Abstract
Non-malignant late effects after hematopoietic stem cell transplantation (HSCT) are heterogeneous in nature and intensity. The type and severity of the late complications depend on the type of transplantation and the conditioning regimen applied. Based on the most recent knowledge, we discuss three typical non-malignant complications in long-term survivors after HSCT, namely pulmonary, cardiovascular and renal complications. These complications illustrate perfectly the great diversity in respect of frequency, time of appearance, risk factors, and outcome. Respiratory tract complications are frequent, appear usually within the first two years, are closely related to chronic graft-versus-host disease (GVHD) and are often of poor prognosis. Cardiac and cardiovascular complications are mainly related to cardiotoxic chemotherapy and total body irradiation, and to the increase of cardiovascular risk factors. They appear very late after HSCT, with a low magnitude of risk during the first decade. However, their incidence might increase significantly with longer follow-up. The chronic kidney diseases are usually asymptomatic until end stage disease, occur within the first decade after HSCT, and are mainly related with the use of nephrotoxic drugs such as calcineurin inhibitors. We will discuss the practical screening recommendations that could assist practitioner in the follow-up of long-term survivors after HSCT.
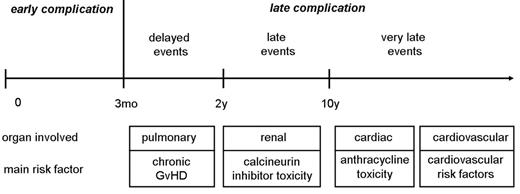
Late complications are conditions appearing after the early phase of hematopoietic stem cell transplantation (HSCT) with clinical consequences on the long-term survivorship. Depending on the type of complication, the threshold between early and late might be set at different time points. Some of the complications with relevant late consequences can start as early as 3 months after HSCT, and other events will become apparent only years or even decades later. Here, we define as late complications all events occurring beyond 3 months (Figure 1 ), and separate them into delayed (3 months to 2 years), late (2 to 10 years) and very late events (>10 years). Late complications after HSCT are the consequence of the conditioning regimen, chronic graft-versus-host disease (GVHD) and its treatment, infectious complications, the treatments used before transplantation, and the pretransplant comorbidity. Many late complications, such as secondary cancer, cataracts, infertility, endocrine dysfunctions, or late bone and joint complications, have been well described. In theory, any organ can be the target of a late effect, and frequently multiple causes are involved. This review will focus on late pulmonary, cardiac and cardiovascular as well as renal consequences after HSCT. It will consider the involved risk factors and the recommended screening practices (Table 1 ).
Delayed Onset Pulmonary Complications
Delayed onset pulmonary complications involving both the airway and lung parenchyma are frequent after HSCT. They include infectious complications in immunocompromised hosts and noninfectious complications of the lung. The most common noninfectious late complications include bronchiolitis obliterans (BO), bronchiolitis obliterans organizing pneumonia (BOOP), and idiopathic pneumonia syndrome (IPS).1 BOOP/COP has also been termed cryptogenic organizing pneumonia (COP) in order to avoid confusion with airway diseases such as bronchiolitic obliterans syndrome (BOS).2 These pulmonary complications, belonging to the delayed events, appear usually within 3 months to 2 years after HSCT. However, the functional consequences often persist for years after HSCT. There are differences between autologous and allogeneic HSCT, particularly in respect of time of appearance. In autologous but not in allogeneic HSCT, pulmonary complications are unusual after 3 months. In a retrospective analysis, the 2-year cumulative incidence of delayed onset noninfectious pulmonary complications was 10% among 438 patients surviving more than 3 months, and 15.6% among those with chronic GVHD.3 The 5-year overall survival was significantly worse in patients with a pulmonary complication, compared to those without. In the unrelated HSCT setting, the incidence of delayed onset noninfectious pulmonary complications is higher and the clinical outcome of these patients worse.4 Chronic extensive GVHD and advanced-stage disease is associated with the development of delayed onset pulmonary complications.
