Abstract
The wide clinical spectrum of von Willebrand disease (VWD), its complex pathophysiology and its classification into distinct quantitative (type 1 or type 3) and qualitative (type 2) types with further subtle distinctions have prevented most clinicians from establishing a straightforward approach to diagnosing and treating this inherited bleeding disorder. The results of studies involving large cohorts of patients with a wide range of bleeding manifestations and variable von Willebrand factor (VWF) reduction have recently become available. These data have allowed the proposal of minimal criteria for a clinically useful diagnosis and for differentiating patients with mild VWD from subjects with borderline or only slightly reduced VWF levels who will not benefit from a specific diagnosis. These criteria are based on measurement of VWF ristocetin cofactor (VWF:RCo), VWF antigen (VWF:Ag), factor VIII and a standardized bleeding score (BS). Demonstration of the inheritance of the disorder could help to classify patients for whom insufficient hemostatic challenges may produce a falsely reassuring BS (like in children). Using this approach, mild VWD appears to be mostly composed of type 1 cases. Complemented by the results of desmopressin trial infusion, these parameters form the basis for a clinically oriented classification of all forms of VWD and may be useful for selecting the best treatment according to the severity of the disease. Although few molecular data have revealed practical utility, there is no doubt that the clarification of the molecular pathophysiology of VWD has allowed the unification of this complex disorder into a simple conceptual framework. This framework underlies the proposed utilization of simple phenotypic markers for optimizing treatments in individual patients.
The main goal of the management of von Willebrand disease (VWD) is to provide effective control of bleeding manifestations while maintaining the highest quality of life. In addition, patients should receive adequate counseling, proportional to the actual severity of the disease and respectful of personal values. Achieving these goals is usually more difficult in VWD than in other mild bleeding disorders. In fact, the pathogenesis of bleeding in VWD is quite complex due to the pleiotropic effects of the genetic defect, which result in a variable reduction of plasma factor VIII and in a variable quantitative and/or qualitative deficiency of the circulating and intracellular multimeric species composing von Willebrand factor (VWF). Thus, bleeding manifestations are greatly variable in type, frequency and severity.
Molecular and clinical markers useful for predicting the clinical profile of the disease in individual patients are being continually identified and investigated. Despite some limitations, a major goal during the patient’s laboratory workup should be to retain only those genetic and phenotypic features useful for the patient and the treating physician. The patient should understand the nature of the bleeding manifestations and the risks and limitations that they may pose. The physician should individualize and optimize treatment.
Three broad approaches are usually considered. The first, limited to the mild forms of VWD, is centered around a supportive approach based on education of the patient and family regarding the use of ancillary measures or drugs such as anti-fibrinolytic agents. Specific treatments based mainly on administration of desmopressin or of VWF/Factor VIII (FVIII)-containing products are reserved for patients with intermediate or severe forms, depending on the disease characteristics and/or requirements of specific circumstances. In rare particular cases, long-term prophylaxis may be considered.
In this overview the authors attempt to offer guidance on how to select and use some of the phenotypic and molecular markers that can be collected during the diagnostic process to optimize patient management in light of the above mentioned principles. A brief description of VWD pathophysiology limited to features that might be useful for providing a treatment-focused classification is a good starting point.
Pathophysiology of VWD
VWF is a large non-enzymatic, multimeric glycoprotein required for the adhesion of platelets to the subendothelium as well as for platelet-to-platelet cohesion and aggregation.1,2 VWF also acts as the carrier of plasma FVIII. The building block of the multimers is a dimer composed of two pro-VWF subunits joined at their C-terminal ends through disulphide bonds. After moving into the Golgi apparatus, dimers are joined at their N-terminal ends to form multimers of increasing molecular weight (up to 20,000 Kd). After removal of the pro-peptide, multimers may be released into the circulation or secreted abluminally. Specific domains present in each VWF subunit promote interaction with platelet glycoproteins (Gp) Ib and Gp IIb/IIIa, FVIII and subendothelial collagen.
