Abstract
People with sickle cell disease (SCD) are living longer, but their lives are impacted even more by the unpredictable intermittent or constant pain that is often poorly managed over a lifetime. To address this problem, an interdisciplinary team approach is needed that brings the medical professionals together for optimal compassionate care that is coordinated from the beginning of life and throughout the patient’s lifespan.The hematologist, whenever possible, should take the lead. Effective models that have been developed to address SCD pain, such as the Day Hospital, The Recidivism Project and various Home Care models, need to be integrated into an overarching plan on a continuum with an underpinning philosophy that will sustain such a program. While the focus of palliative care has been end of life, its philosophies fit the chronic disease model and support an integrated team approach to the SCD pain management dilemma. The patient-focused and family-oriented interventions can be modeled to fit “any place, any time,” outpatient, inpatient or the home, with homecare and other support systems. Such are the needs in SCD: appropriate, timely and compassionate care to prevent or relieve suffering and to preserve dignity, meaning, value and quality of life with improvement that is translational from birth until the end of life.
Introduction
Pain and its management pose major public health challenges around the world.1 The management of pain in sickle cell disease (SCD) has never been viewed with a straightforward approach by many practicing hematologists, pain experts, or generalists, especially for the adult patient. Pain is the hallmark feature of SCD and the lifetime bête noire for many of the patients.2 Greater understanding of a disease whose pathogenesis is associated with increased mortality and complex quality-of-life issues and of the lethality of pain itself has made the care of patients with sickle-related pain more urgent and more challenging.3,4 While improvements in the care of SCD have resulted in people with SCD living longer, their lives are impacted even more by the unpredictable intermittent or constant pain that is often poorly managed over a lifetime.
The clinical negligence of this hallmark feature and the disparity in funding for research regarding pain and its management have resulted in vicious cycles that perpetuate dire effects on persons with the disease and their families, as well as in inadequacies of practitioners and health care systems. Only 1% of the NIH budget goes to primary pain research of any kind, yet pain brings people to the hospital more than any other condition.4 The systematic search for evidence in guideline development of the American Pain Society uncovered that pain in SCD has received relatively little research attention.5 In this era that focuses on evidence-based practices, most of the evidence for pain management in SCD must be extrapolated from other pain states.5 There has never been a report of a randomized control trial on chronic pain in SCD and over the past 30 years, there has been fewer than 10 masked randomized controlled studies of acute pain in SCD;6,7 not very encouraging for a hallmark, sentinel, protean feature of a devastating disease. It is encouraging that recent workshop results indicate that the Division of Blood Disease and Resources of the National Heart Lung and Blood Institute will issue an RFA on the neurobiology of pain in SCD. The immediate need for treatment demands that we must also engage now in efficient and cost-effective ways of accelerating the evaluation of available therapies.
Extensive guidelines that reference and detail specific pharmacologic and non-pharmacologic therapies may be found elsewhere.5 –7 The focus here is to establish an operational framework of pain care practice models that can be applicable in any setting and at any stage of development. We must institute systems that maximize and ensure the evaluation of and accessibility to effective care that both benefits patients and provides evidence for the study designs for available and new therapies while these proposed programs are being formulated.
Phenotypic Diversity of Pain and Other Disease Manifestations
The pathogenesis of clinical disease is exceedingly complicated, and affected individuals display a perplexing degree of phenotypic diversity regarding pain and other aspects of SCD.3,8,–10 Pain is not only a protean manifestation of SCD but the pathophysiology of unrelieved pain can also be a driving force for SCD pathophysiology.2,5,11 Biological, social, and psychological perturbations that include emotional stress, mood, and physical stress, adhesion of sickle cells to vascular endothelium, elaboration of inflammatory mediators, molecular and cytochemical factors result in microvascular occlusion, tissue hypoxia, and tissue injury and influence the wide variability of the pain responses observed clinically.5,7,8,10,11 Efforts are underway to define the diverse phenotypes in SCD.
