Abstract
Survival of children with acute lymphoblastic leukemia (ALL) is often described as the success story for oncology. The improvements in the treatment of ALL represent the work of cooperative groups at their best. Fifty years ago a pediatric oncologist would have never considered using the term “cure” in a discussion with a family whose child was diagnosed with ALL. Today the term is not only used in the initial discussion but referred to frequently thereafter. However, as we all know, cure is not assured and is not obtained without sequelae. This review will focus on the improvements in treatment for newly diagnosed ALL in children and adolescents according to risk group and some of the challenges that remain despite the improved outcome.
Survival
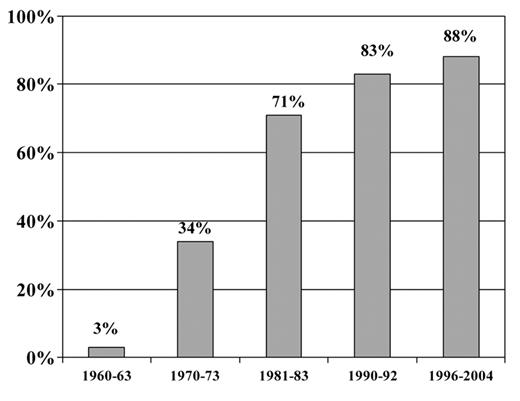
Improvements in the 5-year survival rate for ALL in children continue to be seen. In the l996–2004 SEER data the 5-year survival for patients with ALL was 84% for children and young adults less than 19 years of age and 88% for children and teens less than 15 years of age. This is in comparison to 3% reported in the 1960’s.1 Multiple factors have contributed to this improvement, including a better understanding of the immunobiology of ALL and disease burden, recognition of sanctuary sites and integration of presymptomatic central nervous system (CNS) prophylaxis, use of effective drugs and intensification of treatment, delineation of prognostic factors with risk-adapted treatment and improvements in supportive care. Large randomized clinical trials have been responsible for the majority of these advances. Despite the reporting of subgroup results in children with ALL, the overall outcomes are fairly similar, and more than 95% will attain remission and close to 85% will survive free of leukemia recurrence at least 5 years from diagnosis.2
Risk Classification Systems and Risk-Adapted Therapy
Since intensification of treatment has contributed to the improvement in event-free survival (EFS) in children with ALL, this approach results in some patients being exposed to more aggressive therapy than necessary for cure. Nevertheless, patients identified at diagnosis as having better risk features still account for most treatment failures. This has formed the basis for “risk-adapted therapy.” Children who have historically had a very good outcome are treated with modest therapy and spared toxicity, while allowing children with a historically lower probability of long-term survival to receive more-intensive therapy to maximize cure. The Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG) adopted a common set of risk criteria in 1993 at an international conference supported by the National Cancer Institute (NCI).3 The NCI criteria were based on factors that had international acceptance and reproducibility, including age, initial white blood count (WBC) and the presence of extramedullary disease at diagnosis. Both POG and CCG refined therapy based on additional risk factors such as ploidy, blast karyotype, and early morphologic response. As a result of the merger between CCG and POG, the Children’s Oncology Group (COG) developed a consensus classification strategy for treatment assignment based on the retrospective analysis of over 6000 children and adolescents with ALL from CCG and POG data. Based on this analysis, patients with precursor B-cell ALL are initially assigned to a standard-risk or high-risk group based on age and initial WBC (ages 1 to 9.99 years, and WBC < 50,000 cells/μL is considered standard risk). All children with T-cell disease phenotype are considered high risk regardless of age and initial WBC. Early treatment response and cytogenetics are subsequently used to modify initial risk-group classification. Patients are classified as very high risk if they have any of the following features (regardless of initial risk group): t(9;22) and/or BCR/ABL, hypodiploidy less than 44 chromosomes, and induction failure.4
Different approaches to risk classification are being used by other cooperative groups. The Berlin-Frankfurt-Munster Group (BFM) categorizes risk almost solely on treatment response criteria. Minimal residual disease (MRD) measurements at two timepoints are used in addition to prednisone prophase response. All patients with either t(9;22) or t(4;11) are considered high risk, regardless of marrow response.5 The Dana-Farber Cancer Institute (DFCI) ALL Consortium is testing a new risk classification system for patients with precursor B-cell ALL. Patients are initially classified according to age and WBC and presence of CNS disease as standard or high risk and then, based on the level of MRD at the end of induction, postinduction treatment may be intensified. At St. Jude Children’s Research Hospital (SJCRH), risk classification is based mainly on MRD level (assessed by flow cytometry) after 6 weeks of remission induction therapy.
