Abstract
Allogeneic stem cell transplantation can cure patients with myelodysplastic syndromes. However, more than 50% of the patients who underwent allogeneic stem cell transplantation have failed to benefit from this treatment approach either due to treatment-related mortality or to relapse. The introduction of toxicity or dose-reduced conditioning has significantly reduced the treatment-related mortality but did not affect the risk of relapse. New effective drugs, such as hypomethylating agents, histone-deacetylase inhibitors or lenalidomide, can be used alone or in combination to improve the pretransplant remission status before allogeneic stem cell transplantation or after transplantation to prevent relapse as maintenance or consolidation therapy. This review will focus on these new possibilities and give some perspectives as to how the results of allogeneic stem cell transplantation can be further improved
Introduction
The myelodysplastic syndromes (MDS) are a heterogeneous group of clonal stem cell disorders characterized by hypercellular bone marrow, peripheral cytopenias, and dysplastic features in blood and bone marrow. The clinical course of the disease varies from an indolent course over several years to a more rapid progression into secondary acute myeloid leukemia.
The treatment strategy with the highest curative potential is allogeneic stem cell transplantation (SCT). After standard myeloablative conditioning followed by HLA-identical sibling transplantation, disease-free survival (DFS) rates between 29% and 40% were reported. The non-relapse mortality ranged between 37% and 50% and the relapse rate between 23% and 48%.1–3
Therefore, despite the curative potential of allogeneic SCT, more than 50% of the patients will fail to benefit from this treatment approach either due to treatment-related mortality (TRM) or to relapse after transplantation. The most important factors that influenced survival after allogeneic SCT are the age of the patient (by higher risk of TRM and relapse), number of blasts before transplantation (by higher risk of relapse), abnormal cytogenetic (by higher risk of relapse) and the comorbidity index (by higher risk of TRM). Large studies from the EBMT and the IBMTR confirmed age, number of blasts and cytogenetic abnormalities as independent risk factors for lower survival rates.1,3
Therefore, to improve the outcome after allogeneic SCT strategies should focus on (a) reducing TRM and (b) reducing the risk of relapse (Table 1 ).
General Considerations and Options to Reduce TRM and/or Risk of Relapse
Source of stem cell
A retrospective study of the EBMT compared bone marrow with peripheral blood stem cells as stem cell source in HLA-identical sibling transplantation. The patients with MDS who received peripheral blood stem cells (PBSC) had a faster engraftment and a lower relapse rate, resulting in improved DFS rates, than patients who received bone marrow. Furthermore, while the incidence of acute graft-versus-host disease (GVHD) was similar between both groups, patients who received PBSC had a higher incidence of chronic GVHD.4 Similar results have been reported from the Seattle group with a 3-year DFS of 68% for PBSC transplant compared with 48% for bone marrow.2
Modifying standard preparative regimen
As reported by the EBMT, most MDS patients received either a busulfan/cyclophosphamide-based regimen (52%) or a total-body irradiation (TBI)–based (44%) regimen.1 A report of the National Marrow Donor Program (NMDP) for unrelated SCT revealed a lower relapse rate and a better survival rate for patients with busulfan/cyclophosphamide conditioning in comparison to those with the TBI regimen.5 Improved outcome using the busulfan/cyclophosphamide regimen has also been reported by the Seattle group. They used a target level of busulfan (800–900 ng/ mL) to avoid severe toxicity.2 Patients with refractory anemia/refractory anemia with ringed sideroblasts had a 68% probability of DFS after HLA-identical transplantation and a 70% probability if they received a graft from an unrelated donor. The non-relapse mortality was 12% at 100 days and 31% at 3 years, while relapse occurred in only 5%. For advanced patients with refractory anemia with excess blasts (RAEB) a 45% probability of DFS after HLA-identical sibling transplantation and a 40% DFS at 3 years after unrelated transplantation was reported.2
Since cyclophosphamide is not stem cell toxic but contributes to non-hematological toxicity, attempts are made to replace cyclophosphamide with fludarabine. Targeting busulfan in combination with fludarabine has been investigated in patients with myeloid malignancies. In 38 patients with MDS the non-relapse mortality at day 100 and 1 year was 7% and 24%, respectively.6 Further improvement in non-relapse mortality has been reported by using intravenous busulfan in combination with fludarabine, resulting in a non-relapse mortality of only 3% at 1 year.7
Attempts to reduce the relapse rate by using an intensified regimen have been made by the Fred Hutchinson Cancer Center, evaluating the combination of busulfan (7 mg/kg), cyclophosphamide (120 mg/kg), and TBI (12 Gy) in advanced MDS (RAEB, RAEB in transformation [RAEB-t], and chronic myelomonocytic leukemia). Compared with a historical control group, the relapse rate could be lowered (28% vs 54%); however, this advantage was offset by a higher non-relapse mortality (68% vs 36%) resulting in a worse relapse-free survival rate (23% vs 30%).8 Even when cyclophosphamide was replaced by fludarabine in 50 patients with advanced MDS and secondary acute myeloid leukemia (sAML), the non-relapse mortality was still very high at day 100 (38%) resulting in a disappointing 3-year overall survival rate of only 26%.9 Therefore, intensified conditioning regimens do not appear to be beneficial.