VWF is synthesized by endothelial cells and megakaryocytes. VWF is constitutively secreted by endothelial cells, but most of the circulating VWF is released from specialized storage sites through a stimulus-responsive pathway. Thus, physiologic agonists such as adrenalin and thrombin (or pharmacological agents such as desmopressin) release VWF from endothelial Weibel-Palade bodies or from platelet α-granules.3 VWF released from storage sites is composed of “unusually large” multimers that are usually not found in the circulation. A specific plasma protease called ADAMTS-13 rapidly proteolyses the bond between Tyr 1605 and Met 1606 (Tyr 842 and Met 843 of the mature subunit), generating the entire spectrum of circulating VWF species, ranging from the single dimer to multimers made of up to 20 dimers.4 Since larger multimers show increased affinity for platelet GpIb and GpIIb/IIIa receptors, they are considered to be more potent.
Based on its genetic transmission, plasma VWF/FVIII measurements and multimeric analysis, VWD is commonly classified into two distinct quantitative (type 1 and 3) types and one qualitative (type 2) type.5 Mechanisms responsible for the molecular defects of VWD and their effects on subunit and multimers are outlined in Tables 1 and 2 .
Type 1
Type 1, representing more than 90% of cases in non-specialized centers, is characterized by a partial quantitative deficiency with a variable and concomitant reduction of VWF activity (measured as ristocetin cofactor, VWF:RCo) and antigen (VWF:Ag). Thus, the VWF:RCo/VWF:Ag ratio is invariably near 1; FVIII is also reduced, although to a lesser extent. Multimers are usually normal or show only minor abnormalities not usually detectable with standard methodology.6 In the simplest case, a single allele missense mutation hampers the correct dimerization process. If only wild-type dimers undergo multimerization and subsequent secretion, VWF plasma levels of around 25 U/dL are expected (dominant negative effect on the normal allele). Thus, a qualitative defect at the molecular level appears as a purely quantitative defect at a plasma level. In some cases, compound heterozygosity, such as the co-inheritance of a missense mutation with a null mutation7 explains variable penetrance and expressivity within the same family. In other cases, rapid clearance of the secreted molecule may limit its exposure to ADAMTS-13 so that unusually large multimers may remain detectable, giving rise to a particular subtype called type 1 Vicenza (see Table 1 ).
As reflected in the updated classification, some cases can be classified as type 1 VWD even in the absence of any mutation and/or linkage with the VWF gene.8–10 Improved technology and molecular analysis carried out on c-DNA might well lead to the discovery of additional mutations in some of these cases. Nevertheless, patients usually show a very mild bleeding tendency and only borderline or minimally reduced VWF levels (ranging from 40 IU/dL to the lower limit of the reference interval). In some of these cases, epistasis exerted by modifier genes that also regulate VWF expression (like ABO blood groups) has been proposed. Genetic interaction should amplify the effect of a single null allele or of mutations not otherwise exerting a significant dominant negative effect on the normal allele. We suggest that these patients should be regarded more as people with a “risk factor” for bleeding rather than a genetic bleeding disorder, and we will refer to them as “low VWF subjects” (LVWFS)11,12 (see also the accompanying article by EJ Sadler, beginning on page 106).
Type 3
Type 3 VWD is a recessive disease (homozygous or double heterozygous) with very low prevalence (1–2 cases/million), characterized by the absence of VWF (or only trace amounts) in plasma and platelets, and by FVIII below 5 IU/dL.