Age
Age has remained an independent predictor of outcome. Children (ages 1 to 9 years) have a better disease-free survival than older children, adolescents or infants. This is partly explained by the more frequent occurrence of favorable cytogenetics in the leukemic blasts including hyperdiploidy (> 51 chromosomes) or the t(12;21)(TEL-AML1 translocation). Children and adolescents aged 10 to 20 years have a slightly worse outcome that has been associated with a higher incidence of precursor T-cell disease and lower incidence of favorable cytogenetics. ALL cells from children less than 10 years of age when compared to ALL cells from older children and adults tend to be more sensitive to multiple antileukemic drugs.6 Infants with ALL have a very high risk of treatment failure. This is partly related to the high incidence of unfavorable very immature proB-ALL phenotype and the presence of mixed lineage leukemia gene (MLL) gene rearrangements.7 Infants whose leukemia has a germline MLL gene frequently present with CD10/cALLa-positive precursor B-cell immunophenotype and have a much better outcome than infants with the ALL and MLL gene rearrangements.8,9
Treatment
Treatment of ALL with multiagent chemotherapy is divided into four stages: remission induction, CNS-directed treatment and consolidation, reinduction, and maintenance.
Remission-induction
The goal of induction treatment is to induce a morphologic remission and restore normal bone marrow hematopoiesis. A three-drug induction consisting of vincristine, prednisone/dexamethasone plus l-asparaginase with intrathecal therapy results in complete remission rates of greater than 95%.10 Patients with a higher risk of treatment failure may be treated with a more intense induction regimen consisting of the addition of an anthracycline (daunomycin) in addition to the vincristine, glucocorticoid and L-asparaginase. The majority of children with newly diagnosed ALL will be in a morphologic complete remission (CR) by the first 4 weeks of treatment. Of those who fail to achieve a CR within the first 4 weeks, about half of them will experience a death due to toxicity and the other half will have resistant disease. Patients whose disease requires more that 4 weeks to go into remission have a poorer prognosis.5 Outcome is also less favorable for patients who demonstrate more than 25% blasts in the bone marrow or persistent blasts in the peripheral blood after 1 week of intensive multiagent chemotherapy.11,12
CNS-directed therapy
Only 3% of patients have detectable (CNS) involvement at the time of diagnosis (≥ 5 WBC/μL with lymphoblasts present). Patients with CNS involvement at diagnosis are treated with intrathecal therapy and subsequent radiation. Other groups of patients such as patients with the precursor T-cell phenotype and high WBC are treated with intrathecal therapy and cranial irradiation (12–18 Gy) in the absence of obvious CNS involvement. Unless specific therapy is directed toward the CNS, 50% to 70% or more of children will develop overt CNS leukemia.13 Therefore, early CNS therapy is critical for eliminating clinically evident CNS disease at diagnosis and preventing CNS relapse in patients without overt CNS disease. Generally this is started at the beginning of induction, intensified during consolidation and continued throughout maintenance. The goal is to achieve effective CNS therapy while minimizing neurotoxicity. This is usually accomplished by weekly or biweekly intrathecal therapy along with systemic drugs such as high-dose methotrexate, 6-mercaptopurine, dexamethasone, l-asparaginase, cyclophosphamide or cytarabine.
Reinduction therapy
The intensity of the postinduction period varies, but all patients receive some form of intensification following achievement of remission and before beginning continuous maintenance therapy. Reinduction therapy or delayed intensification most often use drugs similar to those used during induction and consolidation, but may also use intermediate- or high-dose methotrexate, or different drug combinations with little known crossresistance to the induction therapy drug combination, the extended use of high-dose l-asparaginase, or combinations of all of these.