Reduced-intensity or reduced-toxicity regimen
The rationale to decrease the intensity is to shift tumor reduction or eradication of tumor cells from cytoreduction to an immunologically mediated graft-versus-tumor effect. Furthermore, reduction of the intensity will also reduce transplant-related toxicity and therefore transplant-related mortality. This approach allows transplantation of older patients and those who were not eligible for a standard myeloablative conditioning regimen.
The most commonly used regimen is busulfan (8 mg/ kg) in combination with fludarabine with or without Campath or antithymocyte globulin (ATG). In 62 patients the Bu/Flu/Campath regimen has been investigated either from related or unrelated donors. The relapse-free survival was about 60% at 1 year, and the non-relapse mortality was 5% for related and 21% for unrelated SCT.10 The busulfan/ fludarabine and ATG regimen (for unrelated SCT) was used in studies from the German Cooperative Transplant Study Group, resulting in non-relapse mortality of 32%, a relapse incidence of 32%, and a DFS rate of 38% at 3 years.11 Busulfan plus fludarabine without any T-cell depletion in HLA-identical sibling transplantation resulted in a low TRM of only 5% at 1 year and a DFS of 66% at 1 year.12 The low-intensity approach with 2 Gy TBI +/− fludarabine in 78 patients with MDS had a 25% TRM and an estimated 3-year DFS of 20%.13 Another regimen included treosulfan, an alkylating agent, in combination with fludarabine and ATG for elderly patients with MDS and sAML., resulting in a day 100 non-relapse mortality rate of 28%, a relapse rate at 2 years of 21%, and a 2-year DFS rate of 34%.14 The EBMT performed a retrospective study comparing dose-reduced (RIC) and standard conditioning in 836 patients with MDS who underwent HLA-identical sibling transplantation. They found a significantly lower incidence of TRM (P = .015), but a significantly higher incidence of relapse (P = .001) in the RIC group, resulting in comparable disease-free and overall survival rates in both groups.15 However, it is of note that patients transplanted with RIC were older and often suffered from comorbidities and were, therefore, not eligible for a standard conditioning regimen. For this reason the EBMT has started a randomized trial comparing standard and RIC therapies (http://www.ebmt.org).
Donor selection
Since most patients with MDS are lacking an HLA-identical sibling as stem cell donor, unrelated SCT has been also investigated; however, this has been associated with a high non-relapse mortality between 30% and 58%.16,17 Recently, however, the results have considerably improved due to lower non-relapse mortality. The National Marrow Donor Program reported a relative risk for DFS of 1.43 (95%CI:1.01–2.01; P = .03) for transplantation performed between 1988 and 1993 versus more recent transplantations.5 Additional further reduction of non-relapse mortality was observed after targeting busulfan/cyclophosphamide conditioning.2 Unpublished results of the EBMT registry for MDS transplantation in recent years showed no difference in survival of unrelated SCT in comparison to HLA-identical sibling transplantation after adjustment for age and disease status. A retrospective study of the EBMT compared unrelated SCT with autologous SCT in patients in first CR. DFS was 50% for unrelated SCT and 41% for autologous SCT (P = .01). The better survival of unrelated SCT was due to a lower risk of relapse (24% vs 62%), despite the higher non-relapse mortality (38% vs 17%).18 However, the success of unrelated SCT depends on HLA matching: high resolution DNA for HLA-A, HLA-B and HLA-C and DRB1 alleles of donor-recipient HLA is associated with improved survival.19
One out of 300 patients with MDS has a syngeneic twin. Therefore, syngeneic SCT can be performed only rarely. The EBMT reported results for 38 patients with MDS/ sAML who received a syngeneic graft after standard myeloablative conditioning20 and compared the result with 1444 patients who received SCT from an HLA-identical sibling within the same time period. The engraftment after syngeneic transplantation was faster and the non-relapse mortality was lower (27% vs 38%). The relapse rate was only slightly higher after twin transplantation (39% vs 32%), resulting in a trend for improved DFS at 5 years (34% vs 28%). Therefore, a syngeneic donor will be the donor of choice if available.