Pain in SCD can be acute, chronic or mixed, can be related to tissue injury (nociceptive), nerve injury (neuropathic), or causes unknown (idiopathic).2,5,11 Clear delineation between or among types of pain is not always possible. Moreover, it can be disease related, therapy related, or unrelated to disease or therapy. Thus, pain in SCD is a heterogeneous entity and its management must not only factor in its mechanisms but also its complications and comorbidities. In addition to the pain syndromes, clinical sickle disease includes hemolytic anemia, injury, inflammatory changes, acute and chronic vasculopathies and complications related to organ damage.3,8,12,13
Evidence of distinct clustering of symptoms or clinical phenotypes into a twofold model was put forth in a Jamaican Cohort Study9 that classified patients into two groups: Group 1 consisted of individuals with more frequent painful crises and other complications such as dactylitis, meningitis/septicemia, acute chest syndrome and stroke and less frequent leg ulcers; Group 2, leg ulcer phenotype consisted of more frequent leg ulcers and less frequent other complications. Seventy-eight percent of patients were classified with 95% confidence into one of these groups. The emergence of these manifestations showed some order regarding age specificity. The occurrence of dactylitis, acute splenic sequestration and the susceptibility to infection were most marked before the age of 5 years; painful crises and leg ulceration emerged as major problems during late adolescence and early adult life; and chronic organ damage to the kidneys and lungs assumed greater importance after the age of 30 years (Figure 1 ).
Consequences of Unrelieved Pain: Quality of Life and Symptom Burden
There needs to be a clear understanding of and appreciation for the untoward consequences of unrelieved pain or the pathophysiology of pain.14,15 It is more than a symptom and has a pathophysiology that, if left unchecked, can ultimately become a disease itself. Although many individuals with SCD remain remarkably resilient to the consequences of frequent unrelieved pain, inadequate pain management is amplified in a subset of these patients, sometimes to life-consuming and even life-threatening proportions.2,14,16,17 The harmful effects of unrelieved pain are many and involve multiple symptoms and essentially all body systems, as noted in Table 1 .18 Uncontrolled pain has a universal and profound negative effect on the quality of life in all ages and with all types of pain. Of all of the adverse effects of unrelieved pain, the greatest harm of all may well be decreased quality of life.18
Pain is a common experience in children beginning as early as 4 to 6 months of age, and dactylitis in infants is an early prognostic indicator for increased risk of complications in children.5,9,19,20 The failure to address the pain early on can have lifelong implications on their health, generating a vicious cycle of fear, avoidance and pain and poor coping strategies. Unrelieved pain can not only have negative consequences such as missed days of school, other life activities or fear or mistrust of health care providers, but also can lead to amplified responses to subsequent pain experiences and sensitivity to pain later in life.20,21 Frequent painful episodes are associated with early mortality in adults with SCD.16,22 Some patients become profoundly disabled from chronic pain20,23,–25 and require substantial social and healthcare resources that are not consistently available.26,27 In most cases, contrary to commonly held beliefs, most pain problems do not begin when the patient is transitioned (or transferred) from Pediatrics to Adult care but occur on a continuum wherein the debilitating effects often only become clearly evident during adulthood.
Disease and Symptom Management
The ability to treat the underlying disease is of primary importance in the treatment of pain. Hydroxyurea therapy instituted by practitioners in medical offices and in outpatient clinics as prophylactic treatment will decrease the frequency and severity of acute painful crises and acute chest syndrome by nearly half in aggregate in homozygous SCD (SCD-SS) who take it.28,29 A recent consensus development conference has acknowledged the strong evidence that supports this efficacy in adults and that is encouraging in children.30 However, there are extensive barriers and, as with pain, “little research exists on the barriers at the patient, parent/family/caregiver, provider, and system levels.”30 Since there are no approved disease-modifying therapies during an acute event, only supportive care therapies such as oxygen, fluids and electrolyte therapy are currently available to manage associated hypoxia and dehydration.2,11,12 Analgesics are almost always required, not only for acute severe vaso-occlusive pain but also for chronic intermittent or persistent pain. Adjuvant treatments are used to improve analgesia or for treatment of side effects.5,6
Building a Model Framework for Evidence and Effectiveness
The fundamental underlying concept of model development for the successful management of pain is individualization of analgesic therapy. These care models should result in improved access to and quality of care.
