Abstract
The decision to proceed to transplant for adult patients with acute lymphoblastic leukemia (ALL) is not clear-cut. Relapse and nonrelapse mortality continue to plague the outcome of hematopoietic stem cell transplantation (HSCT) even when undertaken in complete remission (CR). Those considered to be at high risk for relapse often are considered for HSCT in first complete remission (CR1) while those at lower risk may not be referred until they have relapsed, when their chances for cure are very poor. In some patients who have a suitable histocompatible sibling, disease- or patient-related factors may override the potential benefit of allogeneic HSCT. Because many patients do not have a suitable histocompatible sibling, one has to consider the relative merits of autologous transplantation versus use of an alternative allogeneic stem cell source, such as a matched-unrelated donor (MUD), umbilical cord blood (UCB) donor, or haploidentical donor. Deciding among these options in comparison to chemotherapy even in high-risk patients is difficult. In the review, the risks and benefits of these choices are discussed to determine whether and by what means to proceed to HSCT in adult patients with ALL who are in CR1. Presented are two patients with ALL and a discussion of how the data we provide would lead to a decision about the selection of therapy.
Would You Recommend Stem Cell Transplantation for Either of These Patients?
Case 1
A 32-year-old woman with no significant past medical history presented to her physician with a 2-week history of abdominal discomfort, a 20-pound weight loss over 2 months, and hepatosplenomegaly. Laboratory studies revealed a white blood cell (WBC) count of 14,200/μL with circulating blasts expressing CD34, CD45, CD19 and CD20. Cytogenetics revealed a normal karyotype. The final diagnosis was pre–B-cell acute lymphoblastic leukemia (ALL), Philadelphia-chromosome–negative (Ph–). She was given induction therapy and attained a complete remission (CR). She has an HLA-identical brother.
Case 2
A 64-year-old man was evaluated for a 4-month history of progressive cytopenias. Bone marrow exam showed extensive, diffuse infiltration by mostly small- and intermediate sized mononuclear cells that, on flow cytometry, expressed CD45dim, CD38 and CD19 and the karyotype revealed t(4:11). The final diagnosis was pre-B cell ALL, Ph–, with a t(4:11) karyotype abnormality. He was treated with daunoru-bicin, vincristine and prednisone. Repeat bone marrow study revealed persistent leukemia. The patient was believed to be refractory to induction therapy. He also had fever and typhlitis. He later was given high-dose methotrexate, cytarabine, cyclophosphamide, vincristine, doxorubicin and dexamethasone. The bone marrow examination revealed no residual leukemia. He attained a CR and has an HLA-identical sister.
Discussion
Contrary to the excellent results achieved in pediatric patients, the outcomes for ALL in adult patients are disappointing. There is considerable evidence that the biologic and clinical characteristics of this disease change dramatically from childhood to adult age groups, including immunophe-notype and karyotype. These differences must be recognized when making treatment recommendations for adult ALL patients. While in recent multicenter trials more than 90% of adult patients with ALL younger than 60 years attain CR, conventional chemotherapy is a long arduous process and will result in long-term survival in only one-third of patients. A number of prognostic factors identified at diagnosis are associated with low probability of cure in adult patients with ALL receiving conventional therapy. Such factors include high WBC count at diagnosis (>30,000/μL for B-cell lineage and >100,000/μL for T-cell lineage), specific clonal cytoge-netic abnormalities such as the presence of Ph, age older than 35 years, extramedullary disease and time to attain CR longer than 4 weeks, although the latter was not observed in the largest ALL study conducted in adults.1
Adverse Factors
The presence of the Ph chromosome affects the chance of remission after induction, as well as the risk of relapse, when using conventional chemotherapy. A recent publication by Moorman et al2 described other karyotypes that portend a poor prognosis. Age remains an important prognostic factor. In many studies including the French protocol LALA-87,3 age older than 35 years decreases the chance of remission as well as increases the risk of relapse in patients given conventional chemotherapy. In the international MRC-ECOG UKALLXII/E2993 trial, patients older than 35 years with high WBC counts at presentation had a dismal outcome when using conventional chemotherapy.1 It remains unclear whether that outcome can be improved with transplantation.
