Abstract
Hemoglobin (Hb) E is one of the world’s most common and important mutations. It results in a heterogeneous group of disorders whose phenotype range from asymptomatic to severe. Hb E trait and Hb EE are mild disorders. The combination of Hb E and Hb S (Hb SE) results in a sickle cell disease syndrome similar to sickle β+ thalassemia. It is important to distinguish Hb E disorders diagnostically because of this marked difference in clinical course among different genotypes. Screening tests, including hemoglobin electrophoresis and high-pressure liquid chromatography (HPLC), may suggest other mutations, unless one is familiar with the findings. E β-thalassemia, the most serious form of E syndromes, affects a million people worldwide and is increasing in North America. Its phenotype ranges from mild anemia to severe transfusion-dependent thalassemia major. Several genetic modifiers affect the phenotype, including the type of β-thalassemia mutation, Hb F levels, and co-inheritance of α-thalassemia. However, the cause of the phenotypic variability is largely unknown. A prospective natural history study of E β-thalassemia in Sri Lanka suggests that environmental modifiers are prognostically important. The clinical course of E β-thalassemia is punctuated by acute and chronic complications that may cause serious morbidity and mortality. Recent studies indicate these patients are at high risk for thromboembolism secondary to a hypercoagulable state increased by splenectomy. Morbidity from iron overload in nontransfused patients secondary to increased gastrointestinal iron absorption is common. Cardiopulmonary disease, including pulmonary hypertension, requires ongoing monitoring and is secondary to iron overload, thromboembolism, and hemolysis-induced nitric oxide deficiency. These patients are excellent candidates for Hb F–modulating agents because moderate changes in hemoglobin may result in marked improvement in phenotype. Recent studies with hydroxyurea indicate 40% of patients will clinically improve with hydroxyurea.
Introduction: Demography
Hemoglobin (Hb) E is one of the world’s most common and important mutations.1–4 The resistance of Hb AE red cells to invasion by Plasmodium falciparum is most likely the cause for its high prevalence throughout the world.5 E β-thalassemia affects at least a million people worldwide. Since its classic description by Chernoff et al,6 it has been noted to be an important health problem in the Indian subcontinent and Southeast Asia. It has replaced β-thalassemia as the most common thalassemia disorder in many regions, including coastal North America. The frequency of Hb E approaches 60% in many regions of Thailand, Laos and Cambodia. The World Health Organization (WHO) estimates that in Thailand at least 100,000 new cases of Hb E β-thalassemia are expected in the next few decades.1,2,7 High estimates are predicted for India, Sri Lanka, Malaysia, and southern China. In the last two decades, most immigrants in North America came from areas where Hb E mutations were prevalent.8 These demographic changes have resulted in E β-thalassemia becoming a health problem in North America. The prevalence of Hb E in California parallels the rise in Asian births. In California, 1 in 4 Cambodian births and 1 in 9 Thai/Laotian births are Hb E carriers.9 The natural history of Hb E thalassemia is highly variable. The phenotype, for patients with similar mutations, can range from asymptomatic to transfusion dependent.
Diagnosis
Hb E is caused by a substitution of glutamic acid by lysine at codon 26 of the β-globin gene. This mutation also activates a cryptic mRNA splice site, which results in reduced synthesis of the β-E chain and leads to a thalassemia phenotype. Hb E has a weakened α/β interface, leading to some instability during conditions of increased oxidant stress. Hb E trait has no clinical significance. Patients may have mild microcytosis without anemia (see Table 1 ).10 The picture may be confused with iron deficiency unless laboratory studies are completed. The red cell morphology may show targeting Hb EE individuals are asymptomatic with very mild anemia and microcytosis.11 The red cell morphology picture may be similar to other thalassemia traits or mild thalassemia intermedia conditions. Targeted and irregularly contracted cells are commonly seen. Occasionally, patients have splenomegaly.