Alternative stem cell sources, such as cord blood or haplo-identical SCT, are being used increasingly for patients lacking a suitable donor, but detailed reports about these alternative stem cell sources in MDS patients are lacking so far.
Specific Strategies to Reduce the Risk of Relapse
The most striking factors for TRM are age, comorbidities, intensity of the conditioning regimen and donor selection. A careful donor selection and an appropriate choice of conditioning regimen will significantly reduce the treatment-related morbidity and mortality. The most significant reduction in TRM can be achieved by an RIC regimen. However, one should be aware that it is unlikely that reducing the intensity of the conditioning regimen will have a positive impact of the risk of relapse in MDS patients. Moreover, retrospective studies suggest that the incidence of relapse will be even higher if the preparative regimen consisted of RIC.12 Therefore, for further improvement of allogeneic SCT additional efforts are needed to reduce the risk of relapse. This can be done by either improvement of the pretransplant remission status or by post-transplant strategies, such as maintenance or consolidation therapies.
Improving pretransplant remission status
Patients with less advanced stages of MDS and lower number of blasts, such as RA or RARS, may benefit most from allogeneic SCT, with long-term DFS of more than 50%.1,21,22 The outcome of patients with RAEB and RAEB-t is less favorable due to a higher relapse rate. The EBMT reported a 5-year relapse rate of 44% for RAEB and 52% for RAEB-t,21 and the Fred Hutchinson Cancer Research Center (FHCRC) reported a 49% relapse rate for RAEB patients compared with only 4% for patients without any bone marrow blasts.22 Therefore, it seems reasonable to lower the number of blast prior to transplantation in order to improve outcome by reducing risk of relapse.
Chemotherapy
The use of pretransplant intensive AML-like chemotherapy in patients with advanced MDS (high number of blasts) before allogeneic SCT is currently common practice in several institutions, but the clinical benefit remains controversial.23 A French trial of therapy-related MDS reported a substantially better outcome in those patients who achieved remission with pretransplant chemotherapy.24 However, patients who failed to achieve remission after induction chemotherapy and underwent in non-CR allogeneic SCT had a worse outcome after transplantation than those who did not receive pretransplant chemotherapy. These results suggest that the pretransplant chemotherapy selects patients with an improved post-transplant outcome. Prospective randomized trials addressing this issue have been started within the EBMT (http://www.ebmt.org). A more recently investigated approach is performing induction chemotherapy followed immediately by the conditioning regimen and SCT. Kolb et al used induction chemotherapy with fludarabine, amsacrine and high-dose cytosine arabinoside followed by a 3-day rest by TBI (4Gy) and cyclophosphamide with or without ATG and SCT with preemptive DLI in patients with MDS (n = 67) or sAML (n = 90). The overall survival rate for the MDS patients at 10 years was 50%, significantly higher than that for patients with sAML (26%). Preemptive DLI appeared to prevent relapse when compared with that to patients not given DLI.25 Alternatively, other active drugs, such as adenosine nucleoside analog clofarabine, in combination with cytosine arabinoside have been used as induction therapy followed by RIC in 10 patients with acute leukemia or MDS with a median age of 62 years. All patients engrafted and achieved 100% donor chimerism, and only 1 patient died from treatment-related causes.26
Epigenetic modulation
Epigenetics is referred to several biochemical modifications of chromatin without altering the sequence of DNA. Epigenetic deregulation plays an important role in development of malignant diseases. The primary epigenetic modifications are DNA methylation and histone modification, both of which are potentially reversible.27,28 DNA methylation plays an important role in gene regulation. DNA methyltransferases (DNMT) mediate methylation by incorporating a methyl group into position 5 of the cytosine ring resulting in 5-methyl cytosine.27 This modifications occurs most frequently in the DNA sequence called cytosine phosphodiester guanine (CpG) dinucleotides that