Abstract
The majority of disease-specific complications in chronic lymphocytic leukemia (CLL), notably infection and autoimmunity, relate to the underlying alterations in immune function. Both cellular and humoral immunity are impaired with qualitative and quantitative defects in B cells, T cells, NK cells, neutrophils and the monocyte/macrophage lineage. Virtually all patients have reduced immunoglobulin levels, even in early stages, and this is associated with an increased frequency and severity of infection. Although prophylactic intravenous immunoglobulin may be of clinical benefit in selected patients, it does not reduce mortality and is certainly not cost-effective. Autoimmune complications occur in up to a quarter of CLL patients and predominantly target blood cells. Autoimmune hemolytic anemia (AHA) is the most common manifestation; immune thrombocytopenia, pure red cell aplasia and autoimmune neutropenia are less common, while non-hematological autoimmunity is rare. The UK CLL4 trial is the largest prospective trial in CLL to examine the significance of both a positive direct antiglobulin test (DAT) and AHA. The study confirmed the usefulness of the DAT in predicting the development of AHA or not, demonstrated that AHA occurred more frequently in patients receiving treatment with chlorambucil or fludarabine alone compared with the combination of fludarabine and cyclophosphamide, and showed that a positive DAT and the development of AHA were poor prognostic markers. Management of CLL-associated autoimmunity rests on good supportive care and the use of immunosuppressive therapies such as steroids and cyclosporine. Splenectomy remains useful, and monoclonal antibodies (rituximab and alemtuzumab) have given promising results.
Introduction
Chronic lymphocytic leukemia (CLL) is characterized by a dysregulated immune system. Indeed, recent evidence suggests that specific antigenic selection is involved in the pathogenesis of the disease.1,2 The clinical course in CLL is dominated by events associated with immune dysfunction, manifested predominantly as an increased susceptibility to infection and/or autoimmunity. The introduction of new therapeutic regimens has led to improved progression-free and overall survival for CLL patients. As patients live longer, disease-specific complications become more common and the risk of developing treatment-related effects increases, both of which make management more complex. In addition, the increased risk of second malignancies seen in CLL is likely to be related to the underlying immune impairment. An improved knowledge of the complex immune alterations in CLL may lead to a clearer understanding of the associated complications and also may help to identify suitable targets for therapy of these events and potential preventative strategies.
Here, following a brief background to impaired immunity, the main focus will be on presentation, diagnosis and management of the more common autoimmune complications in CLL.
Impaired Immunity
There are both quantitative and qualitative defects in immune effector cells that result in abnormal cellular and humoral-mediated immune responses.3 The function of T cells, NK cells, neutrophils and the monocyte/macrophage lineage is impaired (Table 1 ). In addition, production of immunoglobulin from normal B cells and antibody response to different antigens are significantly reduced. These defects arise through a number of mechanisms, including release of immunomodulatory cytokines (e.g., IL-6), cell-cell interactions and direct cytotoxicity. There are bi-directional interactions between non-neoplastic stromal and immune cells and the CLL cells that are important for both maintenance and progression of the CLL clone and for induction of the immune dysfunction. Treatment may also affect the normal immune system by depleting specific subsets of cells.
T-cell dysfunction
Interestingly, T-cell numbers are increased in untreated CLL with oligoclonality of both CD4+ and CD8+ subsets. Some of the latter are specific for cytomegalovirus (CMV).4 There are deficient T-helper function and increased T-suppressor activity with a reversal of the CD4/8 ratio and a dominant Th2 response. The immunophenotypic markers on T cells are altered in CLL, e.g., reduced CD28 and CD152 expression,3 and this may be related to the compromised function of these cells. The CLL cells are capable of inducing changes in normal CD4+ and CD8+ T cells through direct cell-cell contact and mediated via soluble factors, in particular inhibitory cytokines such as IL-6, IL-10, TNF and TGF-β.5,6 Supernatant from CLL cell cultures inhibits activation and proliferation of normal autologous and allogeneic T cells. Although these T-cells appear morphologically normal, functionally they are significantly impaired. Specifically, production, storage and transportation of cytolytic molecules in CD8+ T-cells are faulty.5 Gene profiles show changes in expression of genes key to these cytotoxic functions. Defects in T-cell expression of many antigens and ligands, e.g., adhesion molecules (LFA-1 and ICAM-1), CD28, CD152, IL-2 and CD25, mean that interaction with B cells in the process of antigen presentation is impaired. There has been shown to be both abnormal CD30 response and a reversible acquired CD40 ligand deficiency.7 These changes all contribute to the inability of T cells to successfully initiate, maintain and complete an immune response in patients with CLL.