Maintenance therapy
Maintenance for ALL generally uses daily oral mercaptopurine and weekly oral methotrexate. Maintenance therapy is the longest therapy phase for ALL and generally continues until 2 to 3 years of continuous complete remission. In some protocols additional pulses of vincristine and corticosteroids may be added.
Controversies and Challenges in Therapy
Despite this basic framework for treatment for ALL, there remain numerous areas of controversy and challenge. Several of those areas will be discussed.
Asparaginase
Several forms of L-asparaginase are available for the treatment of children with ALL. Escherichia colil-asparaginase is the most commonly used. PEG–l-asparaginase is an alternative form of l-asparaginase that has a much longer half-life than native E colil-asparaginase. A single intramuscular dose of PEG–l-asparaginase given in conjunction with vincristine and prednisone during induction therapy appears to have similar activity and toxicity as 9 doses of intramuscular E colil-asparaginase (3 times a week for 3 weeks). In a comparison between PEG L-asparaginase versus native E coli asparaginase in which each agent was given over 30-week period following remission, similar outcome and similar rates of asparaginase-related toxicities were observed for both groups.14 In a randomized trial in which patients with standard risk ALL were randomized at diagnosis to receive PEG–l-asparaginase or native E coli asparaginase during induction and two delayed intensification phases, the use of PEG–l-asparaginase was associated with more rapid blast clearance and a lower incidence of neutralizing antibodies.15 Currently COG protocols use PEG–l-asparaginase for all patients with ALL. Patients who develop an allergic reaction to PEG–l-asparaginase should be switched to Erwinial-asparaginase. Erwinia has a much shorter half life and therefore requires more frequent administration and a higher dose. There have been two studies in which patients were randomly assigned to receive Erwinial-asparaginase on the same schedule and dosage as E coli–l-asparaginase and they demonstrated a significantly worse EFS.16,17 Questions regarding the optimal doses, intensity, forms and route of administration of asparaginase are being addressed in current and planned studies. Patients who develop antibodies to asparaginase preparations remain a challenge in how they should be handled to avoid compromising their antileukemic therapy. Issues with availability of asparaginase preparations persist. Newer preparations of asparaginase are undergoing clinical testing as well as intravenous administration.
Presymptomatic CNS involvement
The approach to presymptomatic CNS involvement centers around the findings that long-term intrathecal (IT) therapy is as effective as radiotherapy with comparable EFS and survival rates. High-dose methotrexate, which has been used in protocols to prevent CNS relapse, is not as effective as radiotherapy in preventing CNS relapse. However, intravenous methotrexate reduces systemic relapses.18 In addition, CNS-directed therapy is influenced by systemic therapy. Patients with standard-risk ALL treated on CCG 1922 who were randomized to receive oral dexamethasone had a 50% decrease in the CNS relapse rate as compared with patients receiving oral prednisone (both groups received IT methotrexate alone for CNS prophylaxis).19 The optimal IT chemotherapy is not clear. Standard-risk patients were randomized in CCG 1952 between triple IT chemotherapy (methotrexate, hydrocortisone, cytarabine) or single IT (methotrexate). The results showed an isolated CNS relapse rate of 3.4% for triple IT therapy and 5.9% for single IT therapy (P = .004). There were more bone marrow relapses in the group that received triple IT therapy leading to a worse overall survival in this group (90.3%) compared with the single IT therapy group of 94.4% (P = .01). When the analysis was restricted patients with precursor B cells with a M1 day 14 bone, there was no difference in the CNS relapse rate, EFS or OS.20 Certain groups of patients may require more intensive IT therapy such as patients with blasts in the cerebrospinal fluid but less than 5 WBC and patients with a traumatic lumbar puncture with blasts at the time of diagnosis who are at an increased risk of CNS relapse.21,22 In summary, radiotherapy has been replaced by long-term IT therapy to prevent CNS relapses. It is not clear whether triple IT therapy has an advantage over IT methotrexate alone. Systemic therapy can be a factor in CNS-directed therapy. Certain high-risk groups of patients continue to undergo presymptomatic cranial irradiation. Current studies are ongoing in the pediatric cooperative groups to look at presymptomatic CNS therapy. St Jude Children’s Research Hospital is testing whether patients with clinically evident CNS disease at diagnosis can be treated with intensive IT and systemic chemotherapy without radiation.