Pulmonary function tests
Restrictive and obstructive ventilatory defects and gas transfer abnormalities are common after HSCT. A decrease in forced expiratory volume in 1 second (FEV1) and the FEV1/forced vital capacity (FVC) ratio is the hallmark of airflow obstruction. Restrictive defects are measured by the total lung capacity (TLC) and may be associated with impaired diffusing capacity for carbon monoxide (DLCO). Pulmonary function evaluated retrospectively in 69 patients with a minimum of 5-year follow-up after allogeneic HSCT showed a late decrease from baseline in 31 (45%) of the patients, with a restrictive pattern in 25, and an obstructive pattern in 6. Twelve of the 31 (38%) patients with abnormal pulmonary function were symptomatic.5 Abnormal pulmonary function before transplantation and chronic GVHD were independently associated with late decrease in pulmonary function compared with baseline. In children, a significant proportion have abnormal function tests after HSCT.6 They involve mainly abnormalities of DLCO and TLC, implying restrictive lung disease and diffusion abnormalities. Obstructive abnormalities are less frequently observed. In a prospective study of the Late Effects Working Party of the EBMT, cumulative incidence of lung impairment evaluated in 162 children by pulmonary function was 35% at 5 years. Chronic GVHD was the major risk factor for reduced lung function. In most children the deterioration of pulmonary function was asymptomatic.7
Bronchiolitis obliterans
Bronchiolitis obliterans is a severe pulmonary manifestation characterized by a nonspecific inflammatory injury affecting primarily the small airways. At the initial stage, it is typically an obstructive respiratory disease (Figure 2A/ 2B; see Color Figures, page 495). At a more advanced stage, due to the progressive peribronchiolar fibrosis, BO often presents obstructive and restrictive functional changes. The incidence of BO varies widely in different reports, ranging between 0 and 48%. Among 2152 allogeneic HSCT recipients reported in 9 studies the incidence of BO was 8.3%.8 BO is strongly associated with chronic GVHD, suggesting that BO is a pulmonary manifestation of chronic GVHD.9 However, despite the fact that BO rarely develops in patients without GVHD, single cases of BO have been reported after autologous HSCT. Following peripheral blood progenitor cell transplantation patients were shown to have a 3-fold increase in the risk of BO compared with those who had bone marrow transplantation.10 Other potential risk factors include the use of methotrexate for GVHD prophylaxis, older age of the recipient and/or the donor, busulfan-based myeloablative conditioning, antecedent respiratory viral infection, and low levels of serum immunoglobulin.
The presentation of BO is usually insidious, with a median onset approximately 1 year post-HSCT. The main symptoms are dry cough, progressive dyspnea, and wheezing. Fever does usually not occur, unless there is a concomitant infection. Asymptomatic presentation with abnormal functional tests is observed in 20% of the cases. In the early stage chest X-ray is normal; thus, the presence of parenchymal changes suggests an infection or an unrelated process. In more advanced phases, evidence of hyperinflation may be found. High resolution computed tomography (HRCT) of the chest with inspiratory and expiratory images is the radiological procedure of choice to assess the structural changes in the lung with suspected BO. Pulmonary lobules with normal airways increase their density during expiration, while areas with obstructed airways and air trapping remain radiolucent. This provides a characteristic mosaic image that is highly suggestive of BO. The sensitivity to detect air trapping for the diagnosis of BO ranges between 74% and 91% and specificity between 67% and 94%.11–13 The predictive negative value is higher than 90%. Hence, when no air trapping is seen on expiratory HRCT the diagnosis of BO is very unlikely. At the early stage, pulmonary function tests show air flow obstruction with decreased FEV1, normal TLC and DLCO. A > 20% decline in FEV1 from the pretransplant value, or < 80% of the predicted FEV1 should alert clinicians. Recently, an international workshop on chronic GVHD by the National Institutes of Health defined biopsy-proven BO as the only diagnostic criteria of chronic GVHD in the lung (Figure 2C; see Color Figures, page 495). BO is clinically diagnosed when the following conditions are met: (1) FEV1/FVC ratio < 0.7 and FEV1 < 75% of predicted value; (2) evidence of air trapping or small airway thickening or bronchiectasis in HRCT; and (3) absence of infection in the respiratory tract.14
There are no prospective clinical trials on the treatment of BO. So far, the therapeutical recommendations are mainly derived from retrospective studies and from expert opinion.8,15,16 The management is based on the treatment of chronic GVHD. Early detection and prompt immunosuppressive treatment are likely to contribute to a more favorable outcome. Inhaled corticosteroids with bronchodilatator have shown some utility in the management of obstructive airway disease after HSCT.17,18 Further treatment consists of high-dose, systemic corticosteroids and the institution or augmentation of immunosuppressive therapy. Corticosteroids in a dose of 1 to 2 mg/kg/day for 2 to 6 weeks remain the mainstay of the treatment. Higher doses of corticosteroids have not shown higher efficacy. Cyclosporine is often used concomitantly. The addition of a third immunosuppressive agent such as azathioprine, thalidomide, anti-thymocyte globulin, anti TNF-α, or the use of macrolide antibiotics have been shown to be beneficial in some cases. Prevention of Pneumocystis jirovecii and the early treatment of superinfection is an important component of the treatment strategy. However, prognosis of patients with BO remains poor, and mortality remains high. In a majority of cases, death is attributed to progressive respiratory failure or opportunistic infections.