Molecular Monitoring
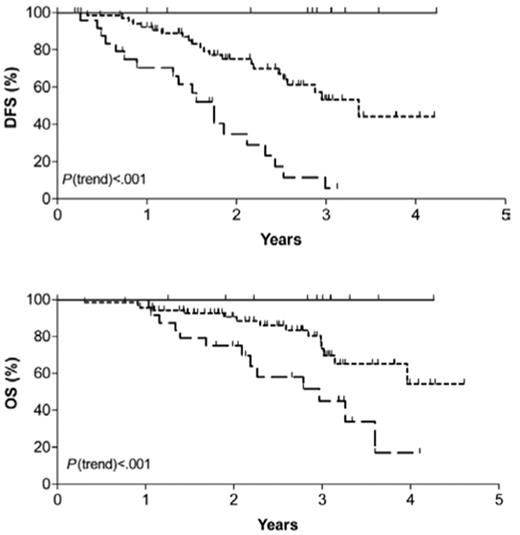
Several investigators have introduced more sensitive molecular techniques such as clonal immunoglobulin or T-cell receptor gene rearrangements to detect minimal residual disease (MRD) that can accurately predict relapse in patients with ALL.4 Pediatricians have recognized that molecular persistence of residual ALL at the end of induction chemotherapy or afterward effectively predicted relapse independent of other risk factors.5,6 Molecular marker monitoring has been used less extensively in adult ALL but appears to be an extremely powerful tool that has yet to be exploited. Brüggemann and colleagues7 of the German Multi-Center Study Group for Adult ALL reported that patients with standard-risk ALL who had a rapid decline in MRD within the first month of therapy had a 0% 3-year relapse rate while those with molecular persistence of ALL by week 16 of therapy had a 94% 3-year relapse rate (Figure 1 ). In a subset of patients treated on the MRC-ECOG adult ALL trial discussed above, detection of MRD (especially in the pre–B-cell subtype) had prognostic significance.8,9 In their hands, the detection of MRD by the completion of the second month of induction therapy appeared to be the optimal timepoint to ascertain ultimate prognosis.8,9 The measurement of MRD has been incorporated into the successor MRC-ECOG–led Intergroup trial in order to help restratify low-risk patients (based on clinical prognostic features) into those patients who now would be considered high-risk due to detection of MRD at the end of induction therapy. The use of this technique will require standardization for methodology when it is used in the clinical course of the patient.
How allogeneic hematopoietic stem cell transplantation (HSCT) is affected by or impacts upon these risk factors has been less well studied. Although the risk of relapse decreases with allogeneic HSCT, the concomitant treatment-related mortality (TRM) eliminates the potential survival benefit. Doney et al10 reported on 182 ALL patients with ALL in first CR (CR1) or beyond and found improved disease-free survival (DFS) and overall survival (OS) in younger patients and those in CR1. Allogeneic HSCT conferred similar OS and relapse rates for Ph+ patients compared with those with normal cytogenetics supporting a graft-versus-leukemia (GVL) effect that is particularly beneficial in that group. Although other karyotypic abnormalities have been shown to portend a poor prognosis with conventional chemotherapy,2 available data are insufficient to determine if there is a benefit to allogeneic HSCT in these patients. Gorin for the European Group for Blood and Marrow Transplantation (EBMT)11 reviewed data on 1402 patients with ALL given allogeneic HSCT in CR1 and noted that time to transplant was a major prognostic factor. Of 414 patients who received transplants within 3 months after achieving CR, patient age, attainment of CR1 with 1 or more induction courses and recipient/donor sex combination predicted outcome. Patients with no unfavorable factors (age younger than 35 years, less than 1 induction course, female donor graft to male recipient) had a 3-year leukemia-free survival (LFS) of 56% compared with 48% for those with 1 adverse factor and 29% for 2 or 3 adverse factors. OS rates were 65%, 53% and 29%, respectively. Although risk factors have been defined, the impact of transplantation on relapse and survival in affected patients is not clear.
Related Donor Allogeneic HSCT: Ph–
There are no trials in the field of HSCT in which patients with a suitable sibling-matched donor have been randomized to an allogeneic HSCT or chemotherapy. “Genetic randomization” or assignment, along with intent-to-treat analyses, have been the established methods of comparing allo-geneic HSCT with chemotherapy treatment.12 Gupta and colleagues13 at the Princess Margaret Hospital reported a small single-institution series in which adult patients with ALL in CR1 age 55 years or younger were offered a myelo-ablative allogeneic HSCT. They found no difference in outcome between the donor versus no-donor groups. High-risk adults in CR1 (which included Ph+ patients) who had a sibling matched-related donor (MRD; N = 41) were assigned a myeloablative allogeneic HSCT, while the others proceeded to an autologous HSCT (N = 115) in the GOELAMS-02 trial.14 Six-year DFS and OS were statistically superior in those who underwent allogeneic HSCT, 75% versus 39% (P = .0027). The French LALA group has addressed this question prospectively in their LALA-94 trial.15 Because of the suggestion of a benefit in LALA-87 to allogeneic HSCT in patients under age 55 years who did not have standard-risk disease,3 they designed a trial of risk-adapted postremission strategy in adult ALL. Patients ages 15 to 55 years with any high-risk ALL features and an HLA-identical sibling were assigned to allogeneic HSCT. Five-year DFS in the group with Ph– high-risk ALL in first CR was improved in the allogeneic HSCT (45% vs 23%, sibling donor vs those with no sibling donor). In multivariate analysis, both age and WBC count were found to be prognostic factors for DFS; however, the impact on the two groups (donor vs no donor) was not discussed.