Other immune defects
NK cells lack azurophilic granules and this may explain their inability to function normally.8 In a study by Nuckel et al human leucocyte antigen-G (HLA-G) overexpression in CLL correlated with the degree of immunosuppression and was thought to contribute to the immune dysfunction.9 Neutrophils and monocytes are also defective in their phagocytic and bactericidal function as well as in migration and chemotaxis.10 In addition they may be reduced in numbers due to advanced disease and/or cytotoxic treatment. Levels of different components of the complement pathway are reduced in CLL patients and accompanied by aberrant activation and binding. These abnormalities correlate with stage of disease and have been shown to be present in all patients with advanced disease.11
Hypogammaglobulinemia
The majority of CLL patients will develop reduction in normal immunoglobulin (Ig) levels during the course of their disease. All 3 classes (IgG, A and M) are affected, although predominantly IgG3 and IgG4. The severity tends to increase with the duration and stage of the CLL, with about 70% of patients developing hypogammaglobulinemia within 7 years of diagnosis. However, in one study, 73% of patients with Stage 0 CLL were found to have diminished levels of at least one serum immunoglobulin, indicating that this is a significant finding even early stages.12 There does not appear to be any correlation between Ig levels and IgVH mutation status.13 The primary serum antibody response is also affected early in the disease. Even in successfully treated patients, there is rarely recovery of Ig levels, although a recent study has suggested that use of high doses of rituximab in CLL can improve immune dysfunction and restore, in part, the production of immunoglobulins.14
Decreased immunoglobulin synthesis is probably related to the cell-cell contact with malignant B cells and release of inhibitory cytokines by the latter. There may be some relationship between tumor burden and reduction of Ig levels.15 The inhibition of immunoglobulin secretion is mediated via CD95L/CD95 interactions between plasma cells (PC) and B-CLL cells, which result in PC apoptosis.16 T-cell dysfunction may also play a role.
There is a direct correlation between low levels of IgG and the frequency and severity of bacterial infections, most commonly Streptococcus pneumoniae and Haemophilus influenzae. Deficiencies in IgA and IgG4 have been correlated with increased frequency of respiratory tract infections. IgG3 is important in protection against herpes infections.
The use of prophylactic intravenous immunoglobulin (IVIg) remains controversial. In a randomized study against placebo, regular infusions of 18 g IVIg every 3 weeks were shown to reduce infections in CLL patients with particular benefit for those with IgG levels less than 3 mg.17 A subsequent randomized study comparing a lower dose of IVIg also showed benefit.18 However, IVIg administration does not appear to improve quality of life or survival.19 Notably, IgM and IgA are not replaced by IVIg. There have been no randomized comparisons between IVIg and prophylactic antibiotics, which are cheaper and more readily available, in this “at-risk” patient population. In selected patients with marked hypogammaglobulinemia and severe recurrent bacterial infections requiring hospital admissions (especially with encapsulated bacteria and without evidence of pneuomococcal antibodies), regular IVIg infusion every 4 to 6 weeks may be beneficial, but is unlikely to be cost-effective. For this reason, general use in those with low Ig levels but without infections cannot be recommended.
Infectious complications
The majority of infections in untreated CLL are bacterial, arising in the lower respiratory tract. Common organisms include S pneumonia, S aureus, H influenzae.20 Recurrent infections are common and may lead over time to chronic sinusitis and bronchiectasis in susceptible individuals. Herpes virus reactivation is relatively common, with H zoster being most frequent. H simplex may be implicated in episodes of Bell’s palsy occurring in CLL patients. In addition, viruses have been implicated in transforming events in CLL; Epstein-Barr virus (EBV) in Richter’s transformation and human herpes virus 8 (HHV8) in Kaposi’s sarcoma. Fungal and opportunistic infections are rare in untreated patients.