Bronchiolitis obliterans organizing pneumonia, cryptogenic organizing pneumonia
Bronchiolitis obliterans organizing pneumonia, also termed cryptogenic organizing pneumonia2 (BOOP/COP), is a clinicopathological syndrome involving bronchioles, alveolar ducts, and alveoli. The lumens of the alveoli become filled with buds of granulation tissue. It has been first described in non-transplanted patients, associated with infections, drugs, collagen vascular disease irradiation or present as an idiopathic form. In the setting of HSCT, BOOP/COP presents as an interstitial pneumonia rather than an airway disease. Compared to BO, it occurs earlier in the course of HSCT, mainly between 1 and 12 months post transplant, and with an incidence of less than 2%. However, delayed onset BOOP/COP, occurring years after HSCT, can be observed. The clinical presentation is usually acute, with dry cough, dyspnea and fever. The chest X-ray presents peripheral patchy consolidation, ground glass attenuation and nodular opacities. In contrast to BO, the pulmonary function tests of most patients with BOOP/COP show a restrictive pattern with decreased TLC and DLCO, but normal FEV.11,19 Bronchoscopy with bronchoalveolar lavage (BAL) is useful for ruling out pulmonary infection. The definitive diagnosis is based on histopathology. In a retrospective review on 817 surgical lung biopsy specimens performed over a period of 22 years, 49 cases of histological proven BOOP/COP were detected after allogeneic HSCT. In a case-control study with 161 matched controls, these 49 patients with BOOP/COP were more likely to have acute and chronic GVHD.20 Despite the lack of clinical evidence, systemic corticosteroids or even inhaled corticosteroids can be considered as first line therapy for BOOP/COP after HSCT. With corticosteroid therapy, BOOP/COP resolved or remained stable in 78%, and progressed in 22%. Patients with progressive disease have poor prognosis. Of 11 patients with progressive disease, 8 died of respiratory failure attributed to BOOP/COP.20 Among HSCT-patients with histological diagnosis of BOOP/COP, 5-year survival was estimated at 33%. In a retrospective study, BOOP/COP was observed in patients treated with unmanipulated peripheral blood progenitor cells, but not when the transplant has been T-cell depleted in vitro.21
Idiopathic pneumonia syndrome
Idiopathic pneumonia syndrome (IPS) usually occurs within 120 days after transplantation and is related with TBI, pretransplant chemotherapy, GVHD, and older age at HSCT.22 However, delayed-onset interstitial pneumonitis occurring years after HSCT have been reported,4,8,23 often in patients with severe chronic GVHD, especially with sclerodermatous cutaneous GVHD.24 Clinical presentation and radiographic findings are non-specific and do not differentiate from infectious pneumonia. Pulmonary function tests show a restrictive pattern, with decreased TLC and DLCO, but normal FEV1. It is rare for delayed-onset IPS to progress to respiratory failure, and these cases are probably related to delayed lung toxicity due to chemotherapy and irradiation. A delayed pulmonary toxicity syndrome, different from IPS, characterized by interstitial pneumonitis and fibrosis, has been described in about 72% of patients treated with cyclophosphamide, carmustine and cisplatinum and autologous HSCT for breast cancer.25 Conditioning regimens including carmustine and TBI are more likely to be associated with pulmonary toxicity.
Late Cardiac and Cardiovascular Complications
Long-term survivors of HSCT are at risk for a variety of cardiac and cardiovascular late effects. However, publications on long-term cardiac complications after HSCT are scarce. Compared to relapse of the primary disease and other non-relapse–related complications, cardiac late effects are considered to be a rare complication, and have so far not been a major concern in long-term HSCT survivors. After autologous HSCT, 43% of the late deaths were attributed to treatment related causes, and 2.4% of them were due to cardiac events.26 After allogeneic HSCT, mortality was attributed to transplant-related causes in 25%, and cardiac toxicity was the cause of death in 3%.27 However, the follow-up time after HSCT might still be too short to assess full magnitude of risk.