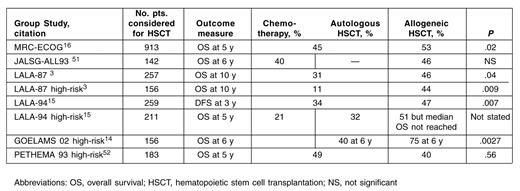
In the largest trial reported to date (MRC-ECOG UKALLXII/E2993), 1826 adult patients with ALL were given induction therapy that resulted in a 91% CR rate.1 All patients younger than 50 years old, irrespective of risk, with a sibling MRD were recommended to undergo a myelo-ablative HSCT using a total body irradiation (TBI)–containing conditioning regimen.16 For 919 patients in CR, 389 with a donor were compared with 524 patients with no donor (Table 1 ). Event-free survival (EFS) and OS were statistically superior in the donor group: 50% versus 41% (P = .009) and 53% versus 45% (P = .02). As noted historically with most HSCT reports, in general, the optimal allo-geneic effect for EFS and OS were observed in the standard-risk rather than the high-risk group (where high risk was defined by age and WBC count). Although in both risk groups the relapse rates were significantly lower in the donor group compared with the no donor group, supporting a strong GVL effect, the TRM rates at 2 years were significantly increased in the high-risk group, 39% versus 20%. Such findings, in large part, reflect poor tolerance to a myeloablative conditioning regimen in those patients older than 35 years. Thus, the majority of the data in Ph– patients favor an improved outcome in younger patients who underwent a MRD allogeneic HSCT, an effect lost when older patients are conditioned using a myeloablative regimen. The number of patients with sibling donors, however, far exceeds the usual ratio of success in finding HLA-matched siblings (such as observed in acute myeloid leukemia HSCT trials). Although the number of patients with a donor appears high, it is consistent with some of the previously published ALL HSCT studies. For example, in the LALA-94 study, for the high-risk group 100 patients had a donor compared with 159 without a donor.15 Table 2 compares several large trials for ALL CR1 patient outcome. These and other data support the use of a MRD myeloablative alloge-neic HSCT for the 32-year-old woman in CR1 described in Case 1. Due to advanced age, however, the 64-year-old man described in Case 2 is not a viable candidate for a MRD myeloablative allogeneic HSCT.
Allogeneic HSCT: Ph+
Although Ph+ patients have a lower remission rate and higher relapse rate using conventional chemotherapy compared to those with Ph– disease, allogeneic HSCT is particularly effective in Ph+ patients. Dombret and co-workers17 reported the LALA-94 trial data for 154 Ph+ patients who proceeded to transplant (N = 103) after attainment of CR1. Sixty patients who underwent allogeneic HSCT (n = 46 MRD and n = 14 MUD) had the same 2-year TRM as 43 patients who underwent autologous HSCT, but OS at 3 years was significantly superior, 37% versus 12% (P = .02). Attainment of BCR-ABL–negative status conferred a significantly better OS as well, 54% versus 12% (P = .006). The French Bone Marrow Transplantation Society18 reported outcome of 121 patients with Ph+ ALL, including 102 adults, of whom 76 were in CR1 at time of transplant. Two-year OS in the CR1 patients was 50% and the 2-year relapse incidence rate was 37%. Stirewalt and colleagues19 at the Fred Hutchinson Cancer Research Center retrospectively analyzed 90 patients with Ph+ ALL aged 2 to 56 years (median, 33 years) who underwent myeloablative allogeneic HSCT. Morphologic or cytogenetic evidence of ALL at time of transplantation as well as autologous or MRD use predicted for highest risk of relapse, while development of chronic graft-versus-host disease (GVHD) after transplantation lowered the relapse rates. These investigators proposed that patients without molecular evidence of disease (“low risk”) undergo a less intense (nonmyeloablative) HSCT. They advocated a more aggressive conditioning be used in “high-risk” patients who have minimal residual or overt disease at time of transplantation including consideration of post-transplantation imatinib consolidation. Gupta and coworkers13 reported a study in which unrelated donor (URD) allogeneic HSCT was offered to a small number of patients with Ph+ ALL in CR1. Three-year DFS and OS were 46% and 57%, respectively; OS did not differ when compared with those Ph– patients at their center who underwent allogeneic HSCT. Finally, in the largest prospective allogeneic HSCT study (conducted during the pre-imatinib era), 5-year OS was superior in those who underwent MRD allogeneic HSCT compared with chemotherapy or autologous HSCT.20 Early relapse, prior to transplantation, however, was common supporting referral for alloge-neic HSCT as soon as possible after attaining CR.20 These and other data suggest that allogeneic HSCT provides significant GVL effect in CR1 patients with Ph+ ALL.