Access to care
Many patients with SCD do not have access to adequate pain management and endure unnecessary waits in the emergency departments and long lengths of hospital stays due to poor pain control; they are often discharged with persistent moderate to severe pain, and have difficulty getting opioid prescriptions filled at neighborhood pharmacies.31,32 Other factors that impact the disparity in access to treatment are the attitudes of nurses, physicians, and other health care providers concerning race and ethnicity, opiophobia or opiophilia regarding opioid requirements, and biases towards those persons who present with recurring and sometimes frequent complaints of pain.2,17,33,34
Basic quality practices
The practices that are employed are based on principles of care and take place in a sequence of stages.2 Briefly, the first stage, assessment, involves self-appraisal of attitude and readiness in determining the type of the SCD, the nature of the pain and the assessment of its significance for the patient. The second stage, treatment, is concerned with the planning and execution of an assessment based management of the illness and pain. The third stage in the process is reassessment that involves the evaluation of the effectiveness of the treatment executed during the second stage. This processing system includes feedback loops or regimen-adjusting strategies (titration, maintenance, rescue and taper dosing) so that if the treatment is not evaluated to be effective, the health care provider may alter original and/or subsequent treatment based upon assessment of the SCD and pain, or both.
Biopsychosocial interpretation
The second feature of the approach is a biopsychosocial interpretation of the information contained in the objective features of the SCD and the sensory and reactive dimensions of pain that direct method of managing the treatment of SCD pain. The sensory qualities of pain include intensity, sites, characteristics and the underlying physiological or neurological mechanisms. The reactive components include the interference of pain with physical functioning and distress associated with the patient’s reactive affective or emotional feelings or suffering due to the combination of the illness and pain.
Pain Care Models and Quality Of Life
Model I. The Day Hospital Care Model as an Alternative to the emergency department
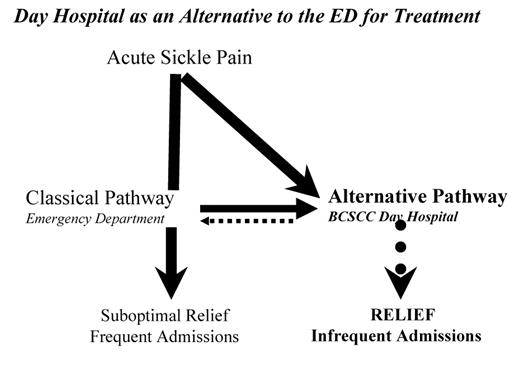
The first pain care model at the Bronx Comprehensive Sickle Cell Center (BCSCC) was the Day Hospital (DH), established approximately 20 years ago in the SCC as an alternative to the Emergency Department (ED) for improved access and quality treatment of acute painful episodes in individuals (Figure 2 ).35
In the DH, individualization of care is accomplished through the basic practices of rapid assessment, early treatment, frequent reassessment and aggressive titration to relief. Pain assessment is a quintessential part of pain management. There is little doubt in this field that pain evaluation is a vital task that becomes very complex and difficult if performed inconsistently or inattentively to appropriate dimensions of pain. Although simple self-reporting scales such as a numerical scale ranging from 0 to 10, with 0 representing “no pain” and 10 representing “the worst possible pain imaginable” have proved invaluable, the meaning of the level of pain ranking fluctuates from one pain episode to another in the same patient. An artist’s graphic rendition of unrelieved pain entitled “Ten redefined” illustrates a deep emotion of pain and suffering that essentially challenges the notion that any given number can reflect the multiple facets of acute or chronic pain experienced by individuals with SCD.5,15,35
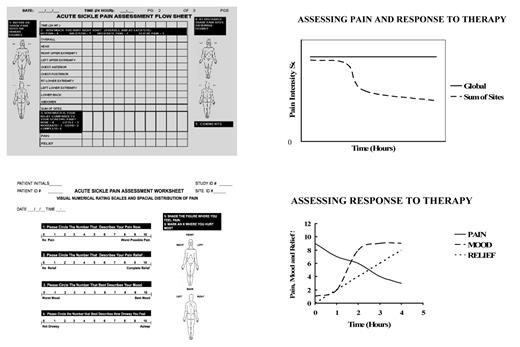
The common practice of evaluating patients solely on the basis of an 11-point numerical rating scale (0–10) and basing analgesic treatment solely on the score can lead to suboptimal treatment.36 A version of this scale depicting various degrees of facial pain expression in children has been validated and serves a useful tool in pediatric clinics.5 Nonetheless, it is important to recognize the multidimensional nature of pain and cautiously evaluate dramatic changes in intensity scores, particularly in a single pain episode. The periodic assessment of acute or chronic pain that is integrally interwoven into a chronic illness should be performed in multiple domains.37 The six recommended domains include physical and emotional functioning, and social and symptom domains. We find that by also rating the pain at each site and obtaining a sum of sites and relief score, response to therapy is more discernable (Figure 3 ).