Type 2
Type 2 VWD missense mutations usually have one of two main distinct effects or a combination of them. First, they may intrinsically impair specific functional domains of the basic subunit responsible for binding to GpIb (and possibly to IIb/IIIa) or to FVIII without a major impact on the multimerization process (type 2M, type 2N). Second and more frequently, mutations may severely affect multimerization (type 2A).5 In these cases, the diminished activity of VWF is mainly related to the selective lack of the intermediate and large multimers. In both instances, the qualitative nature of the defect can be inferred from a greatly reduced VWF:RCo/VWF:Ag (type 2M and type 2A) or factor VIII/VWF:Ag (type 2N).13 Type 2B is characterized by gain-of-function mutations in theA1 domain causing increased affinity of VWF for platelet GpIb. Large multimers spontaneously bind to platelets and are subsequently removed from circulation so that these patients have a decreased proportion of large multimers and variable thrombocytopenia.
Minimal Diagnostic Criteria for a Clinically Useful Diagnosis of VWD
The diagnostic process for VWD should be initiated if one or more of the following is present: low VWF or a member of the family with established VWD or bleeding symptoms of unknown origin.14 Diagnosis of VWD should rest on three main criteria: demonstration of the congenital or inherited nature of the disorder (not required in recessive type 3 cases), a significant bleeding history and a low VWF.
Different practice or consensus-based-criteria have been proposed.15,16 Both of the two of the following criteria should be satisfied for VWD diagnosis: a bleeding score (BS, see below) that is significantly elevated (> 3 in men or > 5 in women) and VWF:Ag or VWF:RCo levels less than 40 IU/dL (either confirmed or consistently reported in patient clinical records). For children and young subjects with few historical hemostatic challenges, the requirement for a high BS is less stringent. VWD type 2N may only become apparent with a reduced FVIII:C level.
These minimal criteria stem from the results of several recent international studies17–20 and were explored using a Bayesian approach in type 1 VWD.21 For the definition of significant bleeding, the overall bleeding tendency of the patient may be more useful than the presence of a single severe symptom. Therefore, the patient should be interviewed about the lifelong bleeding history to collect information about all possible individual symptoms. Using a standardized questionnaire and quantification method (the BS),21,22 we consider a bleeding history significant and specific when the BS is > 3 in adult males or > 5 in adult females.17 With these “minimal” criteria the Bayesian calculation allows the estimation of the odds of having VWD (against not having it) as approximately 4 to 1 [in terms of probability, 80/100 (80%) of such patients will have VWD]. Our group has also explored the contribution of different ranges of VWF levels and of evidence of inheritance to the certainty of final diagnosis using this approach.21 Inheritance may be particularly useful for mild forms of VWD. The reader is referred to a recent publication that extensively discusses all of these aspects.23
The Clinical Spectrum of VWD and Its Implications for the Management of Patients
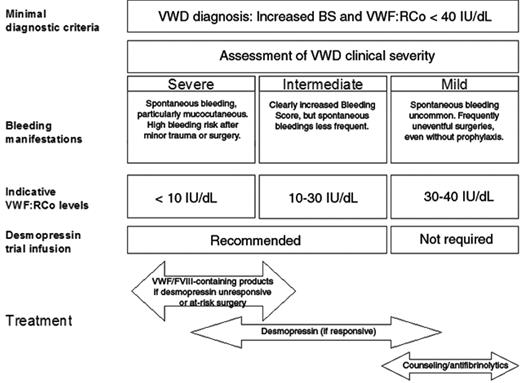
Although the proposed criteria exclude most irrelevant and/or dubious cases and LVWFS from being diagnosed with VWD, the clinical spectrum of “true” VWD remains highly variable. Classifying patients into four broad categories (severe, intermediate, mild VWD, non VWD) has an immediate practical impact (Figure 1 ).
