Abstract
Primary mediastinal B-cell lymphoma is a discrete clinicopathologic entity. Molecular analysis reveals it to be distinct from other types of large B-cell lymphoma, and retrospective analysis suggests that it may respond better to multi-agent chemotherapy regimens than to the more commonly used CHOP. The addition of rituximab may mitigate such differences, and may also diminish the role of consolidation radiotherapy, which is often used to treat residual mediastinal masses. For the future the role of FDG-PET scanning requires prospective examination, and it is hoped that this may allow the de-escalation of treatment if it can be shown to yield reliable prognostic information. The relative rarity of this type of lymphoma necessitates international collaboration in clinical trials, with a prospective clinicopathologic study, IELSG 26, already underway.
First recognized in the 1980s, primary mediastinal (thymic) large B-cell lymphoma (PMBL) was formally established as a distinct subtype of diffuse large B-cell lymphoma (DLBCL) in the revised European and American classification of lymphoid neoplasms and more recently the World Health Organization classification.1,2 It represents less than 3% of all non-Hodgkin lymphoma cases, but is over-represented in the young adult population.3 An increasing body of knowledge regarding the clinical features, pathology, genetics and transcriptional profile of this disease now distinguishes it from other types of DLBCL.
Understanding more about the underlying pathobiology may help to identify new treatment targets in future, but even now there are many questions about the optimal management.
Clinical Features
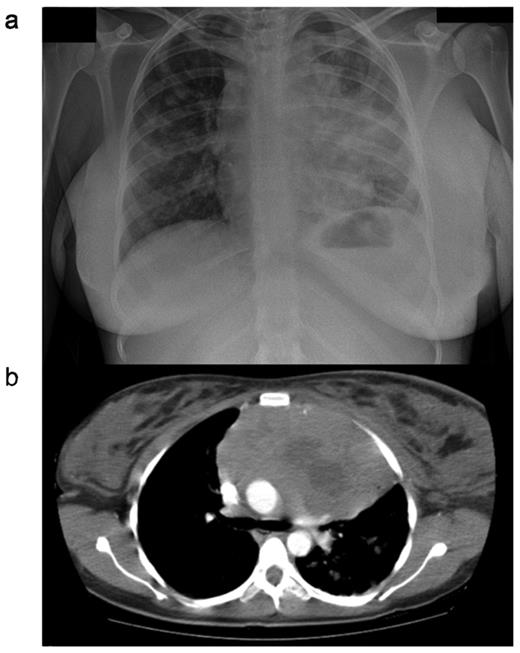
PMBL normally presents with a bulky tumor in the anterior mediastinum that is rapidly progressive and may give rise to local compressive effects including dyspnea, cough, dysphagia and superior vena caval obstruction4 (Figure 1 ). The disease affects females more frequently than men, and peaks in incidence in the third or fourth decades. This is in contrast to the more elderly population affected by DLBCL.5 Tumor extension is local, invading lungs, chest wall, pleura and pericardium, often resulting in effusions (Table 1 ). Breast edema is common and hoarseness may reflect recurrent laryngeal nerve damage. Because the local effects are seen early, most cases present when the disease is limited to within the thorax. Extranodal sites, however, may be involved, particularly at the time of disease recurrence, with a propensity for involvement of the kidneys, adrenals, liver, ovaries and central nervous system. Bone marrow infiltration at presentation is rare.
Pathology
PMBL shows a diffuse growth pattern, comprising large cells with polymorphic nuclei that have an abundant rim of clear cytoplasm. Fibrosis commonly results in compartmentalization of the neoplastic cells.1,2,6–8 Immunophenotyping demonstrates the presence of B-cell antigens in all cases (CD19, CD20, CD22 and CD79a). Bcl-2 is expressed in 80% of cases, CD10 is infrequently expressed and CD21 is negative. Surface immunoglobulin (sIg) expression is absent, and HLA class I and II molecules are expressed at low levels or not at all. The mechanism of such loss has been demonstrated not to be a function of gene deletion given that at the transcription level mRNA expression is present.9 CD30 staining is common, but weak, making the distinction from nodular sclerosing Hodgkin lymphoma (HL) difficult at times. In contrast to HL, the transcription factors PAX5, BOB.1, Oct-2 and PU.1 are always expressed and CD15 is negative.