After a period of aggressive titration to relief, 90% of patients who presented to the DH were discharged home after an average of 4.5 hours of treatment.35 Prior to the opening of the DH, the ED admitted 92% of the SCD patients who presented complaining of acute pain and some of those admissions resulted in prolonged hospitalizations. As a clinical pain research unit, we demonstrated that aggressive titration to relief can lessen or eliminate many sequelae of unrelieved pain and can result in a decrease in the time in pain, in the need for hospitalization, in the cost to the patient and the cost to the healthcare system.35 The success of the DH and the contribution to improving not only the care but also the opportunity for an improved quality of life is well recognized, and has far reaching implications and actual impact on the management of acute sickle pain. The fundamental principle of QOL is that it is assessed by the patient. As an alternative to unnecessary hospital time in pain, the patients view improved pain control and prevention of hospitalizations and accompanying prolonged hospitalizations as providing increased time for activities of daily living.
The Model is transportable and we have helped centers in the U.S. and abroad in various stages of the development and implementation of their day hospital. The reports of similar outcomes in journals and at national meetings attest to the replicability of the approach. Day Hospitals and Day Care Centers are alternatives to emergency departments for outpatient treatment of moderate to severe acute painful episodes in Europe, North America, and the Caribbean.35,38,39 These dedicated units are access-friendly and apply assessment-determined packages of care that generally include opioids as the mainstay of therapy, NSAIDs, anti-histamines, and non-pharmacologic physical, cognitive and behavioral measures.
Model II. Recidivism Model Project for frequently recurring “acute” pain
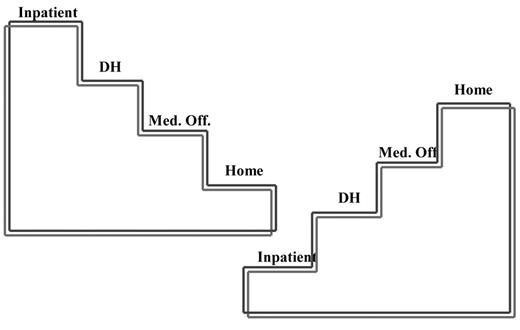
The second Model is a chronic pain model, the Recidivism Project for frequently recurring acute pain/chronic pain.2 In a single-center study of acute pain recidivism in SCD at Montefiore Medical center, we set out to address patients who still presented with great frequency to the DH or the ED as direct visits or often as rapid bouncebacks after discharge from inpatient care. Biopsychosocial assessments were performed and long-acting opioids were used as an alternative to short-acting opioids in an attempt to prevent the occurrence of persistent and escalating pain that might be associated with the neuroadaptive phenomena of tolerance and physical dependence that can occur with chronic opioid use. Tapering was employed to prevent withdrawal and long-acting opioids were used as an alternative to short-acting opioids in an attempt to stabilize the pain and to normalize the stress response via the hypothalamic pituitary adrenal axis that can become hyperresponsive during opioid withdrawal.40 We put forth a stepladder schema to reflect the goal of reversal of management strategies that would stabilize pain, improve functioning and thereby change the course from a life spent predominantly in inpatient and outpatient hospital-based utilization to the possibility of a home-based life focused on activities of daily living (Figure 4 ).