Severe VWD
Severe VWD patients invariably report a life-long history of spontaneous bleeding symptoms (particularly mucocutaneous). They may present clinical symptoms, including arthropathy, indistinguishable from those of moderate (levels 1 – 5 IU/dL) hemophilia A and B (eg, type 3 patients with a homozygous null allele) and are at high risk of bleeding even after minor trauma or surgery
Intermediate VWD
Intermediate VWD patients usually have a less striking bleeding history, although their BS is almost always increased. Distinguishing severe from intermediate patients may be relevant for counseling and prognosis. For treatment, severe and intermediate patients may be grouped together, with the notable distinction that lifelong prophylaxis, a practice that should still be regarded as experimental, should be reserved only for severe patients with frequently recurrent major bleedings.24 Severe/intermediate patients should always receive anti-hemorrhagic prophylaxis before surgery or invasive procedures. In addition, any alarming bleeding episode that interferes with patient daily activities should be adequately treated. Desmopressin is the first choice of treatment in all patients who prove to be responsive to this agent, and patients who do not show a satisfactory response to desmopressin should receive VWF/FVIII-containing products.
Mild VWD
Patients with mild VWD (mainly type 1) only rarely suffer from spontaneous bleedings that are significant, and a history of uneventful surgeries is not uncommon, even without prior anti-hemorrhagic prophylaxis. From a practical point of view, patients with mild VWD should be managed conservatively. Our practice is to warn these patients against inappropriate use of antiplatelet drugs, to advise them about the use of anti-fibrinolytic drugs for invasive procedures or surgery on mucous tissues (eg, dental extraction), and to offer consultation on an as-needed basis. Patients with mild VWD almost invariably have desmopressin-responsive type 1 VWD; thus, for the rare circumstances of inadequate control of bleeding with the previous measures, we recommend resorting to desmopressin even in the absence of results of a previous trial infusion.
Molecular and Phenotypic Tools in the Management of VWD and Selection of Candidates for Desmopressin Trial Infusion and Treatment
There is no single clinical or laboratory tool that convincingly classifies a patient within one of the above mentioned clinical severity categories, and although some phenotypic or molecular data might be useful, their use and interpretation is still rather subjective.
Bleeding Symptoms
The predictive value of the bleeding history has been evaluated in few retrospective studies. In the international multicenter study, only 1 patient experienced severe bleeding requiring plasma transfusion out of 15 obligatory carriers of VWD who had a normal bleeding history at the time of evaluation (6.6%).17 Furthermore, when the association between spontaneous, mucocutaneous bleeding symptoms (epistaxis, cutaneous bleeding, menorrhagia) and bleeding after surgery or tooth extraction was evaluated in patients with type 1 VWD, the BS was comparable or superior to the VWF measurement for the prediction of surgical bleeding.19 Thus, patients with a low BS may possibly be considered as at a lower bleeding risk.
Measurement of VWF
As mentioned above, measurement of plasma VWF is the second basis for assessing severity of VWD patients. VWF levels below 30 IU/dL have been shown to be strongly associated with a significant BS19,20 and with a higher probability of linkage with the VWF locus8,25 or the presence of a specific mutation.9,10 Given these data, plasma VWF levels below 30 IU/dL (measured as VWF:Ag or VWF:RCo in type 1 and 3 or as VWF:RCo in type 2) should be considered as a marker of intermediate VWD. Patients with VWF levels between 30 and 40 IU/dL should be considered for diagnosis of VWD only if the BS is higher than 3 (or 5 in females). This criterion has not been validated for children who often have fewer hemostatic challenges and accordingly a lower score. Patients with type 2 usually have VWF:RCo levels below 30 IU/dL and almost invariably intermediate VWD. Patients with severe symptoms may be found for all types of VWD when VWF:RCo and/or factor VIII is very low (< 5–10 IU/dL) and functional VWF can not be released by the regulated pathway. The lack of releasable pool of functional VWF is reflected by the results of the desmopressin infusion trial. Given the intra-individual variability of VWF measurements over time, measurement of VWF on at least two occasions is recommended and the average value should be considered.