PMBL is considered to arise from a small population of B-cells within the thymus,10,11 and indeed thymic components such as Hassall’s corpuscles may be identified in the pathology specimen. Thymic B-cells and PMBL both show a pattern of somatic hypermutation of both IGH and the 5′ non-coding region of BCL6, consistent with transition through the germinal center. Either these cells subsequently migrate to the thymus, or hypermutation may be occurring within the thymus itself outside the classical germinal center.12,13 Of note, expression of sIg is lost despite the presence of isotype-switched Ig genes.14
Genomic Aberrations
With conventional comparative genomic hybridization (CGH) techniques and PCR fingerprinting, regions of genomic gain are identified more frequently than regions of genomic loss in PMBL. The most commonly observed gains are at +9p, +12q and +Xq.15–17 Using array based CGH, where the resolution is greater, more regions of loss have been demonstrated, but these are generally small in size. The median size of regions of loss is 6 Mb, compared to genomic gains which are a median size of 29.5 Mb.18 Common regions of loss are at 1p, 3p, 13q, 15q and 17p.18,19 These patterns of genomic changes in PMBL are distinct from those observed in other subgroups of DLBCL that have been defined by transcription profiling.20
Molecular Aberrations in PMBL
Attempts to characterize the molecular mechanisms underlying PMBL have demonstrated a relatively low frequency of commonly conserved oncogenic events.21,22 Missense mutations in TP53 have been identified in < 20% cases. c-MYC rearrangements and point mutations within the non-coding region occur at a similar frequency, and inactivation of CDKN2A has been detected by homozygous deletion, mutation and altered methylation in 15% of cases. Translocations of BCL2 have not been documented, nor have RAS mutations.21–23 Translocations involving the transcriptional repressor BCL6 occur in up to 33% of cases of PMBL, although they appear to have little effect upon mRNA expression and no clear repression of target genes in the cases that harbor a translocation.24
A number of other aberrations common to PMBL have, however, been identified. The membrane-bound protein MAL, associated with later stages of intra-thymic T-cell differentiation, is expressed in 70% of PMBL cases but in only 3% of non-mediastinal DLBCLs.12,25,26 Of note, some cases of classical HL (10% to 20%) may also demonstrate MAL expression.26,27 Among normal B cells, MAL expression is largely restricted to a sub-population of thymic medullary B cells, from which PMBL is believed to arise. No mutations have been identified in MAL, and the mechanism of MAL protein overexpression in PMBL is unclear.
A further molecular marker that may distinguish PMBL is the interleukin 4 inducible gene, FIG1.28 The transcript of this gene encodes a signal peptide, expressed at low levels in normal lymphoid tissue. In PMBL expression is high, while it is present mostly at a low level in non-mediastinal DLBCL. The presence of high FIG1 expression points towards constitutive activation of cytokine signalling pathways in pathogenesis of this disease.
NF-κ B Activation in PMBL
Frequent gains of 2p14-16 have been documented in PMBL, implicating the REL protooncogene, encoding a member of the NF-κ B family, in disease pathogenesis.16–20 By florescence in situ hybridization (FISH) analysis, genomic gains have been identified in 75% of cases at this loci.19,29 There is good evidence for constitutive activation of NF-κ B pathway in PMBL. PMBL cell line survival is critically dependent upon NF-κ B, and the target gene profile of NF-κ B in PMBL is clearly directed towards genes promoting cell survival and inhibiting apoptosis.30 Inactivating mutations in the gene encoding the NF-κ B inhibitory protein Iκ B (gene; IKBA) have been postulated as a potential mechanism for activation of the pathway; however, no mutations in this gene have yet been detected in PMBL.31
BCL11A encodes a zinc finger transcriptional repressor critical to lymphoid development; it also been implicated as the oncogene driving selection of the 2p amplification in PMBL. In PMBL, BCL11A is present at increased copy number in 75% of cases, although this increase correlates to changes in transcript and protein levels in only a minority of cases.32 High nuclear protein expression is, however, seen in 88% of cases of PMBL, suggesting that a BCL11A-related program of transcriptional repression may be provide a survival advantage to the malignant B cell.