The addition of imatinib to the armamentarium for treating Ph+ ALL has significantly improved the CR rate from approximately 60% to 90% and has greatly enhanced the access of such patients to allogeneic HSCT by reducing early pretransplantation relapses, a large problem in the past. Yanada et al21 recently reported a significantly improved OS in 49 adult patients with Ph+ ALL who were given post-transplantation imatinib therapy compared with historic controls, i.e., 1-year EFS and OS, 60% and 76%, respectively. Although median follow-up was short at 1 year, both patients who underwent allogeneic HSCT recipients and patients who did not had significantly better outcome. Further, imatinib appeared to prevent earlier relapse, enabling a greater percentage of patients to proceed to allogeneic HSCT. de Labarthe and coworkers22 similarly showed that imatinib in combination with conventional chemotherapy provided results comparable with alloge-neic HSCT. On the other hand, Pfeifer, for the GMALL, recently reported the prevalence of kinase domain mutations in newly diagnosed and imatinib-naive Ph+ ALL.23 BCR-ABL mutations conferring high-level imatinib resistance were present in a substantial proportion of patients with de novo Ph+ ALL and eventually gave rise to relapse. These data support the recommendation to search for a well-matched donor in this population. Until there are published reports of larger studies with longer follow-up, imatinib therapy cannot be considered the standard treatment, and allogeneic HSCT, in general, remains the preferred approach in this subgroup of patients. Imatinib’s positive effect on pretransplantation PCR status, i.e., rendering patients tumor-negative before transplantation, could improve outcome. One can speculate, however, that in the post-transplantation setting, imatinib therapy is only likely to provide time until a GVL effect can develop to eradicate disease. Prophy-lactic imatinib after HSCT can be given safely.24
Blood Versus Marrow as the Stem Cell Source
Blood has nearly supplanted bone marrow as the hemato-poietic cellular graft source for patients who undergo allo-geneic HSCT. Ringden and the EBMT25 reported a retrospective analysis of their observational database in which they compared outcome for 826 patients (513 in CR1) given marrow versus 345 patients (189 in CR1) given blood after conditioning. Engraftment, as anticipated, was faster with blood, but also resulted in an increased risk of chronic GVHD. LFS and OS did not differ among the groups. In a subsequent retrospective EBMT analysis, blood stem cell grafts obtained from URD were associated with inferior outcome compared with bone marrow grafts in ALL.26 The incidence of 2-year TRM in 36 patients receiving blood grafts was 61% compared with 47% in 66 bone marrow recipients, resulting in significantly inferior 2-year LFS (32% vs 21%) and OS (34% vs 24%) rates, P = .04. Finally, Dahlke et al27 reported a single-center, donor-source comparison in ALL allogeneic HSCT (46 MRD vs 38 URD). Younger patients had a significantly lower TRM, but the relapse rates did not differ, indicating similar antileukemic efficacy. The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) has an ongoing, prospective, randomized clinical trial examining URD blood versus marrow source grafts after myeloablative HSCT. Current data in adults do not indicate definitively that one graft source is superior; however, due to significantly greater ease of collecting stem cells, worldwide, 80% of HSCT procedures are performed using blood rather than bone marrow.