It is important to distinguish Hb E disorders diagnostically because of this marked difference in clinical course. DNA-based diagnosis of Hb E disorders is optimal and necessary before genetic counseling is undertaken. Screening tests may suggest other mutations, unless one is familiar with the findings.12 In testing with alkaline Hb electrophoresis, Hb E migrates with C, O Arab, and A2. In acid pH electrophoresis, it migrates with Hb A2. When used together, the diagnosis can be clearly made. Hb E is better separated on isoelectric focusing, but migrates close to Hb O Arab. In high-pressure liquid chromatography (HPLC) screening programs, Hb E similar retention times as Hb A2 and Hb Lepore. The percentage of Hb E in heterozygotes is approximately 30%. Diagnosis of concomitant α-thalassemia requires DNA testing. The concomitant inheritance of α-thalassemia often occurs and lowers the percentage of Hb E.13,14 In Hb E trait in combination with Hb H, Hb E drops to 10%.13,15 Iron deficiency also lowers the Hb E percentage. In Hb EE, the electrophoresis generally indicates 90% or more Hb E with a mild elevation in Hb F. Hb E β+-thalassemia may have an extremely variable laboratory picture. They usually have a mild anemia of approximately 9.5 g/dL. However, significant ranges of anemia has been observed, with Hb as low as 5.7 g/dL. The mean corpuscular volume (MCV) has been approximately 72 ± 6 fL and mean corpuscular Hb concentration (MCHC) has been 29 ± 2 fL.11 The electropheresis demonstrates both Hb E and Hb A, with a marked range for the amount of Hb A. The differential diagnosis includes Hb E β0-thalassemia. In this disease, Hb E ranges from 40% to 60%, with Hb F markedly elevated. In neonates, DNA analysis is required to differentiate these two syndromes in neonates and is increasingly being used routinely for diagnosis of Hb E disorders.
There are several compound heterozygotes with Hb E and an uncommon β-globin mutation. Hb E Lepore and Hb E δ β-thalassemia are compound heterozygotes with mild phenotypes. Hb SE is being more frequently diagnosed due to changing population migration patterns and intermarriage of at-risk individuals. It is often initially misdiagnosed as HB SC because of the comigration of C and E patterns on electrophoresis. Patients are mildly anemic with microcytic red cells. The electropheretic pattern demonstrates 60% S and 30% E. The clinical phenotype is similar to sickle β+-thalassemia. Medical complications appear to increase as patients get older. Painful episodes, acute chest syndrome, splenic sequestration, and avascular necrosis of hip have all been observed.16
Pathophysiology and Clinical Variability
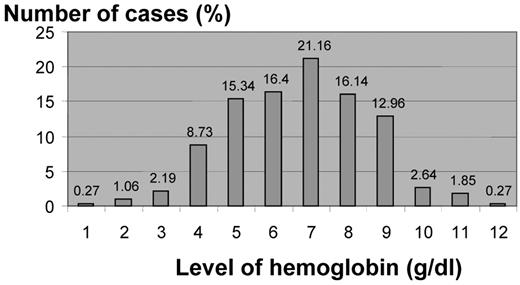
The most serious Hb E syndrome is Hb E β0-thalassemia. The compound heterozygote state of Hb E β-thalassemia results in a variable phenotype ranging from a complete lack of symptoms to transfusion dependency.1,10,17 In review of 378 patients with Hb E-β0-thalassemia from Thailand, the hemoglobin concentrations ranged from 3 to 13 g/dL, with an average of 7.7 g/dL17 (Figure 1 ). Approximately one-half of the patients are phenotypically similar to patients with thalassemia major who require regular transfusion therapy, and the other half resembles thalassemia intermedia.1,10,17
The pathophysiology of Hb E β-thalassemia is complex. Ineffective erythropoiesis, apoptosis, and oxidative damage are central components of the disease and its shortened red cell survival.1,18,19 The instability of Hb E is a minor factor in its pathophysiology, but in febrile patients, may account for accelerated hemolysis.20 The interaction between Hb E and β-thalassemia alleles is the main determinant in the pathophysiology.1 The globin chain imbalance that results from these mutations correlates with the severity of the disease. However, the cause of the striking variability in individuals with E β-thalassemia remains largely unknown. Patients with the same mutations within a family may show significant differences in clinical severity.