occurs in clusters called CpG islands. These islands are often associated with the promoter regions of genes and are usually unmethylated.27,28 Aberrant methylation of such promoter regions can occur in malignancies and correlates with gene silencing, particularly of tumor suppressor genes. Cancer is characterized by regional promoter hyper-methylation of genes, which occurs in a non-random and tumor-specific manner. Promoter hypermethylation of CDKN2B (encoding p15INK4b) has been shown to be restricted to hematological diseases.27,28 It is unclear why MDS is more amenable to hypomethylating agents than are solid tumors, but the exact mechanism of response is unknown. Most studies could not show an association between induction of DNA hypomethylation or histone acetylation and clinical response. At the present time in the US, two DNA hypomethylating agents have been approved for patients with MDS: azacitidine (5-azacitidine) and decitabine (5-aza-2′-deoxycitidine). Both drugs are DNMT inhibitors that have demethylating effects. Azacitidine has been shown superiority in randomized trials regarding time to AML transformation and overall survival compared with best supportive care or other chemotherapeutic interventions.29,30 In the CALGB 9221 trial complete remission was seen in 17% in the 5-azacitidine arm and the median time to leukemic transformation or death was significantly prolonged by 5-azacitidine to 21 months versus 13 months for supportive care (P = .007). In an updated report of the CALGB trial the complete remission rate of azacitidine was 10%. In a European Phase III trial the CR or partial remission rate for 5-azacitidine was 28.5% and the 2-year survival for 5-azacitidine–treated patients was 50.8% in comparison to only 26% for those patients treated with conventional therapy (P < .0001). Decitabine was randomized to supportive care in a Phase III trial, resulting in an increased response rate, but only in a trend toward a longer median time to AML transformation or death compared to supportive care alone.31 In this trial the rate of CR was only 9%, but with a 5-day schedule and a dose of 20 mg/m², the rate of CR increased to 34%.32 Only small Phase II studies investigated the feasibility of using azacitidine or decitabine as induction therapy prior to allogeneic SCT (Table 2 ). Lübbert et al treated 10 patients with MDS or AML and a median age of 65 years with low-dose decitabine before allogeneic SCT. After a median number of 3 cycles, 40% of the patients achieved CR, and 10% achieved partial remission prior to transplantation. After RIC and transplantation from related (n = 3) and unrelated (n = 7) donors, 9 patients engrafted. During follow-up 3 patients died from infectious complications, and 3 patients relapsed.33 The MD Anderson Cancer Center used a similar approach in 12 patients with MDS with a median age of 58 years. After a median of 5.5 cycles of decitabine, 33% of the patients achieved CR and 50% achieved partial remission. All patients underwent allogeneic SCT after reduced (n = 9) or standard conditioning (n = 3) from related (n =5) or unrelated donors (n = 7). Engraftment was seen in 11 patients and after a median follow-up of 11.5 months 9 patients are alive and 2 died due to relapse.34 McCarty et al investigated the cytoreductive effect of azacitidine in 25 patients with high risk MDS or sAML before allogeneic SCT. Patients received at least 4 cycles of azacitidine. Those who improved with marrow blasts continued on azacitidine (n = 13) and those with more than 30% marrow blasts received FLAG chemotherapy (n = 7). Those with increasing blasts were crossed over to FLAG (n = 5). Twenty-one of the patients underwent allogeneic SCT from related (n = 9) and unrelated (n = 12) donors after a busulfan-based conditioning regimen. After a short follow-up of 1 year the median event-free survival for the azacitidine and the FLAG group have not yet reached, while the median event-free survival for those patients who progressed to azacitidine and received FLAG was only 7 months. The TRM and overall mortality and relapse rates were higher in patients who received chemotherapy compared with patients treated with azacitidine; however, in this trial chemotherapy was given only to those patients who did not respond well or progressed after azacitidine.35 The number of patients treated with hypomethylating agents before allogeneic SCT is still limited so valid conclusions can not be drawn. Nevertheless, the major advantage in comparison to conventional induction chemotherapy is lower toxicity, resulting in a better performance status at transplantation