Experiences of cancer survivorship from non-transplanted patients with considerable longer follow-up can be useful for understanding what can be expected after HSCT. Late cardiotoxicity in cancer survivors includes cardiomyopathy, overt congestive heart failure, valvular dysfunction or arrhythmia. Anthracyclines and their derivatives are the main cause of cardiomyopathy. The risk of developing cardiotoxicity is strongly related to the total cumulative dose of anthracyclines. However, there is no safe cumulative dose of anthracycline. Mediastinal radiotherapy can produce inflammation and fibrosis of any structure of the heart, causing a restrictive cardiomyopathy. Fibrosis may also affect electrical conduction pathways, causing intracardiac conduction delay, arrhythmias, and autonomic dysfunction, and develop valvular defects. In long-term survivors of Hodgkin lymphoma, cardiovascular disease remains the major cause of non-relapse morbidity and mortality.28 Mediastinal radiation also increases the risk of myocardial infarction, angina pectoris, and congestive heart failure 2- to 7-fold.
Late cardiac complications
The follow-up in patients treated with HSCT is still substantially shorter than in survivors of Hodgkin lymphoma. Compared to a general population, the risk of late death due to cardiac complications is 4-fold higher after autologous HSCT in females only,26 and 2.3-fold higher after allogeneic HSCT in both males and females.27 In a cohort of patients with aggressive lymphoma treated with autologous HSCT, non-malignant late complications observed within the first 3 years were most often related to infections (19%), or were neurological (18%) and gastrointestinal (15%) late events. Cardiovascular late effects were reported in 6% of the patients, with a 3-year cumulative incidence of 9%.29 All these patients presented with left ventricular ejection fraction dysfunction, but only one third of them had clinical symptoms requiring treatment. More late cardiac complications were noted when mitoxantrone was used as part of conditioning regimen.
In pediatric patients, late cardiac complications after HSCT are less frequent.30 With a median follow-up of 10 years, 87% of 155 long-term survivors after allogeneic HSCT had at least one late effect. Pulmonary dysfunction was observed in 63.2%, but cardiovascular events in only 15% of the patients. In a prospective multicenter EBMT study, cardiac shortening fraction measured by echocardiogram before and yearly up to 5 years of follow-up were evaluated in 119 children treated with allogeneic HSCT.7 The 5-year cumulative incidence of cardiac impairment was 26%. TBI alone and TBI together with pretransplant anthracycline administration were significant risk factors for reduced cardiac function. Of patients who received TBI and anthracyclines, 26% had abnormal cardiac shortening fraction, as compared with only 2% of patients without TBI and an-thracyclines. At last follow-up 5 years after HSCT, only 13% of the patients had cardiac dysfunction and all of them were asymptomatic and in generally good health. In another study on long-term outcome in children surviving 1 year after allogeneic HSCT for a hematological malignancy, the probability of developing cardiac and cardiovascular complications at 10 years increased up to 11%.31 Eleven out of 48 children had abnormal echocardiography, 8 patients had high blood pressure, and 2 patients had cerebrovascular injuries. All patients who developed a cardiac complication had received TBI. Most studies on long-term survivors are based on functional cardiac tests at rest. In a pediatric population, longitudinal assessment showed that cardiovascular performance with exercise testing was significantly impaired after HSCT.32
So far, these studies illustrate that clinically relevant cardiac failure after HSCT appears to be rare. However, lessons from long-term cancer survivorship have demonstrated that cardiac complications will occur decades after treatment as very late events. Over a long period of time, patients develop asymptomatic cardiac dysfunction, as shown by the high frequency of cardiac dysfunction after HSCT, particularly when assessed by exercise testing. Therefore, we have to consider the current optimistic estimates of the risk of cardiac complications after HSCT with caution. Indeed, the real magnitude of risk for cardiac events after HSCT will only be definitively known with time.