Hb F level is the strongest predictor of morbidity.21–25 However, the basis of increased Hb F is usually unknown.23,26 The inheritance of a β-thalassemia chromosome with the Xmn I(+) polymorphism in the promoter region of the G γ-globin gene may be responsible for increased Hb F and a milder clinical course.1,17,22,23 However, the Xmn mutation only explains a small percentage of patients with high Hb F expression.1,10,17 The degree of severity is also affected by the type of β-thalassemia mutation.27 β0-thalassemia mutations generally result in a more severe phenotype than β+ thalassemia mutations. However, some β+ mutations produce minimal amounts of β-globin chains and are similar to β0-thalassemia. The two common mutations, IVS1–5 (G→C) and IVS1–1 (G→A), are severe â+- and â0-thalassemia mutations, and most others cases include â0-thalassemia mutations.1,26 These primary mutations do not explain the marked clinical diversity.1,27–29 Co-inheritance of α-thalassemia mutations decrease the globin chain imbalance and improve the anemia. This occurs in fewer than 15% of patients. Triplicated or quadruplicated α-globin genes increase the severity of E-thalassemia and occur in approximately 4% of the population. Other genetic factors most likely play a major role in its clinical variability. However, screening studies have not uncovered other significant predictors. Sripichai et al screened 1060 patients with Hb E â-thalassemia with a candidate gene approach to uncover secondary genetic modifiers.30,31 The study focused on single nucleotide polymorphisms (SNPs) selected for genes encoding the transcription factors, β-protein 1, the α-hemoglobin stabilizing protein, and genes involved in erythropoiesis. None were predictive of severity.
Understanding the extreme clinical variability of E β-thalassemia requires prospective, laboratory, and clinical studies of the natural history of the disease. In 1997, Weatherall and colleagues initiated a long-term Hb E β-thalassemia study.19,21,24,28 As part of this study, transfusion therapy was stopped in order to better define their baseline clinical condition and laboratory data. Patients were classified into five categories ranging in severity. The mildest group, group 1, never received transfusions, presented at 16 years of age and demonstrated normal growth and development, fertility, with mild iron loading. In group 2 patients had received intermittent transfusions and presented at the age of 10 years; these patients tolerated removal of long-term transfusion therapy and continued to demonstrate normal maturation. Groups 3 and 4 presented at 3.5 years of age, required splenectomy for hypersplenism, and generally required transfusion therapy. Group 5 presented at 1 year of age, were severely affected, and corresponded to the severe β-thalassemia phenotype. The study uncovered several important observations. The difference in the steady-state hemoglobins between mild groups and severe groups was small. Why a small change in hemoglobin has such a profound effect is not fully explained.
Several important genetic and environmental predictors of severity were noted in the Sri Lanka population over time. Age of presentation, growth rate, splenomegaly, and hemoglobin F levels were prognostic indicators of severity. The time of onset of symptoms was useful in predicting the long-term clinical phenotype. Severe anemia with symptoms that begin between 6 and 12 months indicated a thalassemia-major phenotype. A stable clinical condition at 5 years generally indicated a thalassemia intermedia phenotype, at least through early adulthood. Marked splenomegaly, even if responsive to splenectomy, suggested a more severe clinical course. As patients aged, their erythropoietin levels fell and correlated with a decreased hemoglobin level. This observation explains the increased requirements for transfusions in older patients. The high frequency of gallstones in this population was strongly associated with 7/7 genotype of the UGTA1A promoter.32 The study also found environmental factors remain an important modifier of severity. Patients with a positive serology for P falciparum and P vivax were more likely to be severe. It is unclear whether this increased rate of malaria exposure indicates severe patients are more prone to infection or the infection itself changes the chronic phenotypic expression of the disease.
Clinical Course and Treatment Strategies
The clinical course of E β-thalassemia is punctuated by acute and chronic complications that may cause serious morbidity and mortality. The marked expansion of erythropoiesis is responsible for much of the pathology of the disease, including hepatosplenomegaly, extramedullary hematopoietic masses, growth retardation, delayed sexual maturation, and bone deformities.1,10,11,17,33
Splenomegaly often develops in severely affected patients. In the past, splenectomy was routinely performed in an attempt to increase hemoglobin levels. Increasing evidence of the risk of thromboembolism in patients has led to reconsideration of the role of splenectomy.34–36 Splenectomy results in thrombocytosis and an increased exposure of red cell membrane phosphatidylserine.37 These changes induce a hypercoagulable state with endothelial activation.34,38 Thrombi may occur in any organ, but the lung is particularly vulnerable.35 Autopsy data in splenectomized patients often show widespread pulmonary artery thrombosis that may be responsible for the chronic hypoxemia often seen in these patients.1,10
Iron overload in nontransfused patients is common, secondary to increased gastrointestinal absorption of iron.39 End organ failure secondary to iron overload may not be suspected because the serum ferritin level is disproportionately low.39 Hemosiderosis-induced cirrhosis has been observed in nontransfused patients, particularly after splenectomy. Quantitative liver iron measurements are necessary to identify iron overload even in nontransfused patients with Hb E β0-thalassemia.