The JAK/STAT Pathway in PMBL
Frequent gains of 9p are recognized by CGH in PMBL, with a common region of high level amplification localized to the distal part of band 9p23 and 9p24.17 By using FISH gains at this location are observed in 75% of PMBL.16 In classical HL, 9p gains at an identical consensus region are also recognized. This common region spans the JAK2 locus.33 Recent gene expression studies have documented over expression of JAK2 in PMBL and have suggested constitutive activation of the IL-4 and IL-13 pathways as a result.23,34 STAT6, the transcription factor that is primarily regulated by IL-4 and IL-13 is constitutively activated in PMBL.35 Phosphorylated STAT6 was detected in 8 of 11 cases of PMBL, but in only 1 of 10 cases of non-mediastinal DLBCL. However, despite JAK2 amplification and an increase in JAK2 mRNA, only minimal changes in JAK2 protein quantity in PMBL cell lines has been documented.36 However, a clear prolongation of the JAK2 protein half life was observed, resulting in delayed protein turnover. This suggests that upregulation of this pathway is not simply a gene dosage effect but that more complex post-transcriptional factors are involved. Activating mutations in JAK2 might account for pathway activity; however, they have not been detected in PMBL or HL37 and furthermore, recent high resolution genomic studies have suggested that JAK2 is not the target gene in the 9p amplicon as it lies outside the common region of amplification in PMBL.18
The kinase activity of JAK protein is negatively regulated by Src-homology 2 domain containing suppressor of cytokine signalling (SOCS) proteins, which target their substrate for proteosomal degradation. Biallelic deletion mutations of SOCS-1 have been detected in PMBL cell lines, with ectopic expression of wild-type SOCS-1 resulting in a reduction of phosphorylated JAK-2 and growth arrest.36 Mutations in SOCS-1 have been detected in 9 out of 20 primary tissue cases of PMBL examined, although of note the presence of a mutation in SOCS-1 was not mutually exclusive to gain of 9p. Subsequently, a further mechanism of SOCS-1 inactivation through larger biallelic deletions has been documented.18,38 The deletion site may however also include another potential tumour suppressor, PIG7/LITAF, which has a role in the regulation of apoptosis.39 In common with the idea that PMBL and HL share many common features (see below), SOCS-1 deletion mutations have also been detected in HL.40
Transcriptional Profiling
Large-scale analyses of gene expression have shed new light on the biology of PMBL. Rosenwald et al generated a 46-gene signature for PMBL, which included MAL and FIG1, that was clearly able to distinguish PMBL from other types of DLBCL.34 Consistent with clinical observation, cases with a PMBL molecular signature were from younger patients (median age 33) compared to cases classified according to the previously described ABC and GCB subgroups of DLBCL (median ages of 66 and 61 years, respectively). The overall survival (OS) of cases classified as PMBL on molecular analysis was favorable at 64% at 5 years, compared to 46% for the DLBCL group as a whole. JAK2 and other genes located at 9p24 (PDL1, PDL2 and SMARCA2) were all expressed at high levels in the PMBL cases, with PDL2 (a regulator of T-cell response) being the best single discriminator gene between PMBL and DLBCL. Similarly using an alternative gene expression platform, Savage et al were able to identify 100 genes, again including MAL and FIG1, which reliably distinguished PMBL from other DLBCL.23 In both series remarkable similarities in the transcription profile of PMBL and that of HL were identified. Despite this, clear differences in the expression profiles were also apparent, notably the presence of mature B-cell genes in PMBL that are absent from HL.
Prognostic Factors
The utility of the International Prognostic Index (IPI)41 in PMBL is limited by the age distribution of the disease and its usual confinement to the mediastinum. This reflected in the observation that 50% of patients have low IPI scores at presentation.42 The age-adjusted IPI has generally been reported to be of limited predictive value,43–45 although in a recent series of 92 patients there was some clear discriminative value.46 Elevation of LDH to greater than twice the upper limit of normal, age over 40 and performance status ≥ 2 all correlated with reduced survival in a population-based series from British Columbia,43 while in a large series from the International Extranodal Lymphoma Study Group (IELSG) male sex, poor performance status and advanced-stage disease were significant negative predictors of survival.47
Primary Management
Initial therapy is critical in treating PMBL. Salvage therapy for recurrence or progressive disease is of limited efficacy;43,44 thus, the imperative is to cure at the first attempt where possible. There is, however, a tension between delivery of the highest possible cure fraction and minimizing long-term morbidity in this young population. The issues to consider include choice of initial chemotherapy/immunochemotherapy, and any potential benefit from high-dose therapy in first remission. Similarly, the role of consolidation radiotherapy to the chest must be considered, as should the potential of functional imaging to guide treatment choices.
Is a third generation chemotherapy regimen needed for PMBL?
In the treatment of conventional DLBCL no advantage has been demonstrated for the use of third-generation anthracycline-containing regimens over conventional CHOP.48 It is possible that this analysis failed to identify subgroups that might benefit from the more intensive approach: in the case of PMBL there is some evidence to suggest that superior outcomes may be achieved with latter generation regimens. Such conclusions are, however, based upon retrospective analyses.
Early experience in PMBL suggested poor results with CHOP chemotherapy alone.45,49–52 Some centers were, however, able to document improved responses in small numbers of patients when treated with more intensive regimens. Lazzarino et al reported a series of 29 assessable patients. Five of 14 patients (36%) treated with CHOP entered complete remission (CR) compared with 11 of 15 (73%) following the third-generation regimen MACOP-B.53 Similarly, no CRs were documented following CHOP or CHOP-bleomycin compared to 10 of 12 following MACOP-B with or without radiation in a series from Verona.50 Perhaps some of the most impressive results in this disease have come from multicenter non-comparator studies of MACOP-B followed by involved-field RT.54,55 In the largest of the two studies, recruiting 89 patients, the CR rate with this approach was 86% with OS and progression-free survival (PFS) rates of 86% and 91%, respectively, at 9 years54 (Table 2 ).