Intensification of therapy
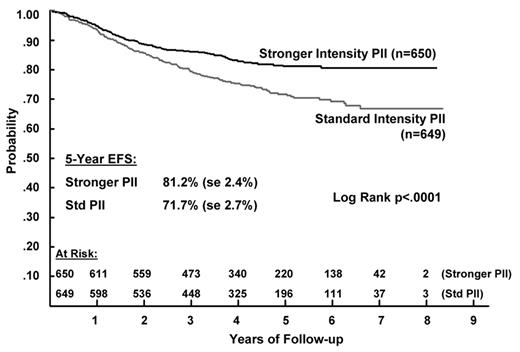
Improvement in the outcome of patients with standard-risk disease have been achieved with a limited exposure to chemotherapeutic agents such as anthracyclines and alkylating agents which are associated with late toxic effects. This has been accomplished through the use of regimens utilizing a limited number of courses of intermediate-dose or high-dose methotrexate, extended use of high-dose asparaginase or by using limited amounts of anthracyclines and alkylating agents in the form of delayed intensification. In higher-risk patients, a number of different approaches have been used, including blocks of intensified therapy such as delayed intensification blocks.5 Augmented postinduction therapy (consisting of 2 blocks of delayed intensification and interim maintenance phases) has been shown to improve the outcome for high-risk patients who show a slow response to 4-drug induction (based on the day-7 marrow) and in patients showing a rapid response to 4-drug induction.23–25 However, for patients showing a rapid bone marrow response to 7 days of induction there was no benefit to an increased duration of intensive therapy—a single delayed intensification/interim maintenance phase was as effective as 225 (Figure 2 ). This regimen, which consists of a single delayed intensification/interim maintenance phase, is the backbone of therapy in higher-risk and precursor T-cell COG studies.
Glucocorticoids
The question of which steroid (dexamethasone vs prednisone) to use in treatment is controversial. Dexamethasone when compared with prednisone has been shown to significantly lower the risk of CNS relapse and bone marrow relapses.19,26 Osteonecrosis (ON), a disorder characterized by segmental death of one or more osseous sites, has only recently arisen as a significant toxicity as increasing numbers of patients with ALL have received dexamethasone-based delayed-intensification therapies with improved disease survival. In CCG 1882, females between 10 and 15 years old and males between 16 and 20 years of age who received multiple courses of steroids had the highest risk of developing ON.27 In CCG 1961 the incidence of ON was decreased in patients who were receiving double delayed intensification phases by alternating the weeks of dexamethasone instead of 3 consecutive weeks.28 Dexamethasone clearance can be influenced by a variety of host- and treatment-related factors that can ultimately lead to increased toxicity. These include the patient’s age, asparaginase allergy and serum albumin level. In standard- and high-risk patients with low albumin as a reflection of asparaginase activity, dexamethasone exposure was prolonged.29
Precursor T-cell ALL
Different approaches have been taken for patients with T-cell ALL. Historically, these patients have had a worse outcome than B-cell precursor patients. With current treatment approaches, outcomes for children with precursor T-cell ALL are similar to those achieved for children with precursor B-cell ALL. The addition of high-dose methotrexate to the DFCI-based chemotherapy regimen resulted in fewer CNS relapses and a significantly improved EFS for patients with precursor T-cell ALL treated on POG-9404.30 Asparaginase (high dose) and doxorubicin were also important components of this protocol. In contrast, CCG-treated patients with precursor T-cell ALL on the same protocols as precursor B cell patients. Treatment assignment was based on age and WBC at presentation and then on the disease response to initial therapy. Most patients with precursor T-cell disease were treated on the Higher Risk protocol, CCG 1961, which showed that a single delayed intensification course produced the best results for patients who were rapid responders to initial induction therapy. CNS relapses were the most common events in this group. When comparing the outcomes for these two studies, the results were similar; however, all patients on POG 9404 underwent cranial irradiation, whereas only patients showing a slow morphologic remission on 1961 underwent cranial irradiation.31 Patients with standard-risk precursor T-cell ALL treated on CCG-l952 and CCG-1991 had an inferior EFS compared to children treated on POG 9404.32 In COG, patients with precursor T-cell ALL are currently being treated on a separate protocol from the patients with precursor B cells that utilizes a post-induction intensification backbone. Standard-and high-risk patients will be treated with nelarabine (a nucleoside analogue that is intracellularly converted to arabinoside furanosylguanine with demonstrated activity in patients with relapsed and refractory T-cell lymphoblastic disease) and randomized between high dose methotrexate and escalating increasing doses of intravenous methotrexate.33 Low risk precursor T cell patients will not undergo cranial irradiation whereas all standard and high risk precursor T cell patients will.