Alternative Donor Stem Cell Transplantation: Unrelated Donor Marrow or Blood
Kiehl and associates28 reported a 12-year retrospective multicenter experience comparing myeloablative HSCT in adult patients with ALL according to donor source. Five-year DFS rates were not statistically different according to donor source, MRD (N = 62) versus URD (N = 32), 42% versus 45%. Similarly, TRM did not differ, 39% versus 31%, respectively. The vast majority of these patients were considered high risk or very high risk. Further, these results were significantly better than if the patients underwent HSCT at CR2 or beyond or at time of active leukemia. Of interest, as observed in sibling MRD HSCT studies, Ph+ patients had no poorer outcome than did Ph– patients. Cornelissen and associates29 reported a multi-national, myeloablative MUD HSCT trial in CR1 patients. Their data again support a strong GVL effect in Ph+ patients, as the relapses were significantly lower in these patients compared with the t[4;11] and t[1;19] groups. Weisdorf et al30 in a retrospective analysis compared outcome and toxicities in 712 patients who underwent HSCT in CR1 or beyond according to donor graft source. DFS was significantly better after the first 6 months in MUD HSCT versus autolo-gous HSCT for younger patients, those in CR2 with a long CR1 duration (more than 1 year) and an initial WBC count less than 50 × 106/μL. At this time, there are insufficient data to conclude whether a myeloablative URD should be offered even to high-risk adult patients with ALL in CR1. Given the GVL effect that seems to be particularly beneficial in Ph+ patients, this patient population may be reasonable to consider for URD transplantations in CR1. Yakoub-Agha et al, for the French Society of Bone Marrow Transplantation and Cell Therapy, recently reported the first prospective study in which outcomes for standard-risk malignancy patients who underwent sibling MRD HSCT were comparable with HLA-allellically matched (10 of 10) unrelated donor HSCT.31 Such data enhance the case for performing MUD HSCT in this patient population. The successor trial to MRC-ECOG UKALLXII/E2993 will investigate whether the benefits of using a sibling-matched allo-geneic HSCT in high-risk patients can be extended to unrelated donor allogeneic HSCT.
Umbilical Cord Blood Transplantation
One single-institution trial compared a myeloablative HSCT regimen followed by either an umbilical cord blood (UCB) graft (N = 100) or a related donor blood or marrow graft (N = 71).32 UCB was safe and effective compared to a related marrow or mobilized blood graft, but only 16 adult ALL patients were included and an ALL diagnosis carried with it a nearly 2.5-fold increased risk for relapse. The analyses of UCB transplantation data are hampered by small patient numbers, heterogeneity of remission status, variability of matching, incomplete information about UCB cell dose (including the use of double UCB grafts), and high engraftment failure rates.33 At present, there are insufficient data to recommend use of this donor stem cell source outside the context of a clinical trial.
Haploidentical Donor Transplantations
This specialized approach has been available at only a very few centers. The high rejection and unacceptable GVHD rates have been lowered by use of more intense preparative regimens and infusion of higher numbers of purified he-matopoietic stem cells.33 When applied to patients not in CR1 or CR2, haploidentical transplantations appear inferior to other alternative donor HSCT results. The major limitations, in addition to relapse in advance disease state, appear to be a proclivity for severe and often-fatal opportunistic infections. Some centers performing this procedure in high-risk CR1 (including Ph+) patients, however, attain results equivalent to MUD allogeneic HSCT.34 Such data need to be corroborated in multi-institutional trials, but this strategy is a distinct option in those patients without a MRD.
Reduced-Intensity Conditioning
Although the GVL effect has been shown to be responsible for some of the benefit in fully ablative HSCT, TRM, especially in the older recipient, may offset the reduction in relapse. As donor lymphocyte infusion (DLI) has been shown to decrease MRD and potentially improve survival in those patients, there may be a role for nonmyeloablative or reduced-intensity conditioning in ALL. Hamaki et al35 investigated reduced-intensity conditioning in 33 ALL patients with a median age of 55 years (range, 17-68 years) using fludarabine-based conditioning regimens followed by MRD or URD grafts; 9 patients died of TRM. The 1-year progression-free survival (PFS) rate for the 19 patients who underwent transplantation in CR1 or CR2 is 31%. Six of 13 patients have a median DFS of 14 months (range, 4-37 months).
The City of Hope recently reported their single-center experience in 21 patients with ALL median age 46 years (range, 6-68 years) given fludarabine-melphalan conditioning followed by a MRD (N = 7) or a MUD (N = 14) graft.36 Ten patients were in CR1 and 11 patients had Ph+ ALL. In this high-risk group, who were either elderly (older than 50 years), had failed a previous HSCT, or had a comorbid condition precluding use of a myeloablative regimen, the 100-day mortality and 1-year relapse rates were 11% and 8%, respectively, while 1-year DFS and OS rates were 77% and 71%, respectively.