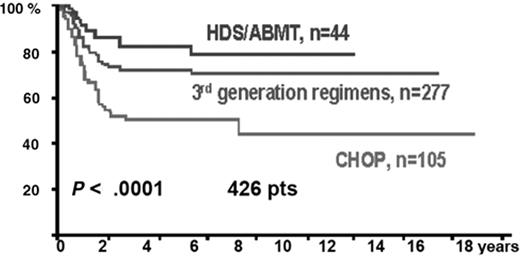
The IELSG retrospectively reviewed the outcomes of 426 previously untreated patients with PMBL.47 The majority of patients who were treated with a third-generation regimen received MACOP-B (n = 204), the rest received either VACOP-B (n = 34) or ProMACE CytaBOM (n = 39). The CR rate following induction chemotherapy was similar between the third-generation subgroup and those treated with conventional CHOP or CHOP-B at 51% and 49%, respectively, improving to 79% and 61%, respectively, following involved field radiation for patients in partial remission (PR). However, the relapse rate at 3 years was significantly lower in the third-generation group compared to those treated with CHOP (12% vs 23%; P = .02) and the projected 10-year OS and PFS were superior at 71% and 67%, compared with 44% and 33% (P = .0001 and P = .0003, respectively) (Figure 2 ). A similar comparison of 138 patients from 13 Italian institutions favored MACOP-B or VACOP-B over CHOP.44 Eighty percent of the former patients entered a CR compared to 51% with CHOP (P < .001); significantly fewer patients failed to demonstrate a response or progressed on therapy (9% vs 42%; P < .01). Both event-free and OS were superior in the MACOP-B/VACOP-B group.
A population-based retrospective analysis of 153 patients from British Columbia reviewed outcomes from a geographical region where treatment choice was mandated by era-specific guidelines.43 Between 1980 and 1992 MACOP-B or VACOP-B was administered, moving to CHOP between 1992 and 2001 and then to rituximab with CHOP (R-CHOP) thereafter. The OS for the cohort was 75% at 5 years, with the OS at 5 years for those treated with MACOP-B/VACOP-B significantly higher at 87% compared with 71% for those patients treated with CHOP (P = .048). Comparison of baseline characteristics, however, demonstrated a greater number of poor-risk patients in the CHOP group. In the multivariate analysis for OS the type of chemotherapy regimen showed a trend towards improved outcomes, but this was not statistically significant.
What has been the impact of rituximab?
There is little clear evidence to quantify the impact of the addition of rituximab to chemotherapy for PMBL, although based upon the results extrapolated from DLBCL56–58 it is now widely adopted. In the population-based analysis of PBML from British Columbia in the pre- and post-rituximab era, there was no apparent survival advantage from the addition of rituximab to CHOP, or similarly when R-CHOP was compared to MACOP-B/VACOP-B.43 At the time of reporting; however, the number of patients treated with immunochemotherapy was small and follow-up limited. Addition of rituximab to dose-adjusted EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide and prednisone) in small numbers of patients with PMBL has been associated with a favorable event-free (P = .036) and OS (P = .023).59 In a small series from Israel, the addition of rituximab appeared to improve PFS, particularly in those patients receiving CHOP, while there was no difference in outcomes in a comparison between either a third-generation regimen with rituximab or CHOP with rituximab (R-VACOP-B vs R-CHOP, 84% and 74%, respectively; P = .44).60
Does high-dose therapy have a role in consolidating first remission?
The low frequency of marrow involvement and the relatively young age of the PMBL patient population has led to consideration of high-dose chemotherapy and peripheral blood progenitor rescue to consolidate first remission. Results from the GEL-TAMO registry have recently been reported.61 Thirty-five patients in first CR, but considered at “high-risk” of relapse, underwent high-dose therapy with various preparative regimens. At 4 years, the overall and PFS were 84% and 81%, respectively, similar to a small series of 12 patients (8 in CR and 4 in PR) reported by Sehn et al.62 Sixty percent of patients also received irradiation either before or after high-dose therapy, and this was the only variable associated with OS in univariate analysis. In the IELSG analysis, a limited number of patients (n = 44) underwent high-dose therapy, which resulted in an estimated OS of 77% at 10 years.47 An early intensification program, cumulating in high-dose therapy in poor-risk patients with PMBL, resulted in 7 of 15 patients achieving CR and a further 7 patients with only minor residual mediastinal changes, with a disease-free survival of 93% reported at a median follow-up of 3 years.63 In the Memorial Sloan Kettering experience high-dose therapy with progenitor cell rescue at first remission was not superior to dose-dense sequential therapy.45
Based upon the results achieved with third-generation regimens and the likely benefit from the addition of rituximab, there is little at present to commend a high-dose therapy approach to consolidate first CR, even in poor-risk patients.