Cardiopulmonary disease is the most common cause of death in Hb E β-thalassemia. Cardiomyopathy, secondary to hemosiderosis, often occurs in chronically transfused patients. The nontransfused patient is at particular risk for pulmonary hypertension and right-heart failure. However, transfused patients have also developed this complication.10 Thromboembolism and hemolysis-induced nitric oxide deficiency result in pulmonary vascular injury and subsequent cardiac disease.34,40 The dysregulated arginine metabolism observed in patients with sickle cell with pulmonary hypertension has also been seen in this population.41 Transfusion therapy and sildenafil may be beneficial.
Patients with Hb E β-thalassemia are excellent candidates for agents directed at elevating Hb F production. A small increase in the steady-state hemoglobin concentration might be of major clinical benefit. In a multicenter trial of 42 patients with E β0-thalassemia treated with hydroxyurea (HU), almost half the patients demonstrated a significant increase in steady-state hemoglobin level.25 HU was started at 7 mg/kg/day and increased to a maximum dose of 20 mg/kg/day without any significant toxicity. Thirty-eight percent of patients maintained a mean hemoglobin increase of almost 1.5 gm/dL for years. This increase in hemoglobin corresponded to a rise in Hb F and a decrease in markers of erythropoietic stress. A high baseline Hb F was the best predictor of response to HU. HU, in combination with erythropoietin, was only beneficial in patients with low baseline erythropoietin levels. Thirteen of 27 patients who were regularly transfused have become transfusion independent and demonstrated a marked decrease in their iron status. Some of these patients were thalassemia intermedia phenotypes placed on chronic transfusions for subjective reasons. While HU therapy may be beneficial to these patients, responses are unpredictable. Clinical trials with decitabine and short-chain fatty acid compounds have recently been initiated and may result in more consistent responses.42
In summary, Hb E disorders are a heterogeneous group of diseases that are rapidly increasing worldwide. The etiology for the marked variation in phenotype remains largely unknown. Correct laboratory diagnosis is essential to separate asymptomatic genotypes from severe mutations. Both transfused and nontransfused patients with Hb E β0-thalassemia are at risk for life-threatening complications and should be followed by a multidisciplinary team. Annual monitoring for complications from anemia and/or iron overload is necessary. Cardiac, endocrine, pulmonary, hepatobiliary, and skeletal systems may be affected. In addition to falling Hb, close monitoring of organ function and quality of life are necessary in order to determine the initiation of chronic transfusion therapy. Chelation therapy may be necessary in both the transfused and nontransfused patients. Comprehensive care, including genetic counseling, psychological support and access to new therapy, are important for all patients with E β0-thalassemia.
Hematologic data in various hemoglobin E syndromes10 (modified).
| . | Hb, g/dL . | MCV, fL . | MCH, pg . | MCHC, g/dL . | RDW, % . | Alkali denaturation test (Hb F),% . | Hb typing . |