Other Specialized Tests
There is little available evidence that measurement of other VWF-related activities, such as collagen binding (VWF:CBA), could add to the diagnosis and management of VWD patients. However, measurement of FVIII:C is frequently used to monitor the response to desmopressin or VWF/FVIII containing products. A reduced FVIII:C/VWF:Ag ratio could be suggestive of the type 2N variant, which calls for testing the VWF affinity for FVIII in patient plasma using a more specific assay. Ristocetin-induced platelet agglutination (RIPA) in platelet-rich plasma allows the identification of type 2B, for which desmopressin is relatively contraindicated due to the risk of inducing thrombocytopenia. Recently, assay of the VWF propeptide has been shown to be useful for the identification of patients with reduced VWF survival rates, such as those with Vicenza type 1.26 Since these patients usually show a sustained, albeit short, response to desmopressin it is still debated whether the use of concentrates should be preferred over desmopressin. In our center, desmopressin remains the first therapeutic option for these patients. Thus, routine measurement of the VWF propeptide is still not recommended.
Molecular Analysis and Therapeutic Correlates
Whether the knowledge of a specific VWF mutation can influence therapeutic decisions is still a matter of debate. Missense mutations in exon 28 of the VWF gene, which codes for the A1 and A2 domains of mature VWF, are the most frequent causes of type 2A and 2B. Some type 2A mutations (see Table 3 ) do not prevent a hemostatically effective response to desmopressin.27 It has been recently observed that patients with some type 2B mutations do not have thrombocytopenia even after desmopressin administration despite showing enhanced RIPA. Thus identification of these mutations may be useful for optimizing treatment.28 Type 2M is caused by a variety of unique mutations in exon 28, usually with dominant negative effects, and with heterogeneous responses to desmopressin.29,30
Missense mutations in the FVIII-binding domain at the N-terminus of VWF are responsible for type 2N.29 These mutations may cause significant symptoms only in the homozygous or compound heterozygous state. The identification of a type 2N mutation is important for genetic counseling to exclude the presence of hemophilia A. The majority of these patients respond to desmopressin even though the half-life of FVIII:C may be rather short.30,31 In case of major surgery, VWF/FVIII-containing products should be preferred.
Molecular genotyping of type 3 VWD may be rather difficult since different types of mutations scattered over the entire gene may occur in homozygous or compound heterozygous states.32 These patients usually do not respond to desmopressin. The identification of patients with homozygous partial or whole gene deletion may be useful since they are at increased risk of inhibitor development with an associated risk of severe allergic reaction upon treatment with exogenous VWF.33
The MCMDM-1 VWD and the Canadian VWD studies9,10 have demonstrated that mutations observed in type 1 VWD largely differ from the typical null alleles observed in type 3 VWD. Not all of the identified mutations show a consistent correlation with the phenotype in all carriers of the mutation (eg, Y1584C), which suggests that some mutations are not entirely causative but that they may interact with other genes and modulate the severity of phenotype. The vast majority of type 1 patients show a good response to desmopressin. However, a subset of patients has been identified who show a shortened survival of FVIII and VWF after desmopressin treatment unrelated to ADAMTS-13 activity.31 R1205H (VWD Vicenza) is the epitome of this subset.34
Selection of Patient Candidates for Desmopressin Trial Infusion or Treatment
Desmopressin is usually administered at a dose of 0.3 μg/kg body weight by a subcutaneous or intravenous route or at a fixed dose of 150 to 300 μg by a metered-dose intranasal spray. A trial infusion with desmopressin should always be considered in severe/intermediate cases irrespective of their type, with the exclusion of type 2B and type 3. Blood sampling at least 1 and 4 hours after infusion is required to identify patients with shortened VWF survival and to schedule further infusions at appropriate times as needed. In type 3 cases, there is a consistent lack of a clinically useful response, and thus they are not worthy of a trial infusion. In most type 2 cases, phenotypic and molecular markers do not correlate with desmopressin responsiveness, and patients with a definite or suspected type 2 diagnosis should not be excluded from receiving a trial infusion during their diagnostic process. On the contrary, patients with mild VWD (VWF:RCo > 30 IU/dL and BS ≤ 3 or ≤ 5 in males and females, respectively) and a VWF:RCo/VWF:Ag near the unit invariably respond to desmopressin, so they may be treated with desmopressin when required without a trial infusion. Details for desmopressin trial infusion schedules and interpretation of the results, treatment protocols and related risks in adults and children can be found in many references.12,23