Late cardiovascular complications
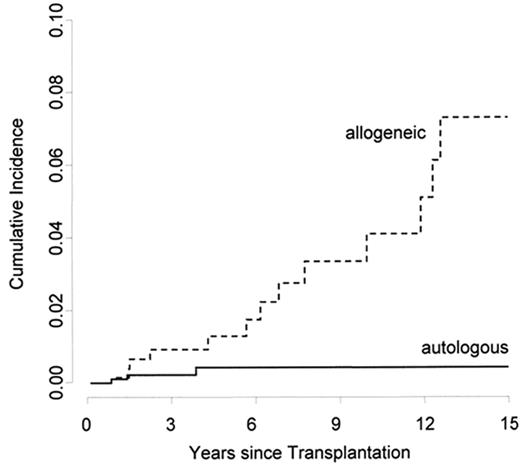
Several transplant centers reported single cases of young patients who developed fatal stroke or coronary artery disease after HSCT. These patients share some common features: They were all treated with allogeneic HSCT and had chronic GVHD. In a series of 11 patients with cardiac GVHD, 2 patients died of coronary heart disease.33 In a retrospective, single-center study including 265 long-term survivors, the cumulative incidence of an arterial event after allogeneic HSCT, such as cerebrovascular disease, coronary artery disease or peripheral artery disease was 22% at 25 years.34 The cumulative incidence was higher after allogeneic than after autologous HSCT. When adjusted for age, patients treated with allogeneic HSCT had a 7-fold increased relative risk of an arterial event at 15 years (Figure 3 ). In a multivariate analysis, the occurrence of established cardiovascular risk factors, including hypertension, dyslipidemia and diabetes, and the type of transplantation were associated with an increased risk of a late arterial event. These results are in line with a retrospective multicenter EBMT study. Twenty (3.6%) of 548 patients from 10 centers developed a cardiovascular event in at least one arterial territory. The cumulative incidence of an arterial event at 15 years was 6%. The cumulative incidence for patients with a high global cardiovascular risk score, defined as presenting ≥ 50% of the risk factors (arterial hypertension, diabetes, dyslipidemia, increased body-mass index, physical inactivity, smoking), was 17%, as compared with 4% in those with a low-risk score.35 In a third study on a cohort of 1089 long-term survivors, this increased risk of cardiovascular complications after HSCT could not be demonstrated.36 These conflicting findings might be explained by a different design. In the first two studies all consecutive patients surviving 2 years were included. The third study was based on a questionnaire filled out by the patients. This ascertainment method excludes long-term survivors who have died and might lead to a bias due to non-reporting patients.
The increased incidence of cardiovascular events after allogeneic HSCT supports the hypothesis that GVHD could be involved in the process. This is in accordance with the data on decreased numbers of microvessels in the subcutaneous compartment in patients with chronic GVHD.37 This loss of microvessels in the skin is independent of the epidermal injury, which is the histopathologic hallmark of cutaneous GVHD. A correlation between GVHD and endothelial injury is also suggested in a study where donor-derived cells contributed to the endothelial repair of GVHD-induced lesions.38 Endothelial injury provoked by GVHD could therefore be responsible for atherosclerotic changes, and lead to premature cardiovascular accidents after allogeneic HSCT. Because of the long latency between the initial vascular injury and the clinical manifestation, cardiovascular complications appear only decades after transplantation and belong therefore to the very late events.
Cardiovascular events are also associated with the appearance of cardiovascular risk factors after HSCT. Hyper-insulinemia, impaired glucose tolerance, hypertriglyceridemia, low levels of HDL cholesterol, and abdominal obesity are more common among patients treated with allogeneic HSCT than among leukemia patients or healthy controls.39 In a large cohort of 1089 long-term survivors, patients treated with allogeneic HSCT were more likely to report diabetes and hypertension than siblings, and more likely to report hypertension than autologous recipients.36 The development of cardiovascular risk factors after allogeneic HSCT can be the consequence of prolonged and intensified immunosuppressive treatment40 or be caused by endocrine dysfunction such as decreased growth hormone secretion in children41 or hypothyroidism. Patients treated with allogeneic HSCT may develop cardiovascular risk factors that in the long term will lead to an increased risk of a cardiovascular disease.
Late Renal Complications
After HSCT, chronic kidney disease may lead to progressive loss of the renal function and terminate in end-stage renal disease. It may remain unnoticed for years until end-stage disease, unless functional tests are performed. Risk factors in the general population include older age, hypertension, diabetes, cardiovascular disease and a family history of kidney disease. Little is known about the magnitude of risk of late renal dysfunction after HSCT. The use of various nephrotoxic agents is strongly associated with renal dysfunction. Substances involved are drugs used for chemotherapy prior to the HSCT and for the conditioning regimen, and anti-infectious agents (antibiotics, antifungal, antiviral therapy), as well as immunosuppressive substances needed to prevent or to treat GVHD.