Adolescents and young adults
Treatment protocols for adolescents and young adults (AYA) with newly diagnosed ALL have been revised based on retrospective analyses that showed improved outcomes for AYAs when treated on protocols for pediatric patients as compared to protocols to treat adult patients. These results have been confirmed in the U.S. and Europe2,34–38 (Table 1 ). There are several potential explanations for these observed differences in outcomes. They include clinical and demographic differences in adolescents receiving treatment at pediatric compared to adult centers, clear differences in protocol design and dose intensity, and potential variations in the degree of adherence to protocol drug administration by adult compared to pediatric oncologists and by the patients treated. Ribera et al showed that for patients treated on a “pediatric type” treatment regimen, there was no difference in outcome for patients 15 to 18 years of age and those 19 to 30 years of age.39 U.S. adult and pediatric cooperative groups are embarking on a prospective trial to address some of the questions related to this group of patients and outcome. Newly diagnosed patients 16 to 30 years of age will be enrolled on an intergroup study that parallels the current COG study for adolescents and high-risk children. This study will examine disease biology, psychosocial disparities between adolescents referred to and undergoing treatment by pediatric oncologists or medical oncologists to assess the ability to administer safely and in a timely manner the same therapy used by the U.S. pediatric cooperative group.34
New Agents
Targeted therapy in ALL holds potential for contributing to improvement in outcome for children and adolescents in ALL. Imatinib mesylate (a selective inhibitor of the BCR-ABL protein kinase) has been combined with conventional chemotherapy in children with Philadelphia chromosome–positive ALL and improves early EFS and reduces minimal residual disease.40 Dasatinib and nilotinib are newer agents in clinical trials that show dual inhibition against BCR-ABL (including mutations). Overexpression of wild-type FLT3 particularly in MLL-rearranged ALL is a target that is being investigated in infant ALL. FLT3 inhibitors such as lestaurtinib (CEP-701), a highly selective small molecule FLT3 tyrosine kinase inhibitor, are being combined with chemotherapy in newly diagnosed infants with ALL and MLL rearrangements.41 Other groups of agents that have shown promising activity in the pediatric preclinical testing program for ALL include a BCL-2 protein inhibitor (ABT-263) and an aurora A kinase inhibitor (MLN8237).42,43 Monoclonal antibodies directed against a variety of targets such as cells expressing CD 19 (SAR3419, XMAb5574), CD 20 (rituximab), CD22 (epratuzumab), CD33 (gemtuzumab) and CD52 (alemtuzumab) are being developed or already in clinical trials.44,45
Summary
The outlook for children and adolescents diagnosed with ALL today is much better than ever before as result of well-designed clinical trials, identification of higher risk features with appropriate treatment changes, and tailoring of therapy according to response and risk groups. This progress should continue with the measurement of MRD, additional understanding about how patients metabolize chemotherapy, genomic profiling and identification of new targets for treatment. Together these should provide further guidance into optimizing treatment and minimizing the toxicity for the future.
