematically reviewed.32 Two prospective studies with similar designs, a Canadian study33 and the Warfarin in the Antiphospholipid Syndrome (WAPS) trial,34 conducted independent trials of 109 and 114 patients with APS that yielded similar results. In both studies, patients were randomly assigned to receive warfarin with a target INR of 2.0 to 3.0 (moderate intensity) or 3.0 to 4.0 (high intensity) to determine whether high-intensity anticoagulation was superior in preventing recurrent thrombosis without significantly increasing the bleeding risks. In the Canadian study, recurrent thrombosis occurred in 2 of 58 patients (3.4%) receiving conventional therapy and in 6 of 56 patients (10.7%) who received the high-intensity therapy (hazard ratio, 3.1; 95% CI, 0.6 to 15). The WAPS trial showed similar results with recurrent thrombosis in 3 of 55 patients (5.5%) receiving the conventional therapy and in 6 of 54 patients (11.1%) receiving the high-intensity warfarin (hazard ratio, 1.97; 95% CI, 0.49 to 7.89). Both studies reached the same conclusion that the appropriate target INR was 2.0 to 3.0 and that high-intensity warfarin was not superior to standard treatment for thromboprophylaxis. The WAPS study also reported an increased rate of minor hemorrhagic complications in the high-intensity treatment cohort.
Several limitations that were reported for these studies should be noted. Most patients in both studies had venous thrombosis. Neither trial reached the targeted sample size, and both trials excluded large numbers of patients who had already experienced recurrent events while receiving oral anticoagulation. The Canadian study also excluded patients with recent strokes, so that 76% of the participants in their study had previous VTEs alone. Additionally, patients in the high-intensity group from this study had sub-therapeutic levels of anticoagulation 43% of the time, which may have contributed to 6 of 8 recurrent thrombotic events in which the INR was between 2.0 and 3.0. The evidence is less clear for ATE, where controversy remains because of previous retrospective studies had shown a lower rate of recurrent thrombosis when the INR was maintained above 3.0.
However, a significant question has been raised regarding treatment of patients with aPL antibodies and the specific situation of stroke. The results of the prospective, randomized, double-blinded APASS and WARSS (mentioned above) showed no significant difference between aspirin and standard warfarin intensity in the prevention of recurrent thrombo-occlusive events regardless of aPL antibody status.29 It should, however, be noted that in the APASS study, aPL antibodies were only tested at the time of the stroke (in contrast to the Sydney criteria for APS diagnosis,13 which require confirmatory retesting after 12 weeks), thereby including patients who might not have this diagnosis by current criteria. The unexpectedly high rate of aPL antibodies in this cohort (41%) suggests that some of these patients may have had transient aPL antibodies rather than definitive APS. Still, clinicians should be aware that there is significant debate regarding treatment of cerebrovascular events associated with aPL antibodies—with some anticoagulating these patients as they would other patients with arterial or venous thrombosis, while others treat with aspirin alone.
Duration of treatment for VTE, arterial events, and CAPS
Patients with the APS and VTE have a significant risk for recurrence if anticoagulation is discontinued after a first episode of venous thrombosis.16,35,36 In a retrospective study of patients with primary and secondary APS, nearly 70% of patients had a recurrence after discontinuation of warfarin, over a median follow-up of 82 months (range, 12 to 258 months), with the rate of recurrence highest during the first 6 months (1.3 per patient year). In contrast, continuation of warfarin (INR 2.5 to 4.0) was associated with a 90% freedom from thrombosis over a 5-year period.35 Long-term anticoagulation is generally recommended in APS as long as the risks of bleeding are considered to be less than the risk of thrombosis.37 Recurrence of VTE while on standard therapy may be treated with higher-intensity anticoagulation or with long-term administration of a heparin in selected situations. High-intensity anticoagulation is recommended after the first arterial thromboembolic event (with the possible exception of cerebral thromboembolism as discussed above), providing there are no contraindications. Furthermore, antithrombotic therapy—whether anticoagulation or aspirin for the subset of stroke patients who are being treated with these drugs—should be continued on an indefinite basis. It has not yet been established whether D-dimer levels, or other markers for activation of coagulation might be helpful in predicting the risk of initial or recurrent thrombosis in APS.