The MRC-ECOG–led Intergroup-planned successor to the MRC-ECOG UKALLXII/E2993 international trial will use a fludarabine- and melphalan-containing regimen in patients considered high-risk by virtue of age older than 40 years. The addition of Campath-1H to the regimen could be beneficial, as this agent has significant antileukemic as well as anti-GVHD effects.37 While intriguing, reduced-intensity preparative regimens must be validated in prospective, multicenter trials. Hence, the 64-year-old man described in Case 2 should be considered a candidate for a MRD reduced-intensity conditioning allogeneic HSCT, but only in the context of a clinical trial.
Autologous HSCT
This modality first was used nearly 50 years ago as treatment for relapsed/refractory ALL. More recently, this approach has been used in patients significantly earlier in their disease course, to provide the potential benefit of myeloablative therapy to those for whom a MRD is not available, as MRD are available only for a minority of patients.38 While long-term DFS has been attained in some patients, few prospective, randomized trials have analyzed sufficiently large enough numbers of patients to determine if this treatment is appropriate therapy.
A variety of preparative regimens have been used for autologous HSCT in adult patients with ALL, but there is no clear benefit to any one approach.39 On the other hand, an analysis from the Center for International Blood and Marrow Transplant Research (CIBMTR) by Marks and associates40 reported in CR1 and CR2 patients with ALL receiving sibling-matched allogeneic HSCT that etoposide or cyclophosphamide in combination in a TBI-containing regimen, or use of TBI dose >1300 cGy were associated with reduced risk of relapse and treatment failure. These allogeneic HSCT data suggest a threshold TBI dose for antileukemia efficacy and could have implications for au-tologous HSCT regimens; these conclusions, however, have to be regarded as speculative based on retrospective analysis of registry data.
Thomas and associates15 for the French LALA-94 trial reported a prospective multicenter trial comparing autolo-gous HSCT, MRD allogeneic HSCT, and chemotherapy in 1000 patients. Ph– patients at high risk for relapse in CR1 who did not have MRD (N = 129) were randomized to an autologous HSCT and 2-year maintenance therapy (6-MP and methotrexate) or chemotherapy. Three-year DFS were 39% in the autologous HSCT versus 24% in the chemotherapy-treated group, not statistically significant. Other small series include reports of long-term survivors in younger patients who underwent autologous HSCT, but such studies do not include control subject comparisons.41 A recent article by Dhédin et al42 updated autologous HSCT results for the LALA-85, -87 and -94 trials. A subset analysis of 180 high-risk CR1 patients showed no differences in either DFS or OS at 10 years for the autologous HSCT versus the chemotherapy treatment groups, 20% versus 12% (P = .10) and 20% versus 13% (P = .78).
The joint MRC-ECOG UKALLXII/E2993 trial16 compared 446 CR1 patients randomized to undergo either an autologous HSCT (N = 223) or 2.5 years of maintenance therapy (N = 223). EFS was statistically superior for the chemotherapy-treated patients, 42% versus 33% (P = .02). While OS also was improved in the chemotherapy group (47% versus 37%), these results were not statistically significant (P = .06). Those assigned to an autologous HSCT did not receive post-transplantation maintenance therapy and continued to relapse at a higher rate. Other groups have reported that use of maintenance chemotherapy after autolo-gous HSCT can prolong remission (see below). These and other data do not support the routine use of autologous HSCT in adult patients with ALL, and patients should be referred for participation in clinical trials addressing this concept.
In Vitro Stem Cell Purging and Post-Transplantation Maintenance Therapy
In vitro purging of the stem cell graft also has been used in a number of autologous HSCT studies in ALL.43 Although such an approach may reduce the number of leukemic progenitor cells in the graft, there has been no correlation between purging efficacy or dose of residual tumor infused and patient outcome. Two groups have suggested that use of maintenance chemotherapy after autologous HSCT can reduce relapse rates.44,45 In the UK, Mehta and colleagues44 noted that increased intensity of treatment was more efficacious but that many patients could not tolerate such an approach, a result similar to the Johns Hopkins group data.45 Until less marrow-toxic agents are readily available to provide a more-targeted therapy, this postautologous HSCT maintenance strategy cannot be recommended for widespread use.