Patients who cluster into this very high utilizer category are generally regarded as not analyzable and are thus excluded from most studies or if included become regarded as outliers that do not get evaluated in efficacy-related data.22 To correct for this, we employed opioid rotation in an N of 1 design wherein the patient served as his/her own control.2 Opioid rotation involves switching the opioid a patient is receiving to another opioid with the objective of reducing limiting adverse effects and/or increasing analgesia. A number of opioids that are available for clinical use would be tested in an individual. If a treatment worked, that treatment would be continued. If the first treatment failed, the patient would receive an alternative treatment until effective treatment was achieved. This approach resulted in multiple rotations to various opioids that were commonly used in patient care. These opioids did not produce stabilization or analgesia that could be maintained. Clinical considerations for the patient demonstrating a decreased effect with the same or increased dose of an opioid analgesic included the development of opioid tolerance, patient-related pharmacokinetic alterations, disease progression, and noncompliance with prescribed analgesic administration and neurotoxic withdrawal or toleranced induced hyperalgesia.
After all other opioids had failed, the first subject in the study was rotated to methadone and improvement occurred within a week. Methadone was added to the list of opioids evaluated in the study. The N of 1 design described above resulted in patients becoming very stable on methadone treatment and subsequently provided the major analgesic therapeutic underpinning for the model. Hospitalizations were reduced 60% to 80% in the Recidivism Project suggesting that many hospitalizations were due to inadequate pain management. Methadone is a potent opioid analgesic that demonstrates incomplete cross-tolerance with other μ-opioid receptor agonist analgesics. Conversion of the opioid-tolerant patient with sickle-related pain to methadone was central to reversing the uncontrolled pain state, with important contributions from adjuvant therapies employing pharmacological, psychological, and physical techniques. The dose of methadone required to establish and maintain analgesia represented a sizeable reduction ranging from 1/5 to 1/10th of the calculated equianalgesic dose.
The resultant model is an Integrated Biopsychosocial Collaboration in six phases:2
Preparatory: Formation of collaborative agreements between the patient and providers.
Stabilization: Establishment of effective drug regimen and stabilizing dosing for each patient utilizing opioid rotation and opioid sparing adjuvant therapies.
Evaluative: Determination of pain mechanisms and response to therapy assessment through the use of the brief pain inventory and the multidimensional inventory.
Therapy adjustment: Self-regulatory techniques are crucial to incorporate through all phases. Participants developed an awareness of therapeutic possibilities and developed skills to deal adaptively with problems included in treatment. Success was attributed to their efforts; that is, to reiterate that they did it.
Adaptation: Functional restoration includes being able to resume work/school and other activities of daily living.
Functioning and motivational enhancement: Improved quality of life through expansion of restoration of function and self-regulatory techniques.
This biopsychosocial model approach underscores that although recurring acute pain and recidivism in SCD pose very difficult and confusing management problems, it is possible to dramatically attenuate them with adjustment in practices. In addition to pain from the disease, a partial contributor can also be a multifactoral heightened sensitivity to pain, hyperalgesia; the hyperalgesia can result from disease processes such as injury and inflammation;15,41 from anxiety that is provoked from pain and/or life issues;42 and in some cases dramatically induced by opioids as tolerance- or withdrawal-associated hyperalgesia.41,43 The latter aspect of management wherein the medicine used to treat pain can reach a threshold of effectiveness after which it causes pain requires special recognition and attention. It is often preventable and when recognized and addressed can lead to dramatic improvement of a magnitude that is consistent with the extent of its contribution.
Opioid-Induced Hyperalgesia: An Emerging Neurotoxicity Syndrome
The aberrant behaviors noted in some patients who present frequently can be the consequence of the failure to recognize neurotoxicity syndromes related to opioid use. Paradoxically, the chronic administration of opioid analgesics to treat pain may lead to similar confusion, by also contributing to or causing pain.15,41,–43 Increased sensitivity to pain may be observed in any clinical setting where recurrent acute or chronic pain occurs. This is often erroneously attributed to either more disease-related pain, or to aberrant drug-seeking or addictive behaviors. There are basic steps to be taken when opioid neurotoxicity exists. First, recognize the syndrome. Delirium, agitation, or restlessness may make the patient seem to be irrational or to be exaggerating the pain. The usually offending opioids are frequently used immediate-release (morphine, hydromorphone or oxycodone) opioids or very high doses of sustained release formulations of morphine, hydromorphone, and oxycodone.41,43,44 An early sign may be clonus, which can be seen while the patient is asleep before it becomes clinically overt. Allodynia and hyperalgesia cause the pain to occur all over and does not follow a reasonable distribution. Rapidly increasing the opioid makes the pain worse. Second, discontinue the offending opioid and rotate to another drug. Third, add additional non-opioid adjunctive medications. Fourth, begin hydration to clear opioid metabolites. Fifth, consider benzodiazepines to decrease neuromuscular irritability but avoid sedation.