The introduction of immunosuppressive therapies (purine analogues, monoclonal antibodies and steroids) may be complicated by increased frequency of more atypical infections, e.g., CMV, Pneumocystis carinii, listeria meningitis and fungal infections.20
The risk of infection increases over time, especially in heavily pretreated patients with active disease, and is usually the cause of death in advanced CLL.
Immunization responses in CLL patients are variable but suboptimal. Vaccination is most successful if used earlier in the disease, when immunoglobulin levels are better preserved, and if protein or conjugated vaccines are used.21 Response to vaccine may be enhanced by the use of adjuvant ranitidine.22 It is important to note the potential danger of administering live vaccines to patients with CLL. These include polio (oral), typhoid (oral), yellow fever, measles, mumps, rubella, BCG and Herpes zoster.
Careful attention to prophylaxis, monitoring for viral reactivation (e.g., CMV) and early treatment can all reduce the risk of morbidity and mortality from infection.
Autoimmunity
Autoimmune complications are well recognized in CLL, occurring in 10% to 25% of patients at some time during their disease course.23 Autoimmunity in CLL predominantly targets blood constituents, and autoimmune hemolytic anemia (AHA) is the most common form of autoimmune disorder. Indeed, CLL is the most common cause of AHA and even in sporadic cases a proportion can be shown to have low numbers of circulating cells with a CLL phenotype.24 The incidence of nonhematological autoimmune phenomena is low, although a wide range of conditions have been reported in the literature.
Auto-antibodies in CLL are polyclonal and differ in specificity and isotype from the immunoglobulins secreted directly by CLL cells. The residual nonmalignant B cells must thus be responsible for producing the auto-antibodies. In cold hemagglutinin disease (CHAD) monoclonal antibodies are produced by the lymphoma cells, but this condition is distinct from CLL.
Autoreactive T cells are present in the normal T-cell repertoire and are usually suppressed by the presence of regulatory T cells (T-regs). The latter are ineffective in CLL and this may result in activation of the autoreactive T-cells. Furthermore, autoimmune disease may be triggered by aberrant antigen presentation by the malignant B cells. CLL cells are not generally good at antigen presentation but may process cryptic antigens differently from normal B cells and thus present a peptide (e.g., Rh) for which there is not immunological tolerance.25
Autoimmune hemolytic anemia
The dominant human red blood cell (RBC) auto-antigens are against Rh proteins.25 Anti-RBC autoimmune responses are more common in CLL patients than the incidence of overt autoimmune hemolytic anemia (AHA) and are not always identified by the direct antiglobulin test (DAT). Latent autoimmunity is therefore underestimated.
The prevalence of AHA is related to stage and progression, with rates of 2.9% observed in stable Binet stage A CLL compared with 10.5% in stage B and C. It appears to be twice as common in patients with unmutated IgVH genes compared to those with mutated.23
Diagnosis of AHA
The diagnosis of AHA is usually based on the presence of an isolated, and often unexpected, fall in hemoglobin (Hb) associated with a positive direct antiglobulin test (DAT), a raised reticulocyte count and a rise in serum bilirubin. However, these test results may not be reliable. Heavy bone marrow infiltration in advanced CLL (Rai stage III and IV; Binet stage C) may mean that there is no reticulocyte response. In addition, the DAT may be negative despite overt hemolysis. Nevertheless, the DAT should always be part of standard assessment of CLL patients: at diagnosis, pre- and during treatment and if the Hb level falls. Serum bilirubin levels may not rise since, in most cases, the liver is capable of metabolizing the increased load. Increased urinary urobilinogen is a more reliable alternative. The lactate dehydrogenase (LDH) is closely linked to other disease factors such as progression, transformation and liver function abnormalities and therefore may not always correlate with hemolysis. A fall in serum haptoglobins may be a helpful independent measure of hemolysis.