What is the role of consolidation radiotherapy?
The role of mediastinal radiotherapy upon completion of chemotherapy remains unclear. The best reported outcomes in PMBL have been achieved with regimens that have incorporated radiotherapy in their planned primary treatment.44,46,54,55,64,65 Furthermore, it is clear from the IELSG series that many patients completing chemotherapy in PR may be converted to CR following radiotherapy47 and that radiotherapy may render active residual mediastinal masses gallium negative.55 Mazzarotto et al report that following induction chemotherapy 42% of patients were in CR, rising to 95% following radiotherapy.64 Univariate and multivariate analysis in two retrospective series have suggested that receiving radiotherapy correlated to better event-free or OS.44,61
However, excellent long-term results have been achieved with chemotherapy alone in some series.45,59,66 Following an era-specific shift in British Columbia towards the use of radiotherapy to consolidate response, there was no difference in PFS or OS by year.43 This observation held even with initially bulky tumors, and indeed in the whole population there was a trend towards improved PFS in the era before routine radiotherapy. From Memorial Sloan Kettering series, only 7% of patients treated with the NHL-15 regimen (comprising intensified doxoroubicin, vincristine and cyclophosphamide) received radiotherapy. Excellent results with OS of 84% at a median follow-up of 10.9 years are reported with this chemotherapy-only approach.45 Similarly the excellent results that have been reported with dose-adjusted EPOCH in combination with rituximab are purported to negate the need for radiation in this disease.59
Given concerns about the long-term toxicity of radiation, a randomized study is needed to address its role, especially now that rituximab is incorporated into therapy for PMBL. The small number of patients and the high rate of cure will, however, require an international collaboration to deliver an equivalence study of convincing power. It may be that response-adapted approaches could offer an alternative.
How should a residual mass be evaluated following therapy?
Owing to the prominent fibrotic component of PMBL, a residual mediastinal mass is often present upon completion of therapy. Distinction is needed between those that have residual disease and those that have simple fibrotic tissue. The 67 Gallium scan has demonstrated utility in the setting of PMBL and has identified patients that are likely to relapse;55,67 however, it is time consuming to perform and spatial resolution is low. Increasingly, the FDG-PET scan has become the tool of choice in such situations. In a series of patients with both HL and NHL and residual mediastinal masses on computed tomography, relapses occurred in all patients with a positive PET scan and only 26% of those with a negative PET.68 Zinzani et al also reported a series of both HL and NHL; from 16 patients with a positive PET at completion of therapy, 14 relapsed within 1 year.69 A systemic review of PET studies has examined post-therapy response assessment in lymphoma.70 In the studies reporting evaluation of residual masses in aggressive NHL, the demonstrated sensitivity of PET ranged from 33% to 87% and the specificity from 75% to 100%. These data clearly indicate that further evaluation is required before modifying planned therapy based upon FDG-PET evaluation alone in PMBL, and the false-positive rate in particular requires definition. A prospective international study is in progress to evaluate this: http://clinicaltrials.gov/ct2/show/NCT00689845.
Is there a role for high-dose therapy in patients with inadequate response or refractory to induction therapy?
As new response criteria incorporating PET imaging become more widely adopted,71 what constitutes a CR or an induction failure in PMBL may become more consistently reported. In previous series based upon cross-sectional imaging, the definition of CR has included residual mediastinal masses that have reduced in size post therapy by varying amounts, making direct comparison and definition of induction failures difficult. By using PET imaging in response assessment it might be that larger mediastinal masses, previously considered as PR might be documented CRs. In many series radiotherapy has been guided by the results of imaging. The GEL-TAMO registry group reported on the results of high-dose chemotherapy in 36 patients who had “not responded,” defined either by the best response being a PR (n = 23) or being completely refractory to induction therapy (n = 13).61 In this population following high-dose therapy OS was 49% and PFS 42%, respectively, at 4 years; however, those undergoing high-dose chemotherapy in PR fared significantly better, with an OS of 64% at 4 years compared with only 23% in those with refractory disease, and PFS of 56% and 16%, respectively. Patients whose lymphomas progress during primary therapy have a poor outlook: of 14 patients in the British Columbia series, the majority were resistant to alternative chemotherapy regimens and there were no long-term survivors.43 Sehn et al, however, reported on 12 patients with refractory disease who at 5 years had a PFS of 58% following high-dose chemotherapy. It seems appropriate in this setting to test conventional chemosensitivity prior to high-dose chemotherapy, but to proceed in those fit enough regardless of outcome and consolidate the response with involved-field radiotherapy.