Epidemiologic Studies and Model Development
Using examples and concepts from the single center Pain in Sickle Cell Epidemiological Studies (PISCES) project, we can outline some of the major issues that remain in the quest to improve models of care: gender differences in pain and health care utilization,46 differences between home and hospital utilization for pain management in adults,47 and catastrophizing as a strong predictor of chronic pain patients’ health-related quality of life.48 Population-based studies also help identify certain models of care that are sometimes poorly characterized.
Pain in adults with SCD is the rule rather than the exception and is far more prevalent and severe than previous large-scale studies have portrayed. It is mostly managed at home; therefore, its prevalence is probably underestimated by health care providers, resulting in misclassification, distorted communication, and undertreatment.47 Interventions in SCD should consider improvements in health-related quality of life as important outcomes.48
Models of care for chronic pain often have a patchy evidence base, partly reflecting a disconnect between clinical trial methods developed for monotherapies and the increasing need for complex interventions for chronic diseases.45 Epidemiological studies of pain have descriptive and/or analytic functions that can help inform models of care by identifying those at risk for poor outcomes, and narrowing down a range of intervention targets. As stated earlier, there are no controlled chronic pain controlled randomized trials in SCD and thus none to inform model development.
Home Care Model
Several studies in children and evidence in epidemiologic studies cited above show the majority of pain is treated at home.4,49,50 While there are several reports on home care, there are few reports that evaluate the effectiveness of family caregivers in that home care. Ethnographics have been employed to recognize the effectiveness of pain management in this setting and the important role that educating family caregivers in pain management principles and practices play in this effort.50 While family caregivers use many techniques to “get through” the episode and avoid the hospital, there is a documented need for professionals to design the most effective methods of home pain management with the families. Religious thinking and prayer have been proposed to improve coping and the management of pain and psychological distress.51 In a recent study,49 it was determined that the events at home and those requiring visits to the hospital constituted a continuum: events of varying pain severity with similar vaso-occlusive pathophysiology. Effective management at home primarily involves mild to moderate pain, while moderate to severe pain is most effectively managed in the hospitals.
Palliative Care Model
The core ideals of integrated palliative care are open communication, timely access to care, intensive symptom management, flexibility in the implementation of interventions, ethical decision making, and attention to the quality of life of the patient, the family, parents, siblings and significant others. The interventions are patient focused and family oriented and can be modeled to fit “any place, any time,” in the hospital or the home, with home-care and other support.52
While the focus of palliative care has been end of life, it has been proposed that the palliative care model be instituted for patients with SCD, that it should be introduced in early childhood and continued throughout the patient’s lifespan.20 The World Health Organization has defined palliative care as the active, total care of patients whose disease is not responsive to curative therapy. It is both a philosophy of care and an organized system for delivering care by an interdisciplinary team. In contrast to the palliative care philosophy in which partnership between patients and health care providers is visible, the sickle cell experience is confounded by adversarial relationships with health care providers.33 Care in SCD is often fragmented and contentious.
Advocates of such an approach in SCD believe that applying palliative care philosophies in the management of pain in patients with SCD would change the public and medical purview of this population’s plight.20 One could argue that in SCD we need both a Revolution (according to Marilyn Gaston in her presentation at the National meeting commemorating the 30th anniversary of the Sickle Cell Disease Program and the Sickle Cell Disease Association of America, Inc.) and a Renaissance53 in order to have a tremendous impact on the health care system, the patients and their families.