Therapy-related AHA
In 1966 a landmark paper suggested that treatment with X-rays or alkylating agents might trigger the onset of AHA in CLL.26 Following the advent of purine analogue therapy in the 1980s there have been a number of single case reports and small series, involving over 100 patients, suggesting that AHA may occur more commonly following treatment with these agents. The Leukaemia Research Fund (LRF) CLL4 trial is the largest prospective trial in CLL to examine the risk of developing both a positive DAT and AHA during treatment27,28 (Table 2 ); 777 patients were randomized to receive chlorambucil or fludarabine, alone or with cyclophosphamide (FC). The incidence of a positive DAT was 14% and AHA 10%. The DAT correctly predicted the development, or not, of AHA after therapy in 83% of cases. The approximate risk of a patient with a positive DAT developing AHA was 1 in 3. Although there were some cases of DAT-negative AHA in the trial these were infrequent, in contrast to a recent report by Borthakur et al for patients treated with FC plus rituximab.29
Of 299 patients tested both pre- and post-treatment, those treated with single-agent fludarabine were most likely to remain DAT positive and to change from negative to positive. Patients treated with chlorambucil or fludarabine were more than twice as likely to develop AHA as those receiving FC (12%, 11% and 5% AHA cases, respectively). Possible explanations for this include the better responses seen in the FC arm which resulted in more rapid and effective control of CLL; the lower total dose of fludarabine administered in FC compared to fludarabine monotherapy, and finally the possible protective effect of cyclophosphamide, an immunosuppressive agent, when administered in combination with fludarabine.
In a multivariate analysis, age, DAT result, stage C disease and treatment were independent predictors of AHA. Other studies have also reported advanced stage and age over 65 as risk factors for AHA.30,31 Four deaths were attributed to AHA after first-line treatment, all on fludarabine monotherapy. These account for 2% of deaths in which CLL was a cause. Development of AHA was associated with poorer response to treatment (66% versus 81% overall response rate) and both shorter progression-free survival (PFS) (9% versus 18% at 5 years) and overall survival (OS) (37% versus 58% at 5 years). Other studies have not shown an independent negative effect of AHA on survival.32 The DAT status at the time of initiation of therapy, with or without associated AHA, was also a poor prognostic marker for PFS and OS. This had not been reported previously. A large retrospective review of CLL patients at the Mayo Clinic showed that patients with cytopenias due to an autoimmune disease had better survival (median 9.1 years) compared to those with bone marrow failure (median 4.4 years) and that it was not an independent adverse prognostic factor.33
It is clear from LRF CLL4 and other reports in the literature that AHA precipitated by therapy may be very severe and difficult to treat (in LRF CLL4 this was particularly the case following single-agent fludarabine). It would not be recommended to introduce the same treatment again in such patients. It would also seem that patients requiring CLL treatment who have a positive DAT or a previous history of AHA may benefit from treatment with FC or FC and rituximab rather than using single-agent chlorambucil or fludarabine.
Treatment of AHA
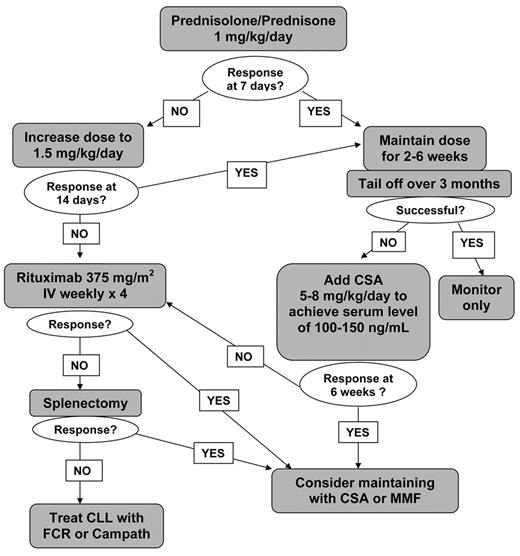
There have been no systematic studies or controlled trials of treatment for AHA, either occurring in relation to CLL or not. Treatment choice is usually based on personal experience and small case reports (Figure 1 ). It is important to remember the role of supportive care, in the form of blood transfusion support and folic acid. Red cell transfusion may be more effective if immediately preceded by infusion of IVIg. The most widely adopted first-line therapy is prednisolone, 1 mg/kg given orally for 2 to 4 weeks and then tailed off over a prolonged period of several months. The majority of patients will respond. If a rapid response is needed, a single high dose of methyl-prednisolone (1 g) may be given or IVIg (0.4 mg/kg/d for 5 days), which has a short-lived effect in about 40% of cases. Steroids should be given with a gastro-protectant and careful monitoring for diabetes. It may also be prudent to give prophylactic anti-fungals. In non-responders or those patients who relapse on steroid withdrawal, the use of cyclosporine (CSA) (5–8 mg/kg/d) or mycophenolate mofetil (MMF) is beneficial. We usually aim for a trough serum level of 100 to 150 ng/mL for CSA and 1 to 3.5 mg/L for MMF. Reported response rates for CSA are 63% with a median duration of 10 months.34 We have been able to maintain patients on CSA for many years without toxicity. Renal function and blood pressure should be monitored. Low-dose cyclophosphamide may also be useful as an immunosuppressant.