Do Patients with PMBL Require Central Nervous System Prophylaxis?
Patients with PMBL frequently present with an elevated LDH, a recognized risk factor for central nervous system (CNS) disease.72,73 Bone marrow involvement is infrequent but involvement of extranodal sites, such as the kidneys and adrenals, which are also considered as high-risk sites for CNS involvement, are a feature of recurrent PMBL.73 At presentation, CNS disease is uncommon;74 however, at the time of first relapse, the incidence has been reported to be as high as 23%.75 In this analysis, parenchymal brain lesions predominated, but free lymphoma cells were not detected on cerebrospinal fluid examination despite the presence of leptomeningeal involvement established by imaging. Bishop et al recommend CSF examination and careful neuroimaging in the presence of multiple extranodal sites or one nodal site when the LDH is elevated.75 It seems appropriate that these at-risk patients are identified and should receive intrathecal prophylaxis, although the evidence base is lacking.
Treatment at the Time of Recurrence
The rate of recurrence appears to be lower in PMBL than DLBCL, perhaps a feature of disease biology or owing to the differences in age and stage. The majority of events occur within the first 12 months, and are rare beyond 2 years from completion of therapy,43,44,47,76 and every effort should be made to confirm histology at the time of recurrence and not to rely on functional imaging alone. Salvage therapy of PMBL has followed that of DLBCL, attempting reinduction with non-crossresistant agents, followed by consolidation with high-dose chemotherapy in those deemed fit enough. The outcomes have been disappointing, however. In the series of Todeschini et al, from a series of 138 patients, all of whom relapsed and died of their lymphoma.44 Similar outcomes have been reported by other investigators.54 By contrast, Sehn et al reported on 11 patients with recurrent disease after an initial response to chemotherapy; 27% remained alive and progression free at 5 years following high-dose chemotherapy, with outcomes that were identical between those with chemotherapy-sensitive and chemotherapy-resistant relapse.62 From the British Columbia series 40% of patients fit enough to undergo high-dose chemotherapy remained alive at 5 years. Among 30 patients from the MD Anderson Cancer Center who showed chemosensitivity at the time of relapse, OS of 72% at a median follow-up of 8½ years is reported.77
Conclusions
In an uncommon disease it is essential that international cooperative trials be designed to answer the outstanding questions regarding management (Table 3 ). There are many barriers to such trials, not the least of which are selection of chemotherapy regimens, recruitment of adequate patient numbers to provide properly powered results, and concerns about PET guided decision making. Currently, the IELSG 26 study in PMBL aims to perform detailed immunophenotypic and molecular characterization and also to establish the PET response rate following initial treatment with a permissive range of rituximab-chemotherapy regimens. Consolidation radiotherapy is administered at the Investigator’s discretion. This will enable some prospective comparison of response rates, both to immunochemotherapy and radiotherapy, which may facilitate the future design of randomized trials.
Presenting clinical characteristic of patients with primary mediastinal large B-cell lymphoma (PMBL) from the British Columbia series. From Savage et al. Ann Oncology. 2006;17:123–130.43
| Clinical characteristic . | Percentage (n = 153) . |
|---|---|
| Median age | 37 |
| Stage | |
| I/II | 74 |
| III/IV | 26 |
| Elevated LDH | 77 |
| Bulk (>10 cm) | 75 |
| B symptoms | 47 |
| Age-adjusted IPI | |
| 0 | 12 |
| 1 | 49 |
| 2 | 27 |
| 3 | 12 |
| Pleural or pericardial effusion | 50 |
| Clinical characteristic . | Percentage (n = 153) . |
|---|---|
| Median age | 37 |
| Stage | |
| I/II | 74 |
| III/IV | 26 |
| Elevated LDH | 77 |
| Bulk (>10 cm) | 75 |
| B symptoms | 47 |
| Age-adjusted IPI | |
| 0 | 12 |
| 1 | 49 |
| 2 | 27 |
| 3 | 12 |
| Pleural or pericardial effusion | 50 |
Studies in primary mediastinal large B-cell lymphoma (PMBL) employing third-generation chemotherapy regimens.