Chronic kidney disease is defined as a sustained decrease in glomerular filtration rate (GFR) below levels of 60 mL/min/1.73m2.42 Using this definition in a retrospective single center study on 266 patients treated with myeloablative allogeneic HSCT, 23% of the patients developed chronic kidney disease, with a cumulative incidence rate at 10 years of 27%.43 This is more than twice as high as seen in a matched general population. Decrease of renal function occurred in about one third of the patients within the first 2 years after HSCT. However, the cumulative incidence of chronic kidney disease continued to increase between 5 and 10 years after HSCT. Most patients with a GFR between 60 and 30 mL/min/1.73m2 were asymptomatic. Severe kidney disease, with GFR below 30 mL/min/1.73m2 was reported in 3% of the patients; half of them had end-stage diseases and needed chronic dialysis.43,44
Risk factors for chronic kidney disease after HSCT are older age at transplantation, female gender, hypertension after HSCT, low pretransplant GFR,43 use of fludarabine and the use of single-dose TBI for conditioning.44 In a cohort study of 1635 patients treated with HSCT, an increased risk of chronic kidney disease was also associated with acute renal failure after HSCT as well as acute and chronic GVHD.45 Cyclosporine and other calcineurin inhibitors have been recognized as an important factor associated with renal dysfunction by causing tubular and vascular changes (Figure 4; see Color Figures, page 496). The most reproducible and reliable diagnostic features are tubular atrophy, interstitial fibrosis, and calcineurin inhibitor arteriolopathy.
Nephrotic syndrome is a rare complication after allogeneic HSCT. It has been described in patients after discontinuation of cyclosporine and in whom remission of the nephropathy was obtained by treatment with steroids and/or reintroduction of cyclosporine.46 After allogeneic HSCT, nephrotic syndrome is now considered as a renal complication of chronic GVHD, usually responding within 12 weeks to treatment with cyclosporine and corticosteroids.47 In nonmyeloablative HSCT, nephrotic syndrome seems to have a higher frequency, with a cumulative incidence at 3 years of 6.1%. In contrast to what is reported in myeloablative HSCT, nephrotic syndrome is not always associated with other symptoms of chronic GVHD. Furthermore, it usually does not improve with re-initiation of immunosuppression, resulting in progressive renal failure necessitating renal dialysis in some of them. Antibody producing residual host B cells surviving after reduced intensity conditioning as well as abrupt withdrawal of post-transplant immunosuppression might predispose to a membranous nephropathy resulting in nephrotic syndrome.48
Radiation-induced renal damage is a process of degeneration and sclerosis of the arterioles and capillaries, with secondary destruction of the glomeruli and tubules associated with interstitial fibrosis. After autologous HSCT, TBI used for conditioning plays an important role in renal dysfunction. This has been demonstrated in a group of children who showed a deterioration of GFR within the first 6 months, with stabilization thereafter for most of them. Chronic kidney dysfunction was observed in 17% of the children, all conditioned with TBI.49 A dose-effect relationship for renal dysfunction could be demonstrated, and fractionation of the radiation dose contributed in reducing chronic renal dysfunction.50
Recommended Screening Practices
General recommendations on screening and preventive practices in long-term HSCT survivors have been published.51 These recommendations are not all based on evidence derived from randomized or other controlled trials, since in most cases preventive practices have not been subjected to such trials. Nevertheless, based on knowledge in analogue non-transplant situations they represent sensible practice for optimizing long-term outcome. However, prospective studies should be performed to determine which test should be done how often and for which patient group (e.g., allogeneic versus autologous; pediatric versus adult) after HSCT. Here, we exclusively focus on pulmonary, cardiovascular and renal recommendations. Pretransplant evaluation should assess baseline condition and enable identification of patients at risk (Table 2 ). A detailed history, including smoking habits, comorbidity, treatment with anthracycline analogues, and radiation therapy, and a full clinical assessment should be obtained. Pulmonary function tests, electrocardiogram, chest X-ray, renal function and cardiovascular risk factors comprise baseline testing for all patients. A more exhaustive evaluation should be performed in patients at risk: elderly patients; patients with cardiopulmonary, vascular or renal comorbidity; and patients who have received anthracyclines, chest radiation or had been exposed to nephrotoxic drugs.