Splenectomy is still indicated in refractory AHA and may be life-saving in patients with vigorous uncontrolled hemolysis. Response is better in patients with IgG alone without complement on the red cells. Laparoscopic splenectomy can be an advantage, especially in patients less fit for conventional surgery.35 Splenic irradiation may be an alternative for patients in whom surgery is contra-indicated. Despite uncertainty about the efficacy of vaccination in CLL, it is recommended that patients should receive immunization against pneumococcus, Haemophilus influenzae B (HIB) and meningococcus, ideally 2 to 3 weeks prior to surgery, with lifelong penicillin (or equivalent) prophylaxis thereafter. In addition, patients should receive information leaflets and carry alert cards.
Rituximab (375 mg/m2/week for 4 weeks) has been used in the treatment of many autoimmune diseases, including AHA.36 In a small study, rituximab was used in combination with cyclophosphamide and dexamethasone with good results.37 More recently alemtuzumab has been reported as effective in controlling refractory AHA.38
The mode of action of any of these therapies in AHA is ill-understood. Depletion of T cells and/or interference with their function appear in some individuals to induce immune phenomena, e.g., after purine analogue therapy, and in others to control it. Although the occurrence of an auto-immune complication is not in itself an indication for CLL therapy, it is clear that in some patients this does not fully resolve until the underlying CLL is brought under control.
Immune thrombocytopenia
Approximately 2% of patients with CLL develop clinically significant immune thrombocytopenia (ITP). There are no clear criteria for diagnosis, and the platelet autoantibody tests lack sensitivity and specificity. Nevertheless, the occurrence of a rapid unexplained fall in platelets, in the absence of evidence of bone marrow (BM) failure or hyposplenism, suggest an immune origin for the thrombocytopenia. This assumption can be supported by evidence of improvement in the platelet count in response to steroids or IVIg. Demonstration of an increase in BM megakaryocytes is important diagnostically. About one-third of cases also have AHA (Evans syndrome). A recent Italian review identified 5% of ITP cases in a series of 1278 newly diagnosed CLL patients.39 The median time from CLL diagnosis to diagnosis of ITP was 13 months and the median platelet count was 14 × 109/L. The development of ITP occurred predominantly in patients who were not receiving therapy, 25% of the cases occurring at, or shortly after, CLL diagnosis. There was no association with use of fludarabine although other cases have been reported in the literature. Less than 10% experienced significant bleeding episodes. A high white blood cell count (WBC), a positive DAT, unmutated IgVH genes and ZAP70+, but not positive CD38 or abnormal FISH, were associated with development of ITP. Although numbers were small, patients with ITP had a significantly worse prognosis than other CLL patients, with 5 and 10 year OS of 64% and 42%, respectively. Early onset of ITP and refractoriness to treatment were associated with the poorest outcomes.
Response to first-line treatment, usually steroids or IVIg, is 50% to 60%; about 20% are refractory. Up to 70% of patients may have a long-term response to splenectomy. Rituximab, as in AHA, has been used with good results. The most effective dose schedule is not clearly defined and a recent study in non-CLL–associated ITP has found a lower dose of 100 mg weekly effective in 4 out of 7 patients.40 Vincristine is effective in some cases given at 1 mg weekly for 4 to 6 weeks.