| Reference . | Setting . | n . | Regimen . | RT . | IPI Low/High (%) . | CR/CR(u) . | PR . | Overall survival . | Progression- free survival . |
|---|---|---|---|---|---|---|---|---|---|
| * CR defined by normal mediastinal contour by CXR, with or without residual minor CT abnormalities | |||||||||
| ** For CR all masses had to have disappeared for 3 months. All other responses were PR | |||||||||
| † CR defined as complete regression of assessable disease of ≥ 80% response of residual mass | |||||||||
| ‡ Near CR is included, defined as ≥ 90% reduction in size of mass | |||||||||
| § CR(u) persistence of mass with having demonstrated ≥ 80% reduction | |||||||||
| Abbreviations: MACOP, methotrexate, leucovorin, adriamycin, cyclophosphamide, vincristine, prednisolone; MACOP-B, MACOP plus bleomycin; VACOP, etoposide, adriamycin, cyclophosphamide, vincristine, prednisolone; DA-EPOCH, dose-adjusted etoposide, vincristine, doxorubicin cyclophosphamide, prednisone; R, rituximab; ProMace, methotrexate, doxorubicin, cyclophosphamide, etoposide; MOPP, mechlorethamine, vincristine, procarbazine, prednisone; ProMace-CytaBOM, prednisone, doxorubicin, cyclophosphamide, etoposide, cytarabine, bleomycin, vincristine, methotrexate, leucovorin; NHL-15, doxorubicin, vincristine, cyclophosphamide, granulocyte colony-stimulating factor. | |||||||||
| RT, radiotherapy; I-CT, induction chemotherapy; RFS, relapse-free survival; EFS, event-free sur vival; FU, follow-up; y, year; CR, complete remission; PR, partial remission | |||||||||
| Todeschini 1990 | Single center | 12 | MACOP-B (12 pts from series of 21) | Yes (67%) | — | 83% | 17% | — | — |
| Bertini 1991 | All stage II | 18 | MACOP-B | Yes (39%) | — | 16/27 | 1/18 | 77% at 3 y | 83% at 3 y |
| Lazzarino 1993 | Single center | 15 | MACOP-B/VACOP-B | Yes | — | 11/15* | 4/15 | — | — |
| Martelli 1998 | Single center (0 post I-CT) | 27 | MACOP-B | Yes | — | 3/27** | 23/27 | 93% at 2.2 y | 91% at 2 y |
| Zinznani 1999 | 2 centers | 50 | MACOP-B | Yes | — | 86% | — | 82% at 8 y | 93% at 3¼ y (RFS) |
| Zinzani 2001 | 8 centers | 89 | MACOP-B | Yes 26% post I-CT | — | 88%† | 4% | 86% at 9 y | 91% at 9 y (RFS) |
| Zinzani 2002 | IELSG mulitcenter retrospective | 277 | MACOP-B/VACOP-B ProMACE CytaBOM | Yes (51% post I-CT) | 82/18 | 79% | 9% | 71% at 10 y | 67% at 10 y |
| Todeschini 2004 | 13 centers | 138 | MACOP/VACOP-B | Yes | 67/33 | 80%‡ | 10% | — | 76% at 5 y (EFS) |
| Hamblin 2005 | Single institution | 68 | NHL-15 | No | 75/25 | 88% | 3% | 84% at median FU 10.9 y | 60% at median FU 10.9 y (EFS) |
| Savage 2006 | Population based | 47 | MACOP-B/VACOP-B | Dependent upon era | — | — | — | 87% at 5 y | — |
| Dunleavy 2006 | Single institution | 44 | DA-EPOCH (n = 18) R + DA-EPOCH (n = 26) | No | — | — | — | 78% at 9.5 y FU 100% at 4.2 y FU | 67% at 9.5 y (EFS) 91% at 4.2 y (EFS) |
| Mazzarotto 2007 | Single institution | 53 | ProMACE-MOPP/MACOP-B/VACOP-B | Yes (81%) | 87/13 | 86% 38% post I-CT | — | 87% at 5 y | 93% at 5 y |
| De Sanctis 2008 | Single institution | 92 | MACOP-B | Yes | 57/43 | 87%§ | 9% | 87% at 5 y | 81% at 5 y |
| Reference . | Setting . | n . | Regimen . | RT . | IPI Low/High (%) . | CR/CR(u) . | PR . | Overall survival . | Progression- free survival . |
|---|---|---|---|---|---|---|---|---|---|
| * CR defined by normal mediastinal contour by CXR, with or without residual minor CT abnormalities | |||||||||
| ** For CR all masses had to have disappeared for 3 months. All other responses were PR | |||||||||
| † CR defined as complete regression of assessable disease of ≥ 80% response of residual mass | |||||||||
| ‡ Near CR is included, defined as ≥ 90% reduction in size of mass | |||||||||