Another epigenetic modulation is histone modification. Histone acetyltransferase (HATs) and histone deacetylase (HDACs) are involved in chromatin modification, which is crucial for regulation of gene transcription.27,28 Histone deacetylation restores a positive charge of lysine residues in core histones, resulting in tight interaction of DNA and histones, which maintain chromatin in a transcriptionally silent state, In contrast, histone acetylation neutralizes the positive charge, thereby disrupting DNA-histone, which facilitates access of transcription factors. This disrupting of DNA-histone led to the hypothesis that histone deacetylase inhibitors (HDACi) lead to derepression of silenced tumor suppressor genes.27,28 Abnormal activity of HATs and HDACs have been demonstrated in myeloid leukemias. So far no reports are available of the use of HDACi as induction therapy for allogeneic SCT, but in an animal model HDAC could reduce GVHD and preserve graft-versus-leukemia effect.51 Valproic acid (VPA) induced a 35% response rate (one partial remission and 15 hematologic improvements) in 43 patients with low-risk MDS.36 Because in-vitro experiments of combining hypomethylating agents and HDACi indicated a synergistic effect in terms of leukemia cell killing and gene reactivation, clinical trials of combination therapy have been conducted. Combining VPA with azacitidine or with decitabine in untreated patients increases the rates of CR up to 33% and 40%, respectively.37,38 Therefore, it could be possible that pretransplant remission status can be further improved by combining azacitidine or decitabine with HDACi, such as VPA, phenylbutyrate, MS-275, vorinostat or other HDACi. Additional studies investigating such combination therapy followed by allogeneic SCT will be of clinical importance.
Induction of cytogenetic remission
Besides the number of blasts and the aforementioned options to reduce their numbers before SCT, cytogenetic abnormalities were also shown to be of importance to the outcome of allogeneic SCT. The EBMT recently reported about the impact of cytogenetic status—classified according to IPSS—on outcome after allogeneic SCT. For 692 patients a complete data set was available. The cytogenetic risk according to IPSS significantly influenced survival: 47% for good risk, 40% for intermediate risk, and 31% for high risk. The relapse rate was 34% for good-risk, 35% for intermediate-risk, and 57% for high-risk cytogenetic status. In addition to age, the cytogenetic risk score remained an independent prognostic factor for relapse and survival in multivariate analysis.39 Another study investigated outcomes of allogeneic SCT in 70 patients with MDS according to their IPSS cytogenetic risk score. The event-free survival for good-, intermediate-, and high-risk cytogenetic subgroups was 51%, 40%, and 6%, respectively. While no difference in non-relapse mortality was seen, the relapse rates were 19% for good-risk, 12% for intermediate-, and 82% for high-risk patients.40 New agents in the treatment of MDS have also impacted on the resolution of chromosomal abnormalities and might be also used as pretransplant therapy. Lenalidomide is a potent immunomodulating drug that has shown activity in terms of transfusion independency and in resolution of chromosomal abnormalities, especially in isolated 5q- but also in complex abnormalities involving 5q-.41 So far only case reports are available for successful resolution of cytogenetic abnormalities by lenalidomide prior to allogeneic SCT.42 There are also some observations that MDS patients with poor cytogenetic abnormalities such as monosomy 7 may benefit from hypomethylating agents.43,44
Post-stem cell transplantation modifications
Besides improving pretransplant status of patients with MDS by inducing clinical and cytogenetic remission, further approaches to reduce the risk of relapse after allogeneic SCT involve post-transplant modifications.
Adoptive immunotherapy: donor lymphocyte infusion
Donor lymphocyte infusion (DLI) can induce durable remission in relapsing patients with hematological malignancies after allogeneic SCT.45 However, the experience of DLI in patients with MDS is limited.