Anticoagulant treatment is generally not sufficient to treat the catastrophic antiphospholipid syndrome (CAPS), a form of the disorder that is marked by disseminated macro-and microvascular occlusions resulting in multi-organ failure. CAPS, which occurs in less than 1% of patients presenting with APS, is defined by thrombotic occlusions in at least three organ/tissues occurring within a week (“thrombotic storm”), with laboratory confirmation of aPL antibodies.38 Even with optimal treatment, the mortality rate is approximately 48%.38,39 Patients with CAPS generally require supplementing anticoagulant treatment with plasmapheresis and immunosuppression. Treatment approaches have included intravenous IgG, corticosteroids, cyclophosphamide, and azathioprine. Rituximab has also been tried, in some cases with apparent success, in patients who have been refractory to other treatments.
Summary of recommendations for treatment of thrombosis
Initial VTE in patients with confirmed APS should be treated with warfarin to a target INR of 2.0 to 3.0 for the long term. Recurrence of VTE in the face of standard treatment should be treated with higher-intensity anticoagulation, or in selected situations with a form of low-molecular-weight heparin (LMWH). The diagnosis of CAPS also generally warrants consideration of trying plasmapheresis and immunosuppressive therapy.
Areas of uncertainty
The appropriate intensity for treatment of arterial thromboembolism is not completely clear. Where the risks are acceptable, treatment with higher-intensity anticoagulation (e.g., INR 3.0–4.0) may be considered. There is a significant controversy regarding the treatment of patients with APS with cerebrovascular disease, with some evidence that supports treatment with aspirin alone.
Treatment of Pregnancy Complications
Recurrent spontaneous pregnancy losses tend to be the most common complication for which patients are referred to hematologists for suspected APS. While not listed in the formal investigational criteria for APS, other complications that have been attributed to the condition include intrau-terine growth restriction (IUGR), oligohydramnios, and the obstetrical “hemolysis, elevated liver enzymes, low platelets” (HELLP) syndrome. The treatment of recurrent miscarriages attributed to APS has recently been reviewed in an analysis of 13 studies that included 849 patients.40 The authors felt that the available studies had significant limits, that about half of the studies had clear evidence of allocation concealment, and that participant characteristics varied between trials. Still, the available studies indicated that unfractionated heparin (UFH) combined with aspirin significantly reduced pregnancy loss compared with aspirin alone, and there was no advantage for high doses over low doses. This treatment is generally extrapolated to the other APS-associated pregnancy complications mentioned above. Pathologic evaluation of placental tissues can be helpful in guiding treatment decisions. The data are sparse with respect to LMWH, which together with aspirin did not significantly reduce pregnancy loss compared to aspirin alone. Still, most clinicians currently treat patients with APS with a combination of prophylactic dosage LMWH plus low-dose aspirin. Most clinicians will initiate aspirin treatment prior to conception and begin the heparin when pregnancy is documented. Treatment is generally modified during the last month of pregnancy treatment, when many clinicians switch the patient to UFH because of its shorter half-life. Anticoagulant therapy with LMWH or warfarin is generally resumed for 6 weeks post-partum in order to reduce the risk of postpartum deep vein thrombosis.
Recent data from an experimental model of aPL antibody–induced pregnancy losses in mice demonstrated a key role for complement activation in this process.12 Intriguingly, heparin, but not other anticoagulants, reversed this process in the mice, suggesting that the therapeutic effect of heparin in the disorder might be due to the inhibition of complement rather than its inhibition of coagulation. These data, if generalizable to human APS-related pregnancy losses, have raised the intriguing possibility of novel nonanticoagulant approaches to treatment.
Summary of recommendations
Treatment with prophylactic dose heparin plus low-dose aspirin (81 mg daily), with modification of administration for delivery, followed by resumption of prophylactic anticoagulation for the puerperium.