| § CR(u) persistence of mass with having demonstrated ≥ 80% reduction | |||||||||
| Abbreviations: MACOP, methotrexate, leucovorin, adriamycin, cyclophosphamide, vincristine, prednisolone; MACOP-B, MACOP plus bleomycin; VACOP, etoposide, adriamycin, cyclophosphamide, vincristine, prednisolone; DA-EPOCH, dose-adjusted etoposide, vincristine, doxorubicin cyclophosphamide, prednisone; R, rituximab; ProMace, methotrexate, doxorubicin, cyclophosphamide, etoposide; MOPP, mechlorethamine, vincristine, procarbazine, prednisone; ProMace-CytaBOM, prednisone, doxorubicin, cyclophosphamide, etoposide, cytarabine, bleomycin, vincristine, methotrexate, leucovorin; NHL-15, doxorubicin, vincristine, cyclophosphamide, granulocyte colony-stimulating factor. | |||||||||
| RT, radiotherapy; I-CT, induction chemotherapy; RFS, relapse-free survival; EFS, event-free sur vival; FU, follow-up; y, year; CR, complete remission; PR, partial remission | |||||||||
| Todeschini 1990 | Single center | 12 | MACOP-B (12 pts from series of 21) | Yes (67%) | — | 83% | 17% | — | — |
| Bertini 1991 | All stage II | 18 | MACOP-B | Yes (39%) | — | 16/27 | 1/18 | 77% at 3 y | 83% at 3 y |
| Lazzarino 1993 | Single center | 15 | MACOP-B/VACOP-B | Yes | — | 11/15* | 4/15 | — | — |
| Martelli 1998 | Single center (0 post I-CT) | 27 | MACOP-B | Yes | — | 3/27** | 23/27 | 93% at 2.2 y | 91% at 2 y |
| Zinznani 1999 | 2 centers | 50 | MACOP-B | Yes | — | 86% | — | 82% at 8 y | 93% at 3¼ y (RFS) |
| Zinzani 2001 | 8 centers | 89 | MACOP-B | Yes 26% post I-CT | — | 88%† | 4% | 86% at 9 y | 91% at 9 y (RFS) |
| Zinzani 2002 | IELSG mulitcenter retrospective | 277 | MACOP-B/VACOP-B ProMACE CytaBOM | Yes (51% post I-CT) | 82/18 | 79% | 9% | 71% at 10 y | 67% at 10 y |
| Todeschini 2004 | 13 centers | 138 | MACOP/VACOP-B | Yes | 67/33 | 80%‡ | 10% | — | 76% at 5 y (EFS) |
| Hamblin 2005 | Single institution | 68 | NHL-15 | No | 75/25 | 88% | 3% | 84% at median FU 10.9 y | 60% at median FU 10.9 y (EFS) |
| Savage 2006 | Population based | 47 | MACOP-B/VACOP-B | Dependent upon era | — | — | — | 87% at 5 y | — |
| Dunleavy 2006 | Single institution | 44 | DA-EPOCH (n = 18) R + DA-EPOCH (n = 26) | No | — | — | — | 78% at 9.5 y FU 100% at 4.2 y FU | 67% at 9.5 y (EFS) 91% at 4.2 y (EFS) |
| Mazzarotto 2007 | Single institution | 53 | ProMACE-MOPP/MACOP-B/VACOP-B | Yes (81%) | 87/13 | 86% 38% post I-CT | — | 87% at 5 y | 93% at 5 y |
| De Sanctis 2008 | Single institution | 92 | MACOP-B | Yes | 57/43 | 87%§ | 9% | 87% at 5 y | 81% at 5 y |
Important clinical research questions in primary mediastinal large B-cell lymphoma (PMBL).
|
|
CXR (a) and CT (b) scan from a female patient presenting with primary mediastinal large B-cell lymphoma (PMBL). Note is the large anterior mediastinal mass, with areas suggestive of central necrosis. Marked breast edema is present.
CXR (a) and CT (b) scan from a female patient presenting with primary mediastinal large B-cell lymphoma (PMBL). Note is the large anterior mediastinal mass, with areas suggestive of central necrosis. Marked breast edema is present.
Overall survival by chemotherapy subtype in the IELSG study of 426 patients with primary mediastinal large B-cell lymphoma (PMBL). Reprinted with permission from
Overall survival by chemotherapy subtype in the IELSG study of 426 patients with primary mediastinal large B-cell lymphoma (PMBL). Reprinted with permission from
Disclosures Conflict-of-interest disclosure: P.W.M.J. declares no competing financial interests. A.J.D. declares no competing financial interests. Off-label drug use: None disclosed.
References
Author notes
Cancer Research UK Clinical Centre; Cancer Sciences Division, School of Medicine, University of Southampton; Southampton General Hospital, Southampton, United Kingdom