Only a small number of patients with MDS who received this approach were reported. Campreger et al reported about 16 patients with MDS who received DLI for relapse after allogeneic SCT. Twenty-two percent achieved a CR, which lasted in 2 patients more than 5 years, but all patients with CR experienced severe acute and chronic GVHD.46 Similar results were reported by the Societe Française de Greffe de Moelle et Therapie Cellulaire in 14 relapsed patients with MDS who received a median of 6.3 × 107 CD3+ cells/kg; 14% achieved CR.47 Kolb et al used preemptive DLI (escalating from 1 × 106/kg to 5 × 106/kg and 1 × 107/kg) after a chemotherapy induction cycle following a 3-day rest by reduced conditioning consisting of TBI (4 Gy) (or busulfan 8 mg/kg) and cyclophosphamide with or without ATG in high-risk MDS (n = 67) or patients with sAML (n = 90). Preemptive DLI appeared to prevent relapse when compared to patients not given DLI.25
Maintenance therapy with azacitidine
Efficacy of azacitidine for relapsing patients after allogeneic SCT in combination with DLI was shown in a small series of 6 patients.52 Five of 6 patients responded to treatment with azacitidine and DLI, either with complete (n = 3) or partial (n = 2) remission. Of note no patients developed acute GVHD and 2 experienced extensive chronic GvHD. The MD Anderson Cancer Center investigated azacitidine at doses between 8 and 24 mg/m² for 5 days given for 1 to 4 cycles as maintenance therapy in 40 high-risk patients with MDS or AML. They observed no severe side effects and no impact of azacitidine on GvHD and chimerism. The recommended dose of azacitidine was 24 mg/m² × 5 days for at least 4 cycles.48 This encouraging approach, either alone or in combination with DLI, needs further evaluation.
Vaccination and T-cell specific immunotherapy
The donor-derived hematopoietic and lymphopoietic system offers a platform for several immunologically based interventions after allogeneic SCT to treat either residual minimal disease and to prevent relapse. Leukemic-specific antigens such as WT1, PR1, RHAMM or cancer testis antigens have been tested as peptide-based vaccinations eliciting immunologic T-cell response and some clinical remission; however, only a very few of the study population suffered from MDS.49,50
Future Directions
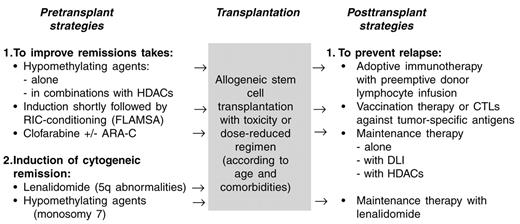
The results of allogeneic SCT in MDS have improved progressively mainly because of a reduction in non-relapse mortality, while no clear reduction of relapse incidence could be observed. The availability of MDS active drugs such as hypomethylating agents, HADCi, lenalidomide and others, offers a new, less toxic option to lower the number of blasts prior to allogeneic SCT, which might reduce the risk of relapse. Furthermore, these agents can be used alone or in combination with immunotherapy (DLI or vaccination) as post-transplant maintenance or consolidation therapy to prevent relapse (Figure 1 ). At the present time, the available studies may be influenced by selection bias and the small number of included patients. Therefore, these new, exciting treatment possibilities have to be investigated or confirmed in well-designed clinical studies to demonstrate a clinical benefit.
Transplantation, patient- and donor-related factors that influence treatment-related mortality and incidence of relapse. Arrows in parentheses: only moderate effect.
| . | TRM . | Relapse . | DFS . |
|---|---|---|---|
| *Including targeting busulfan and i.v busulfan | |||
| Abbreviations: TRM, treatment-related mortality; PBSC, peripheral blood stem cells; BM, bone marrow; Bu, busulphan; Cy, cyclophosphamide; TBI, total-body irradiation; CR, complete response | |||
| PBSC vs BM2,4 | ↔ | ( ↓ ) | (↑ ) |