Adolescent acute lymphoblastic leukemia (ALL) outcome according to treatment protocol.2
| Site . | Age Range, yr . | Number . | 5-year EFS . | Ref. . |
|---|---|---|---|---|
| *7-year EFS | ||||
| Abbreviations: CCG, Children’s Cancer Group; CALGB, Cancer and Leukemia Group B; FRALLE, French Acute Lymphoblastic Leukemia Pediatric group; LALA, Leucémie Aiguë Lymphoblastique de l’Adulte; DCOG, Dutch Childhood Oncology Group; HOVON, Dutch-Belgian Hemato-Oncology Cooperative Group; UKALLXII-United Kingdom Acute Lymphoblastic Leukemia; NOPHO 92, Nordic Society of Pediatric Haematology and Oncology | ||||
| USA | 34 | |||
| CCG | 16–21 | 197 | 63* | |
| CALGB | 16–21 | 124 | 34* | |
| France | 35 | |||
| FRALLE 93 | 15–20 | 77 | 67 | |
| LALA 94 | 15–20 | 100 | 41 | |
| The Netherlands | 36 | |||
| DCOG | 15–18 | 47 | 69 | |
| HOVON | 15–18 | 44 | 34 | |
| United Kingdom | 37 | |||
| MRC ALL | 15–17 | 61 | 65 | |
| UKALLXII | 15–17 | 67 | 49 | |
| Sweden | 38 | |||
| NOPHO 92 | 15–18 | 36 | 74 | |
| Adult | 15–20 | 21 | 39 | |
| Site . | Age Range, yr . | Number . | 5-year EFS . | Ref. . |
|---|---|---|---|---|
| *7-year EFS | ||||
| Abbreviations: CCG, Children’s Cancer Group; CALGB, Cancer and Leukemia Group B; FRALLE, French Acute Lymphoblastic Leukemia Pediatric group; LALA, Leucémie Aiguë Lymphoblastique de l’Adulte; DCOG, Dutch Childhood Oncology Group; HOVON, Dutch-Belgian Hemato-Oncology Cooperative Group; UKALLXII-United Kingdom Acute Lymphoblastic Leukemia; NOPHO 92, Nordic Society of Pediatric Haematology and Oncology | ||||
| USA | 34 | |||
| CCG | 16–21 | 197 | 63* | |
| CALGB | 16–21 | 124 | 34* | |
| France | 35 | |||
| FRALLE 93 | 15–20 | 77 | 67 | |
| LALA 94 | 15–20 | 100 | 41 | |
| The Netherlands | 36 | |||
| DCOG | 15–18 | 47 | 69 | |
| HOVON | 15–18 | 44 | 34 | |
| United Kingdom | 37 | |||
| MRC ALL | 15–17 | 61 | 65 | |
| UKALLXII | 15–17 | 67 | 49 | |
| Sweden | 38 | |||
| NOPHO 92 | 15–18 | 36 | 74 | |
| Adult | 15–20 | 21 | 39 | |
Improvement in survival for children with acute lymphoblastic leukemia (ALL).1 Five-year survival rates for children less than 15 years old with ALL: 1960–2004. SEER Cancer Statistics Review 1975–2005.
Improvement in survival for children with acute lymphoblastic leukemia (ALL).1 Five-year survival rates for children less than 15 years old with ALL: 1960–2004. SEER Cancer Statistics Review 1975–2005.
Five-year event-free survival (EFS) according to the type of postinduction intensification (PII) chemotherapy for higher risk acute lymphoblastic leukemia (ALL) patients.23
Reprinted with permission from
Five-year event-free survival (EFS) according to the type of postinduction intensification (PII) chemotherapy for higher risk acute lymphoblastic leukemia (ALL) patients.23
Reprinted with permission from
Disclosures Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: Vincristine, daunomycin, dexamethasone, prednisone, cyclophosphamide, cytarabine, antimetabolites, methotrexate-most of the chemotherapy that we use to treat children with ALL.
References
Author notes
Senior Investigator, Clinical Investigations Branch, Cancer Treatment Evaluation Program, National Cancer Institute, Bethesda, MD