Selection of Patients for Treatment with VWF/FVIII-containing Products
VWF/FVIII concentrates are the treatment of choice for type 3 and type 2B patients and severe/intermediate patients who are not responsive to desmopressin.35 Replacement therapy is also required for patients responsive to desmopressin who are undergoing major surgery (eg, neurosurgery), procedures with high bleeding risk, or those in which even minor bleeding should be avoided. Furthermore, treatment with VWF/FVIII-containing products should be considered for patients for whom desmopressin may be contraindicated, such as patients with overt cardiovascular disease or very young children. Commercially available VWF/FVIII-containing products feature a variable content of FVIII and VWF, and high post-infusion levels of VWF are consistently obtained with plasma-derived FVIII concentrates.36 Products labeled for VWF content and with a VWF/FVIII ratio higher than 1 should be preferred. The goal of treatment in patients undergoing major surgery is to maintain FVIII plasma levels around 80 to 100 IU/dL for at least a couple of days and above 50 IU/dL for an additional 5 to 7 days thereafter. A loading dose of 50 U/kg of VWF is usually given in severe cases 1 hour before surgery, followed by similar daily doses for the next 2 days. Furthermore, a FVIII increase lasting for up to 24 hours and much higher than predicted is observed because of the stabilizing effect of exogenous VWF on endogenous FVIII, which is synthesized at a normal rate in these patients. High levels of post-infusion FVIII:C have been recently associated with episodes of deep vein thrombosis that have been reported in VWD patients receiving repeated infusions of FVIII/VWF concentrates following surgery.37 Since FVIII:C reliably predicts delayed bleeding, daily monitoring of FVIII:C during the first 5 to 7 days after major surgery is advised. VWF level monitoring is not strictly required. For patients undergoing minor surgery or invasive procedures (eg, dental extractions) a FVIII level around 30 to 50 IU/dL for at least 12 to 24 hours is required. In some instances (eg, patients with a good but short-lived response to desmopressin), treatment with VWF/VWF-containing products may be advisable as an adjunctive treatment. Recombinant FVIII or products obtained by immunoaffinity chromatography (FVIII > 2,000 IU/mg) should be the preferred choice in type 3 patients with VWD with homozygous gene deletions or nonsense mutations, since these patients may develop alloantibodies with the risk of life-threatening anaphylaxis.38
Women with VWD
Women with VWD are at particular risk of bleeding during their lifetime and usually their BS is increased compared with males with a VWD of similar phenotype due to menorrhagia and postpartum bleeding.17 Severe hemorrhage at menarche may be the first manifestation of VWD in young girls.
Menorrhagia
In some studies, up to 20% of women with menorrhagia turned out to have mild VWD.39 Using a standardized questionnaire, two recent studies reported menorrhagia in up to 70% of women with VWD, usually of increased severity as compared with age-matched women with menorrhagia and no bleeding disorders.40 Treatment options are similar to those adopted in women without a bleeding disorder, with the exception of desmopressin or therapy with VWF/FVIII-containing products. In general, it seems preferable to avoid combined oral contraceptives (COC) at menarche or in young girls if the patients respond to antifibrinolytics, such as (oral or parenteral) tranexamic acid (15–25 mg/kg body weight) given 3 times a day for 4 to 5 days or aminocaproic acid (50–70 mg/kg every 4 hours) for the same time period. Oral contraception may be preferred for older individuals. Recently, a levonorgestrel-releasing intrauterine device (trade name: Mirena), proven to be efficacious and safe in primary menorrhagia, was also proposed for use in women with inherited bleeding disorders.41 Iron supplementation is invariably required.