| Bu*/Cy vs TBI/Cy2,5–7 | (↓ ) | ( ↓ ) | (↑ ) |
| Intensified vs standard conditioning8,9 | ↑ | ↓ | ↓ |
| Dose-reduced vs standard conditioning12 | ↓ | ↑ | ↔ |
| Donor: HLA-ident sibling vs matched unrelated donor16,17 | ↓ | (↑ ) | ↑ |
| Donor: Syngeneic vs HLA-ident sibling20 | ↓ | (↑ ) | ↑ |
| Induction chemotherapy: if CR | ↓ | ↓ | ↑ |
| if no CR23,24 | ↑ | ↑ | ↓ |
| Comorbidities13 | ↑ | ↔ | ↓ |
| Higher age1–3 | ↑ | (↑ ) | ↓ |
| Unfavorable cytogenetics39,40 | ↔ | ↑ | ↓ |
| . | TRM . | Relapse . | DFS . |
|---|---|---|---|
| *Including targeting busulfan and i.v busulfan | |||
| Abbreviations: TRM, treatment-related mortality; PBSC, peripheral blood stem cells; BM, bone marrow; Bu, busulphan; Cy, cyclophosphamide; TBI, total-body irradiation; CR, complete response | |||
| PBSC vs BM2,4 | ↔ | ( ↓ ) | (↑ ) |
| Bu*/Cy vs TBI/Cy2,5–7 | (↓ ) | ( ↓ ) | (↑ ) |
| Intensified vs standard conditioning8,9 | ↑ | ↓ | ↓ |
| Dose-reduced vs standard conditioning12 | ↓ | ↑ | ↔ |
| Donor: HLA-ident sibling vs matched unrelated donor16,17 | ↓ | (↑ ) | ↑ |
| Donor: Syngeneic vs HLA-ident sibling20 | ↓ | (↑ ) | ↑ |
| Induction chemotherapy: if CR | ↓ | ↓ | ↑ |
| if no CR23,24 | ↑ | ↑ | ↓ |
| Comorbidities13 | ↑ | ↔ | ↓ |
| Higher age1–3 | ↑ | (↑ ) | ↓ |
| Unfavorable cytogenetics39,40 | ↔ | ↑ | ↓ |
Results of epigenetic modulation prior or after allogeneic stem cell transplantation (allo SCT) for myelodysplastic syndrome (MDS).
| Author . | n . | Indication . | Remission . | Outcome after allo SCT . |
|---|---|---|---|---|
| Abbreviations: CR, complete response; PR, partial response; TRM, treatment-related mortality; ORR, overall response rate; EFS, event-free survival; GVHD, graft-versus-host disease; cGVHD, chronic GVHD; AML, acute myeloid leukemia | ||||
| Lübbert33 | 10 | Decitabine prior allo SCT | 40% CR/10% PR | 33% relapsed/33% alive/33% TRM |
| De Padua34 | 12 | Decitabine prior allo SCT | 33% CR/50% PR | 75% alive/17% relapsed |
| McCarty35 | 25 | Azacytidine prior allo SCT | 52% ORR | Med EFS for azacytidine resp. pts: not reached for azactidine refractory patients: 7 months |
| Czibere52 | 6 | Relapse after allo SCT (azacytidine with DLI) | CR (n = 3) PR (n = 2) | No acute GVHD/cGvHD (n = 2); relapse (n = 3) |
| De Lima48 | 40 (AML+MDS) | Maintenance (azacytidine dose-finding study) | n.a | Mild toxicity, no increase of GvHD, relapse (n = 11) |
| Author . | n . | Indication . | Remission . | Outcome after allo SCT . |
|---|---|---|---|---|
| Abbreviations: CR, complete response; PR, partial response; TRM, treatment-related mortality; ORR, overall response rate; EFS, event-free survival; GVHD, graft-versus-host disease; cGVHD, chronic GVHD; AML, acute myeloid leukemia | ||||
| Lübbert33 | 10 | Decitabine prior allo SCT | 40% CR/10% PR | 33% relapsed/33% alive/33% TRM |
| De Padua34 | 12 | Decitabine prior allo SCT | 33% CR/50% PR | 75% alive/17% relapsed |
| McCarty35 | 25 | Azacytidine prior allo SCT | 52% ORR | Med EFS for azacytidine resp. pts: not reached for azactidine refractory patients: 7 months |
| Czibere52 | 6 | Relapse after allo SCT (azacytidine with DLI) | CR (n = 3) PR (n = 2) | No acute GVHD/cGvHD (n = 2); relapse (n = 3) |
| De Lima48 | 40 (AML+MDS) | Maintenance (azacytidine dose-finding study) | n.a | Mild toxicity, no increase of GvHD, relapse (n = 11) |
Perspectives of allogeneic stem cell transplantation (SCT) in myelodysplastic syndromes (MDS).
Perspectives of allogeneic stem cell transplantation (SCT) in myelodysplastic syndromes (MDS).
Disclosures Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: Azacitidine and decitabine and lenalidomide are not approved for MDS in Europe.
References
Author notes
Clinic for Stem Cell Transplantation, Medical University Hospital Hamburg-Eppendorf, Germany