Other Issues: Approach to Younger Adults
Adolescent and young adult patients appear to be a distinct population compared with older adults. The pattern of referral may influence whether patients are treated by a pediatrician or adult hematologist-oncologist specialist. Emerging data indicate that such patients have a consistent survival advantage when treated on a pediatric regimen.46 –48 This issue is being addressed prospectively in a CALGB-led intergroup study.
Conclusions
Adult ALL remains a challenging disease. While the only treatment that results in long-term DFS in patients with ALL in CR2 is allogeneic HSCT, and outcomes from transplantation are better if performed in CR1 rather than CR2, the decision to proceed to transplantation in CR1 remains a complicated one. Current data indicate that autologous HSCT is inferior to other approaches and cannot be recommended at this time. In patients at high risk for relapse such as those with adverse biologic, clinical, or laboratory factors, allogeneic HSCT with a sibling MRD is the preferred option (Table 3 ). The outcome after salvage therapy with any HSCT or nontransplantation therapy in relapsed patients is extraordinarily poor, as demonstrated by a 5-year OS rate of only 7% in the MRC-ECOG trial discussed above.49 Even in younger patients whose initial CR1 exceeded 2 years, 5-year OS in this subset was only 11%. A similar experience has been reported recently for patients who relapse after receiving therapy per the LALA-94 study.50
The 32-year-old woman in CR1 (Case 1) who has no comorbid conditions should be referred for a MRD myelo-ablative allogeneic HSCT. Although she has no high-risk features, paradoxically, the best results for allogeneic HSCT have been observed in standard-risk patients. On the other hand, the 64-year-old man in Case 2 who did not readily attain CR is not a viable candidate for a myeloablative allogeneic HSCT due to advanced age. Despite the high-risk cytogenetic karyotype abnormality t(4:11), it is unknown if there is a substantial benefit to allogeneic HSCT in this situation. This patient, however, has an extremely poor prognosis given his age and primary induction failure status. He should strongly be encouraged to undergo an allogeneic HSCT using his histocompatible sister as a donor, as the benefits may outweigh the risks. The use of reduced-intensity conditioning HSCT is the subject of ongoing investigation. This approach cannot be recommended routinely at this time due to the paucity of data, but in the near future sufficient data likely will be available to assess the contribution of this strategy. If such a transplantation is to be considered in this patient, it should be performed in the context of a clinical trial.
Finally, some trials have demonstrated that URD transplantations appear to provide results comparable to MRD HSCT.27 Although the use of alternative donor HSCT and reduced-intensity transplantations remain controversial, a number of investigators suggest this option for high-risk patients.
Further studies need to be done to better define high risk, i.e., improve understanding of cytogenetic abnormalities and prognosis, and determine which high-risk disease patients are most likely to benefit from standard myelo-ablative allogeneic HSCT. Age, which repeatedly has been shown to be a factor in defining high-risk disease, also puts patients at higher risk for therapy-related morbidity and mortality and offsets the decreased relapse rate using allo-geneic HSCT. Alternative strategies will be necessary to maximize the risk-benefit ratio in patients who have a high likelihood of relapse without transplantation. Similarly, there is a group of adult patients with ALL who have prognostic factors that suggest high remission and long-term DFS rates. In those who are younger with a low WBC count, allogeneic HSCT appears to further improve their long-term DFS. Further analysis, however, will need to take into account other prognostic factors such as karyotype, time to CR, and MRD. Unlike with conventional therapy, for patients with Ph+ disease, outcomes after allogeneic HSCT are no different than for Ph– disease. There may be a group of patients for whom the risk of relapse is so low that even a reduced TRM incidence may not justify transplant such as T-cell ALL with the TXL1 (Hox11) mutation. As future prospective studies are done, it should allow us to define subsets of patients who are most and least likely to benefit from the various allogeneic HSCT options.
MRC-ECOG UKALLXII/EC2993: outcome after allogeneic hematopoietic stem cell transplantation (HSCT) in Ph−patients who had donors versus those who did not have donors.