Pulmonary function tests should be done in all patients regularly after allogeneic HSCT, particularly in symptomatic patients. Additional tests assessing the respiratory tract (chest X-ray, HRCT, bronchoscopy with BAL, pulmonary biopsy) should be considered when clinically indicated. Late cardiac failure is usually preceded by asymptomatic left ventricular dysfunction. Electrocardiogram, echocardiography and, if indicated, additional cardiac tests should be done in symptomatic patients and in patients at risk (pretreatment with anthracyclines, chest radiation). Regular screening for established cardiovascular risk factors, in particular including a lipid panel, should be performed for all patients. Renal function, GFR, and urine protein analysis should be performed at yearly intervals in all patients after HSCT. Renal biopsy should be considered in cases with unclear chronic kidney disease after transplantation.
Conclusions
A number of late complications can affect the respiratory tract, the cardiovascular system, and the kidney. All these complications may show a great diversity in respect of frequency, time of appearance, risk factors, and outcome. The underlying origin of the complications is often multifactorial; however, each organ complication has known risk factors (Figure 1 ). Respiratory tract complications are frequent, appear as delayed events, are closely related to GVHD and are of poor prognosis. Cardiac and cardiovascular complications are mainly related to cardiotoxic chemotherapy and TBI, and to the increase of cardiovascular risk factors. They belong to the very late events with a low magnitude of risk during the first decade. However, as has been observed in long-term survivors of Hodgkin lymphoma, it can be anticipated that their incidence will increase significantly with longer follow-up after HSCT. The chronic kidney diseases are usually asymptomatic until end-stage disease, are late events, occurring within the first decade after HSCT, and are mainly related with the use of nephrotoxic drugs such as calcineurin inhibitors. In summary, the diversity in presentation and outcome of the long-term complications greatly influences the screening practice, which should be adapted to the respective time window after HSCT. With longer follow-up time late complications may change character. Therefore, long-term survivors should be assessed lifelong after HSCT.
Clinical manifestations, risk factors and interventions in pulmonary, cardiac, cardiovascular and renal late complications after hematopoietic stem cell transplantation (HSCT).
| Organ . | Clinical manifestation . | Risk factors . | Intervention . |
|---|---|---|---|
| Respiratory tract | Bronchiolitis obliterans Bronchiolitis obliterans organizing pneumonia Restrictive lung disease | Graft-vs-host disease (GVHD) Infection Smoking Radiotherapy Chemotherapy Infection | Treatment of infection GVHD treatment Fractionated radiotherapy Steroids Consider lung transplantation |
| Heart and cardiovascular system | Restrictive or dilated Arrhythmia Autonomic neuropathy | Anthracyclines Mediastinal radiotherapy | Treatment of cardiac insufficiency Pacemaker |
| Cerebrovascular events Cardiovascular events Peripheral vascular events | Cardiovascular risk factors GVHD (?) | Correction of cardiovascular risk factors | |
| Kidney | Nephropathy Nephrotic syndrome | Total body irradiation Chemotherapy (platinum) Nephrotoxic drugs Calcineurin inhibitors GVHD | Control of hypertension Treatment of GVHD Dialysis Consider renal transplantation |
| Organ . | Clinical manifestation . | Risk factors . | Intervention . |
|---|---|---|---|
| Respiratory tract | Bronchiolitis obliterans Bronchiolitis obliterans organizing pneumonia Restrictive lung disease | Graft-vs-host disease (GVHD) Infection Smoking Radiotherapy Chemotherapy Infection | Treatment of infection GVHD treatment Fractionated radiotherapy Steroids Consider lung transplantation |
| Heart and cardiovascular system | Restrictive or dilated Arrhythmia Autonomic neuropathy | Anthracyclines Mediastinal radiotherapy | Treatment of cardiac insufficiency Pacemaker |
| Cerebrovascular events Cardiovascular events Peripheral vascular events | Cardiovascular risk factors GVHD (?) | Correction of cardiovascular risk factors | |
| Kidney | Nephropathy Nephrotic syndrome | Total body irradiation Chemotherapy (platinum) Nephrotoxic drugs Calcineurin inhibitors GVHD | Control of hypertension Treatment of GVHD Dialysis Consider renal transplantation |
Recommended screening practices for pulmonary, cardiac, cardiovascular and renal late complications after hematopoietic stem cell transplantation (HSCT).