Pure red cell aplasia and autoimmune neutropenia
It is likely that a number of past reports of pure red cell aplasia (PRCA) and autoimmune neutropenia in CLL were, in fact, associated with what is now recognized as T-cell large granular lymphocyte leukemia (T-LGL). Nevertheless, both do occur in CLL and are probably under-recognized. An examination of the bone marrow to assess the absence of hemopoietic precursors is essential in the diagnosis. In PRCA there is an absolute reticulocytopenia and it is now possible to obtain accurate assessment of anti-neutrophil antibodies in specialist laboratories. It is important to rule out viral infections (CMV, EBV, parvovirus, etc.) before assuming an immune pathogenesis. The treatment approach is the same as for AHA. Response to steroids in PRCA can be monitored by assessing the reticulocyte count. Rituximab has been successful in the treatment of PRCA41 but another study noted that it was less effective in refractory autoimmune neutropenia.42
Nonhematological autoimmune phenomena
Autoimmune conditions affecting nonhematological tissues are rare but well-described, and in some instances have been precipitated by therapy (Table 3 ). Numerous antibodies can be detected serologically in CLL patients but only infrequently correlate with clinical disease. In a study by the GIMEMA group 41% of CLL patients had a least one positive marker of autoimmunity, e.g., antinuclear antibody (ANA) or rheumatoid factor (RF), and 30 out of 194 cases (16%) of autoimmune diseases in CLL patients were non-hematological.31 These were mostly observed in Stage A patients in contrast to hematological autoimmunity, which was much more common in advanced (Stage B and C) disease. However, age-matched controls have a similar prevalence and distribution of serological and clinical autoimmunity, and these may therefore be coincidental findings. Some of the autoimmune conditions described in the literature, such as paraneoplastic pemphigus and glomerulonephritis (due to antineutrophil cytoplasmic antibodies), have been triggered by fludarabine and may therefore be a true complication rather than a chance association. These may be fatal. Angioedema, although rare, seems to be caused by a product of the CLL and is also seen in other lymphoid malignancies.
Conclusions
CLL is marked by the presence of immune defects. These are involved in the initiation and maintenance of the malignant clone as well as in the frequent occurrence of infections and autoimmune phenomena. All components of cellular and humoral immunity are affected. Autoimmunity is largely directed at hemopoietic cells, particularly red cells, and AHA occurs in up to a quarter of CLL patients. A large randomized trial in CLL prospectively examined the significance of the DAT and the development of AHA in CLL patients requiring their first therapy. This study confirmed the usefulness of the DAT in predicting the development of AHA or not, demonstrated that AHA was more frequent in patients receiving chlorambucil or fludarabine mono-therapy compared to the combination of fludarabine plus cyclophosphamide and showed that a positive DAT and development of AHA were each independent markers of poor prognosis.
Immune defects in chronic lymphocytic leukemia (CLL).
| Primary |
|
| Secondary |
|
| Primary |
|
| Secondary |
|
Significance of a positive direct antiglobulin test (DAT) pretreatment and the development of autoimmune hemolytic anemia (AHA) during treatment in the LRF CLL4 Trial.
| DAT Positivity before Treatment |
|
Development of AHA during Treatment
|
| DAT Positivity before Treatment |
|
Development of AHA during Treatment
|
Non-hematological autoimmune complications in chronic lymphocytic leukemia (CLL).
|
|
Treatment algorithm for autoimmune hemolytic anemia in chronic lymphocytic leukemia (CLL).
Note: All patients should receive folic acid 5–10 mg/day and red cell transfusions as necessary to maintain Hb > 8 g/dL.
Treatment algorithm for autoimmune hemolytic anemia in chronic lymphocytic leukemia (CLL).
Note: All patients should receive folic acid 5–10 mg/day and red cell transfusions as necessary to maintain Hb > 8 g/dL.
Disclosures Conflict-of-interest disclosure: The author serves on the speakers’ bureau of Roche and Bayer Schering Off-label drug use: The use of monoclonal antibodies (rituximab and alemtuzumab) will be mentioned as treatment options for refractory autoimmune complications of CLL.
Acknowledgments
The author would like to thank Professor Terry Hamblin for his help, particularly with the development of a treatment algorithm for AHA.
References
Author notes
Department of Haemato-Oncology, The Royal Marsden NHS Foundation Trust and Institute of Cancer Research, Sutton, Surrey, UK