| . | No. . | 5-y overall survival, % . | 5-y relapse rates, % . | 2-y nonrelapse mortality, % . |
|---|---|---|---|---|
| * High-risk is defined as age ≥ 35 years, WBC > 30,000/μL for patients with B-cell disease or WBC > 100,000/μL for patients with T-cell disease, or time to attain CR > 4 weeks. | ||||
| High-risk* | 401 | |||
| Donor | 171 | 40 | 39 | 39 |
| No donor | 230 | 36 | 62 | 12 |
| Standard-risk | 512 | |||
| Donor | 218 | 63 | 27 | 20 |
| No donor | 294 | 51 | 50 | 7 |
| . | No. . | 5-y overall survival, % . | 5-y relapse rates, % . | 2-y nonrelapse mortality, % . |
|---|---|---|---|---|
| * High-risk is defined as age ≥ 35 years, WBC > 30,000/μL for patients with B-cell disease or WBC > 100,000/μL for patients with T-cell disease, or time to attain CR > 4 weeks. | ||||
| High-risk* | 401 | |||
| Donor | 171 | 40 | 39 | 39 |
| No donor | 230 | 36 | 62 | 12 |
| Standard-risk | 512 | |||
| Donor | 218 | 63 | 27 | 20 |
| No donor | 294 | 51 | 50 | 7 |
Recommendations regarding decision to proceed to a sibling-matched related donor (MRD) or matched-unrelated donor (MUD) allogeneic hematopoietic stem cell transplant (HSCT) as therapy for acute lymphoblastic leukemia (ALL) patients < age 55 years.
| Disease indication . | MRD transplant recommended? . | MUD transplant recommended? . | Comments . |
|---|---|---|---|
| *High-risk cytogenetics defined as: t(4:11), t(8:14), complex karyotype (≥ 5 abnormalities), hypodiploidy/near triploidy | |||
| Abbreviations: CR1, first complete remission; CR2, second complete remission; TRM, treatment-related mortality; GVL, graft-versus-leukemia; Ph, Philadelphia chromosome | |||
| CR1 patients < 40 y: | Yes | No | |
| Standard-risk | |||
| CR1 patients < 40 y: | Yes | Yes | For high-risk cytogenetics,* increased relapse risk with standard chemotherapy. Unknown if allogeneic HSCT decreases risk. |
| High-risk | |||
| CR1 patients ≥ 40 y | Possibly, using reduced- intensity conditioning | No | Allogeneic transplantation decreases relapse risk, but TRM offsets benefit; reduced-intensity conditioning regimens being explored. |
| ≥ CR2 | Yes | Yes | |
| Primary refractory disease | Yes | Yes | |
| Ph+ disease | Yes | Yes | Highly potent allogeneic GVL effect in Ph+ disease |
| Minimal residual disease positivity after induction in Ph– disease patients | Yes | Possibly | Patients with minimal residual disease have increased relapse risk with standard therapy alone. |
| Only Ph+ disease shown to benefit from allogeneic HSCT. | |||
| Insufficient data on impact of HSCT in others. | |||
| Disease indication . | MRD transplant recommended? . | MUD transplant recommended? . | Comments . |
|---|---|---|---|
| *High-risk cytogenetics defined as: t(4:11), t(8:14), complex karyotype (≥ 5 abnormalities), hypodiploidy/near triploidy | |||
| Abbreviations: CR1, first complete remission; CR2, second complete remission; TRM, treatment-related mortality; GVL, graft-versus-leukemia; Ph, Philadelphia chromosome | |||
| CR1 patients < 40 y: | Yes | No | |
| Standard-risk | |||
| CR1 patients < 40 y: | Yes | Yes | For high-risk cytogenetics,* increased relapse risk with standard chemotherapy. Unknown if allogeneic HSCT decreases risk. |
| High-risk | |||
| CR1 patients ≥ 40 y | Possibly, using reduced- intensity conditioning | No | Allogeneic transplantation decreases relapse risk, but TRM offsets benefit; reduced-intensity conditioning regimens being explored. |
| ≥ CR2 | Yes | Yes | |
| Primary refractory disease | Yes | Yes | |
| Ph+ disease | Yes | Yes | Highly potent allogeneic GVL effect in Ph+ disease |
| Minimal residual disease positivity after induction in Ph– disease patients | Yes | Possibly | Patients with minimal residual disease have increased relapse risk with standard therapy alone. |
| Only Ph+ disease shown to benefit from allogeneic HSCT. | |||
| Insufficient data on impact of HSCT in others. | |||
Probability of disease-free (DFS) and overall survival (OS) for standard-risk adult acute lymphoblastic leukemia patients. Patients are further stratified into low-risk (LR), intermediate-risk (IR) and high-risk (HR) as determined by extent of minimal residual disease.
Reprinted with permission from
Probability of disease-free (DFS) and overall survival (OS) for standard-risk adult acute lymphoblastic leukemia patients. Patients are further stratified into low-risk (LR), intermediate-risk (IR) and high-risk (HR) as determined by extent of minimal residual disease.
Reprinted with permission from
Ireland Cancer Center, University Hospitals of Cleveland, Cleveland, Ohio