Areas of uncertainty
There is insufficient evidence from randomized clinical trials regarding as to whether there is a benefit for LMWH, as opposed to UFH, in this setting. Most practitioners prefer LMWH for the treatment of pregnancy losses in these patients.
Summary of the Sydney Consensus Statement on Investigational Classification Criteria for the APS.13 Antiphospholipid antibody syndrome (APS) is present if at least one of the clinical criteria and one of the laboratory criteria that follow are met.
| Clinical criteria |
Vascular thromboses:
|
| In studies of populations of patients who have more than one type of pregnancy morbidity, investigators are strongly encouraged to stratify groups of subjects according to a, b, or c above. |
| Laboratory criteria |
|
| Investigators are strongly advised to classify APS patients in studies into one of the following categories: I, more than one laboratory criteria present (any combination); IIa, LAC present alone; IIb, aCL present alone; IIc, anti-β2GPI antibody present alone. |
| Summarized from Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost . 2006 ;4 :295 –306. |
| Clinical criteria |
Vascular thromboses:
|
| In studies of populations of patients who have more than one type of pregnancy morbidity, investigators are strongly encouraged to stratify groups of subjects according to a, b, or c above. |
| Laboratory criteria |
|
| Investigators are strongly advised to classify APS patients in studies into one of the following categories: I, more than one laboratory criteria present (any combination); IIa, LAC present alone; IIb, aCL present alone; IIc, anti-β2GPI antibody present alone. |
| Summarized from Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost . 2006 ;4 :295 –306. |
Types of patients having antiphospholipid (aPL) antibodies.
|
|
Tests used for diagnosis of the antiphospholipid syndrome.
| Immunoassays |
| Biologic false-positive serologic test for syphilis Anticardiolipin antibodies (cofactor-dependent assay) Anti-β2GPI antibodies Antiphosphatidylserine antibodies Antiprothrombin antibodies |
| Coagulation Tests |
| Dilute Russell viper venom time (DRVVT) with confirmatory tests |
aPTT:
|
| Kaolin clotting time |
| Tissue thromboplastin inhibition test |
| Hexagonal phase array test |
| Textarin/ecarin test |
| Immunoassays |
| Biologic false-positive serologic test for syphilis Anticardiolipin antibodies (cofactor-dependent assay) Anti-β2GPI antibodies Antiphosphatidylserine antibodies Antiprothrombin antibodies |
| Coagulation Tests |
| Dilute Russell viper venom time (DRVVT) with confirmatory tests |
aPTT:
|
| Kaolin clotting time |
| Tissue thromboplastin inhibition test |
| Hexagonal phase array test |
| Textarin/ecarin test |
Comparison of lupus anticoagulants (▪) and anticardiolipin antibodies (□) for their association with thrombosis: compilation of 5 studies on 753 patients and 234 controls. Modified from Galli M, Luciani D Bertolini G, et al.24 The reader is referred to that article for the individual studies that are cited.
Comparison of lupus anticoagulants (▪) and anticardiolipin antibodies (□) for their association with thrombosis: compilation of 5 studies on 753 patients and 234 controls. Modified from Galli M, Luciani D Bertolini G, et al.24 The reader is referred to that article for the individual studies that are cited.
Anticardiolipin antibodies and thrombosis: analysis of 11 cross-sectional, case-control, and ambispective studies on 1883 cases and 2469 controls. Odds ratio with 95% confidence intervals (CI) are grouped according to the site and type of thrombosis and the antibody isotype.
Modified from Galli M, Luciani D Bertolini G, et al.24 The reader is referred to that article for the individual studies that are cited.
Anticardiolipin antibodies and thrombosis: analysis of 11 cross-sectional, case-control, and ambispective studies on 1883 cases and 2469 controls. Odds ratio with 95% confidence intervals (CI) are grouped according to the site and type of thrombosis and the antibody isotype.
Modified from Galli M, Luciani D Bertolini G, et al.24 The reader is referred to that article for the individual studies that are cited.
Montefiore Medical Center; Albert Einstein College of Medicine, New York, NY
Acknowledgments
I thank Dr. Miles Levin and Dr. Xiao-Xuan Wu for their assistance in the preparation of this manuscript.