|---|---|---|---|---|---|---|---|
| Abbreviations: CS, Hb Constant Spring; D, decreased; Hb, hemoglobin; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; N, normal; ND, not determined; RDW, red cell distribution width | |||||||
| Normal | M15.9 ± 0.9 F12.5 ± 2.0 | 87 ± 6 | 31 ± 1.1 | 33 ± 0.9 | 13.1 ± 0.8 | 0.5 ± 0.2 | A2 (2.5 ± 0.2) + A |
| Hb E trait | 12.8 ± 1.5 | 84 ± 5 | 30 ± 2.4 | 33 ± 1.8 | 14.1 ± 0.6 | 0.9 ± 0.7 | E (29.4 ± 2.3%) + A |
| Hb E α-thalassemia 2 | 13.1 ± 1.4 | 88 ± 4 | ND | ND | ND | E (28.5 ± 1.5%) + A | |
| Hb E α-thalassemia 1 | 12.5 ± 1.4 | 77 ± 5 | 23 ± 1.1 | 32 ± 1.6 | ND | 0.9 ± 0.4 | E (20.7 ± 1.2%) + A |
| Homozygous HbE | 11.4 ± 1.8 | 70 ± 4 | 22 ± 1.9 | 33 ± 1.7 | 15.6 | 1.8 ± 1.4 | E (87.7 ± 5.9%) |
| Hb E/β0-thalassemia | 7.8 ± 2.6 | 67 ± 6 | 19 ± 3.6 | 28 ± 4.8 | 26.5 ± 5.6 | 42 ± 1.5 | E (58 ± 1.5%) + F |
| AE Bart’s disease | E (13.0 ± 2.1%) + A + Bart’s (2.2 ± 1.8%) | ||||||
| α-thalassemia 1/α-thalassemia 2-Hb E | 9.1 ± 1.1 | 60 ± 3 | 17 ± 2 | 31 ± 4 | ND | 2.0 ± 0.7 | |
| α-thalassemia 1/Hb CS-Hb E | 8.0 ± 0.9 | 67 ± 4 | 19 ± 2 | 29 ± 2 | ND | 2.3 ± 1.4 | CS (1.1 ± 0.4%) + E (13.9 ± 1.8%) + A + Bart’s (3.9 ± 1.5%) |
| Hb EE + Hb H | 8.0 ± 1.3 | 63 ± 6 | 18 ± 2 | 29 ± 2 | ND | 5.8 ± 3.7 | E (80%) + F + Bart’s(5%) or CS (1.9 ± 0.9%) + E (86.4 ± 8%) + F + Bart’s (3.7 ± 1.9%) |
| Hb SE12 | 11.2 ± 1.8 | 75 ± 10 | ND | ND | ND | ND | Hb S (62.8% ± 7.4%), Hb E (33.3% ± 3.6%), Hb F (range 1 – 5.2) |
| . | Hb, g/dL . | MCV, fL . | MCH, pg . | MCHC, g/dL . | RDW, % . | Alkali denaturation test (Hb F),% . | Hb typing . |
|---|---|---|---|---|---|---|---|
| Abbreviations: CS, Hb Constant Spring; D, decreased; Hb, hemoglobin; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; N, normal; ND, not determined; RDW, red cell distribution width | |||||||
| Normal | M15.9 ± 0.9 F12.5 ± 2.0 | 87 ± 6 | 31 ± 1.1 | 33 ± 0.9 | 13.1 ± 0.8 | 0.5 ± 0.2 | A2 (2.5 ± 0.2) + A |
| Hb E trait | 12.8 ± 1.5 | 84 ± 5 | 30 ± 2.4 | 33 ± 1.8 | 14.1 ± 0.6 | 0.9 ± 0.7 | E (29.4 ± 2.3%) + A |
| Hb E α-thalassemia 2 | 13.1 ± 1.4 | 88 ± 4 | ND | ND | ND | E (28.5 ± 1.5%) + A | |
| Hb E α-thalassemia 1 | 12.5 ± 1.4 | 77 ± 5 | 23 ± 1.1 | 32 ± 1.6 | ND | 0.9 ± 0.4 | E (20.7 ± 1.2%) + A |
| Homozygous HbE | 11.4 ± 1.8 | 70 ± 4 | 22 ± 1.9 | 33 ± 1.7 | 15.6 | 1.8 ± 1.4 | E (87.7 ± 5.9%) |
| Hb E/β0-thalassemia | 7.8 ± 2.6 | 67 ± 6 | 19 ± 3.6 | 28 ± 4.8 | 26.5 ± 5.6 | 42 ± 1.5 | E (58 ± 1.5%) + F |
| AE Bart’s disease | E (13.0 ± 2.1%) + A + Bart’s (2.2 ± 1.8%) | ||||||
| α-thalassemia 1/α-thalassemia 2-Hb E | 9.1 ± 1.1 | 60 ± 3 | 17 ± 2 | 31 ± 4 | ND | 2.0 ± 0.7 | |
| α-thalassemia 1/Hb CS-Hb E | 8.0 ± 0.9 | 67 ± 4 | 19 ± 2 | 29 ± 2 | ND | 2.3 ± 1.4 | CS (1.1 ± 0.4%) + E (13.9 ± 1.8%) + A + Bart’s (3.9 ± 1.5%) |
| Hb EE + Hb H | 8.0 ± 1.3 | 63 ± 6 | 18 ± 2 | 29 ± 2 | ND | 5.8 ± 3.7 | E (80%) + F + Bart’s(5%) or CS (1.9 ± 0.9%) + E (86.4 ± 8%) + F + Bart’s (3.7 ± 1.9%) |
| Hb SE12 | 11.2 ± 1.8 | 75 ± 10 | ND | ND | ND | ND | Hb S (62.8% ± 7.4%), Hb E (33.3% ± 3.6%), Hb F (range 1 – 5.2) |
Hemoglobin levels in E/β thalassemia patients (Thailand)1 (modified).
Children’s Hospital & Research Center at Oakland, Oakland, California