A Model of TranQuality: Integrated Interdisciplinary Pain Care on a Continuum
Given the above features, arguably models of care in SCD should include the elements of palliative care. There is no doubt that the philosophy of palliative care is appropriate for pediatric and adult SCD patients, but the organized system to implement such a program currently does not exist in SCD programs or palliative care programs in care facilities. There is a need to help transform the care of people with SCD through rigorous scientific studies, new models of clinical care, and innovative educational programs that integrate biomedicine, the complexity of human beings, the intrinsic nature of healing and the rich diversity of therapeutic systems. A concept that may possess potential utility for clinicians in the current structure is translational analgesia.36 In most cases, a sustained and significant improvement in pain perception that is deemed worthwhile to the patient should “translate” into improvement in quality of life or improved social, emotional or physical function.
Quality of life as a key evidence variable
Many pain patients will experience symptoms and adverse events associated with their illness and pharmacologic treatment that can impact the quality of their lives adversely. Each patient’s definition of quality of life is unique and models must be constructed as to address the uniqueness of the individual.14 We propose incorporating quality of a life as a key evidence variable in evaluating analgesic effectiveness in research and clinical pain care models. It can serve as an efficacy indicator of intended effects or as a differentiator of analgesics; as a gauge of the impact of various modes of drug administration; as a possible critical marker of tolerability and as a determinant of symptom distress due to unintended medicinal effects.
Quality of life was impacted positively in both models developed at the BCSCC as described above. In the day hospital approach, 90% of patients go home after an average of 4.5 hours treatment. Hospitalizations were reduced 60% to 80% in the biopsychosocial recidivism project approach. Most pain management in these models has transitioned back to home care that is coordinated collaboratively during medical office visits and involves the patient, family and professional caregivers.2,35
TranQual analgesia model
Thus, we are advocating an organized “TranQual Analgesia Model” system approach that begins by melding models into an harmonious interdisciplinary integration; that reaffirms the importance of the home and the relationship between practitioners, the patient and family caregivers; focuses on the whole person; is informed by available evidence; and makes use of all appropriate therapeutic approaches, healthcare professionals and disciplines to achieve optimal health and healing. It is at the very least “Translational Analgesia” wherein the patient and provider perceived pain and prescribed therapies clearly result in quality of life improvement and sustenance. The clinician and patient are actively involved in a shared decision-making approach to treatment that maximizes the patient’s readiness to take an active role in pain management. When home and hospital-based outpatient and inpatient care occurs along a continuum with the same principles of care applied relevant to the setting, pain management becomes a manageable aspect of overall care imparting “tranquality” from the first to the last moment of life of the patient.
Harmful effects of unrelieved pain.
| Domains affected . | Specific responses to pain . |
|---|---|
| May be duplicated for use in clinical practice. From McCaffrey M, Pasero C. Pain: Clinical Manual, p. 24.18 Copyright © 1999, Mosby, Inc., with permission. | |
| Endocrine | ↑Adrenocorticotrophic hormone (ACTH), ↑ cortisol, ↑ antidiuretic hormone, ↑ epinephrine, ↑ norepinephrine, ↑ growth hormone (GH), ↑ catecholamines, ↑ renin, ↑ angiotensin II, ↑ Aldosterone, ↑ glucagons, ↑ interleukin-1, ↓ insulin, ↓ testosterone |
| Metabolic | Gluconeogenesis, hepatic glycogenolysis, hyperglycemia, glucose intolerance, insulin resistance, muscle protein catabolism, ↑ lipolysis |
| Cardiovascular | ↑ Heart rate, ↑ cardiac output, ↑ peripheral vascular resistance, ↑ systemic vascular resistance, hypertension, ↑ coronary vascular resistance, ↑ myocardial oxygen consumption, hypercoagulation, deep vein thrombosis |
| Respiratory | ↓ Flows and volumes, atelectasis, shunting, hypoxemia, ↓ cough, sputum retention, infection |
| Genitourinary | ↓ Urinary output, urinary retention, fluid overload, hypokalemia |