| Organ . | Baseline assessment . | Long-term monitoring . |
|---|---|---|
| Respiratory tract | Mandatory in all patients: History of respiratory tract disease Smoking habits Clinical assessment Pulmonary function Chest X-ray | Regular visits (6 mo; annual): History of respiratory track disease and smoking habits since last visit Clinical assessment, inclusively of chronic GvHD in other organs Pulmonary function testing (allo HSCT) |
| In patients at risk: High-resolution CT | If indicated (one or several): Chest X-ray High-resoultion CT Bronchoalveolar lavage Biopsy | |
| Heart and cardiovascular system | Mandatory in all patients: History of cardiac and vascular disease History of cardiovascular risk factors (smoking, familiar risk, hypertension, life style) Exposure to anthracyclines, radiation therapy Clinical assessment Electrocardiogram | Regular visits (annual): History of cardiac and cardiovascular disease since last visit Exposure since last visit (smoking life style) Therapy for cardiovascular risk factors since last visit Clinical assessment Cardiovascular risk factors |
| Patients at risk: Echocardiography 24-hour electrocardiogram Additional functional tests | If indicated (one or several): ECG Echocardiography 24-hour electrocardiogram Radiological and echocardiographic assessment of vascular disease Additional functional tests | |
| Kidney | Mandatory in all patients: History of renal disease Clinical assessment BUN and creatinine GFR Urine protein | Regular visits (6 mo; annual): History of kidney disease and treatment of hypertension since last visit BUN and creatinine GFR Urine protein |
| If indicated: Renal biopsy |
| Organ . | Baseline assessment . | Long-term monitoring . |
|---|---|---|
| Respiratory tract | Mandatory in all patients: History of respiratory tract disease Smoking habits Clinical assessment Pulmonary function Chest X-ray | Regular visits (6 mo; annual): History of respiratory track disease and smoking habits since last visit Clinical assessment, inclusively of chronic GvHD in other organs Pulmonary function testing (allo HSCT) |
| In patients at risk: High-resolution CT | If indicated (one or several): Chest X-ray High-resoultion CT Bronchoalveolar lavage Biopsy | |
| Heart and cardiovascular system | Mandatory in all patients: History of cardiac and vascular disease History of cardiovascular risk factors (smoking, familiar risk, hypertension, life style) Exposure to anthracyclines, radiation therapy Clinical assessment Electrocardiogram | Regular visits (annual): History of cardiac and cardiovascular disease since last visit Exposure since last visit (smoking life style) Therapy for cardiovascular risk factors since last visit Clinical assessment Cardiovascular risk factors |
| Patients at risk: Echocardiography 24-hour electrocardiogram Additional functional tests | If indicated (one or several): ECG Echocardiography 24-hour electrocardiogram Radiological and echocardiographic assessment of vascular disease Additional functional tests | |
| Kidney | Mandatory in all patients: History of renal disease Clinical assessment BUN and creatinine GFR Urine protein | Regular visits (6 mo; annual): History of kidney disease and treatment of hypertension since last visit BUN and creatinine GFR Urine protein |
| If indicated: Renal biopsy |
Sequence of appearance of pulmonary, cardiac, cardiovascular and renal complications after hematopoietic stem cell transplantation (HSCT), and main risk factors. Late complications are subdivided into delayed events (between 3 months and 2 years), late events (between 2 and 10 years), and very late events (> 10 years).
Sequence of appearance of pulmonary, cardiac, cardiovascular and renal complications after hematopoietic stem cell transplantation (HSCT), and main risk factors. Late complications are subdivided into delayed events (between 3 months and 2 years), late events (between 2 and 10 years), and very late events (> 10 years).
The figure shows the cumulative incidence of an arterial vascular event at 15 years adjusted for age. By using an adjusted Cox model, the relative risk is significantly higher after allogeneic than after autologous hematopoietic stem cell transplantation (HSCT). Reprinted with permission from Tichelli A et al. Blood. 2007;110:3463–3471.
The figure shows the cumulative incidence of an arterial vascular event at 15 years adjusted for age. By using an adjusted Cox model, the relative risk is significantly higher after allogeneic than after autologous hematopoietic stem cell transplantation (HSCT). Reprinted with permission from Tichelli A et al. Blood. 2007;110:3463–3471.
Disclosures Conflict-of-interest disclosure: A.T. and A.R. declare no competing financial interests. A.G. has been a consult for Novartis, Roche, Amgen, Bristol-Myers Squibb, Polyphor, and Pfizer, and has received research funding from them. Off-label drug use: None disclosed.
Acknowledgments
We thank Prof. Stephan Dirnhofer for the histology figures of the patients.
Reference List
Author notes
Center for Stem Cell Transplantation, University Hospital Basel, Basel, Switzerland