| Gastrointestinal | ↓ Gastric and bowel motility |
| Musculoskeletal | Muscle spasm, impaired muscle function, fatigue, immobility |
| Cognitive | Reduction in cognitive function, mental confusion |
| Immune | Depression of immune response |
| Developmental | ↑ Behavioral and physiologic responses to pain, altered temperaments, higher somatization, infant distress behavior, possible altered development of the pain system, ↑ vulnerability to stress disorders, addictive behavior, and anxiety states |
| Future pain | Debilitating chronic pain syndromes |
| Quality of life | Sleeplessness, anxiety, fear, hopelessness, ↑ thoughts of suicide |
| Domains affected . | Specific responses to pain . |
|---|---|
| May be duplicated for use in clinical practice. From McCaffrey M, Pasero C. Pain: Clinical Manual, p. 24.18 Copyright © 1999, Mosby, Inc., with permission. | |
| Endocrine | ↑Adrenocorticotrophic hormone (ACTH), ↑ cortisol, ↑ antidiuretic hormone, ↑ epinephrine, ↑ norepinephrine, ↑ growth hormone (GH), ↑ catecholamines, ↑ renin, ↑ angiotensin II, ↑ Aldosterone, ↑ glucagons, ↑ interleukin-1, ↓ insulin, ↓ testosterone |
| Metabolic | Gluconeogenesis, hepatic glycogenolysis, hyperglycemia, glucose intolerance, insulin resistance, muscle protein catabolism, ↑ lipolysis |
| Cardiovascular | ↑ Heart rate, ↑ cardiac output, ↑ peripheral vascular resistance, ↑ systemic vascular resistance, hypertension, ↑ coronary vascular resistance, ↑ myocardial oxygen consumption, hypercoagulation, deep vein thrombosis |
| Respiratory | ↓ Flows and volumes, atelectasis, shunting, hypoxemia, ↓ cough, sputum retention, infection |
| Genitourinary | ↓ Urinary output, urinary retention, fluid overload, hypokalemia |
| Gastrointestinal | ↓ Gastric and bowel motility |
| Musculoskeletal | Muscle spasm, impaired muscle function, fatigue, immobility |
| Cognitive | Reduction in cognitive function, mental confusion |
| Immune | Depression of immune response |
| Developmental | ↑ Behavioral and physiologic responses to pain, altered temperaments, higher somatization, infant distress behavior, possible altered development of the pain system, ↑ vulnerability to stress disorders, addictive behavior, and anxiety states |
| Future pain | Debilitating chronic pain syndromes |
| Quality of life | Sleeplessness, anxiety, fear, hopelessness, ↑ thoughts of suicide |
Pain management and age-related complications and pain on a continuum.
Pain management and age-related complications and pain on a continuum.
The Bronx Comprehensive Sickle Cell Center (BCSCC) Day Hospital approach as an alternative to the emergency department.
The Bronx Comprehensive Sickle Cell Center (BCSCC) Day Hospital approach as an alternative to the emergency department.
Assessment form that rates pain globally and at each painful site. Upper right panel: Assessment of pain and response to therapy illustrates change in sum of sites score when the global score does not reflect change. Lower left panel: modification of the Memorial Assessment Card39 using numerical rating scales for measuring pain, relief, mood and sedation Lower right panel: pain, relief and mood depicting response to therapy. As the pain is reduced, relief and mood increase indicating that mood disturbance was related to pain.
Assessment form that rates pain globally and at each painful site. Upper right panel: Assessment of pain and response to therapy illustrates change in sum of sites score when the global score does not reflect change. Lower left panel: modification of the Memorial Assessment Card39 using numerical rating scales for measuring pain, relief, mood and sedation Lower right panel: pain, relief and mood depicting response to therapy. As the pain is reduced, relief and mood increase indicating that mood disturbance was related to pain.
Recidivism Project: Transition from hospital to home care. Stepladder schema for transitioning from dominance of inpatient and outpatient hospital utilization with very little home time as depicted in the left steps with reversal to home dominance and very little time spent in the hospital as depicted to the right.
Recidivism Project: Transition from hospital to home care. Stepladder schema for transitioning from dominance of inpatient and outpatient hospital utilization with very little home time as depicted in the left steps with reversal to home dominance and very little time spent in the hospital as depicted to the right.
Disclosures Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
References
Author notes
Comprehensive Sickle Cell Center, Montefiore Medical Center, New York, NY