Desmopressin has been successfully used in a concentrated formulation for subcutaneous injection or as an intranasal spray formulation42,43 for home treatment with an effective response in about 85% of instances even though a crossover trial of nasal spray versus placebo failed to demonstrate a substantial benefit.44 Over the last 20 years in our center, we have not performed any hysterectomies related to menorrhagia that is exclusively related to VWD.
Delivery
Women with VWD experience an increased risk of postpartum hemorrhage if untreated (up to 29% compared with an incidence of 3% to 5% in the general population).45 A recent case-control study of 4067 deliveries by VWD patients showed that VWD women have a higher risk of primary postpartum hemorrhage (OR 1.5) and a fivefold greater risk of being transfused.46 However, the risk of severe postpartum bleeding is mainly restricted to type 3 or type 2, although patients with type 1 and severe phenotype (eg, VWD Vicenza) may also quite often experience significant bleeding. Patients with type 2 B may have an exacerbation of an existing thrombocytopenia, which adds to the risk of bleeding in these patients.28 In mild VWD (VWF > 30 IU/dL), FVIII and VWF usually normalize by the end of pregnancy and no specific treatment is required.
Antifibrinolytic agents during puerperium could be of help in cases of excessive bloody uterine discharge (lochia). For intermediate cases, a heterogeneous pattern of pregnancy-related FVIII/VWF changes is observed depending on the type of VWD, so follow-up during pregnancy is always recommended.47,48 Desmopressin is successful in preventing bleeding even in patients with intermediate phenotypes and shortened VWF survival, provided that the results of a previous trial infusions had demonstrated the achievement of safe levels.49 The compound can be administered immediately after umbilical cord sectioning and repeated after 12 to 24 hours up to 3 or 4 times as required. Fluid restriction is advised when repeated doses are anticipated. In women with severe/intermediate VWD who are unresponsive to desmopressin, FVIII and VWF concentrates (usually 40–50 U FVIII/kg b.w.) must be used immediately before delivery and additional lower doses (20–30 U/kg) administered 24 and 72 hours later. FVIII monitoring is advised in these cases to decide the best timing of infusions and dosing.
Social Considerations
In Western countries, there is an increased interest in so-called mild bleeding disorders.14 This interest is understandable in societies striving for the highest standards of efficiency, active life and avoidance of any discomfort. In this light, even a minor bleeding risk might seem unacceptable. On the other hand, it should be considered that current limitations in diagnostic tools could cause an exceedingly high number of false positive or useless diagnoses or, even worse, may result in an unjustified stigma of an inherited disorder, which is particularly true for VWD. An example of this problem can be found in the pursuit of a specific diagnosis in women with isolated menorrhagia.14 In a previous epidemiologic investigation using more relaxed criteria, the prevalence of VWD was found to be around 1%.50 The criteria that have been proposed in this paper try to balance the advantages and disadvantages of the diagnostic approach, and to include in the diagnostic process only those aspects that could generate useful information for counseling and treatment. It is likely that using the proposed criteria, the prevalence of clinically significant VWD could be estimated to be at least one logarithm lower.
Conclusions: A Simplified Classification of VWD for Treatment Decisions?
Outstanding progress has been made in unraveling the molecular basis of the various VWD types and in elucidating the pathophysiology of the disease. Nevertheless, few of these findings have practical implications for clinicians willing to fit patients into simple categories to guide diagnosis and therapeutic options. For this purpose, novel tests and multimeric analysis are not usually required. The contribution of molecular investigations to diagnosis is very limited and at present is reserved for cases with significant bleeding not fitting the proposed criteria. Thus, in the practical setting, diagnosis and treatment of VWD may turn out to be quite simple; clinicians are justified in stopping pursuit of diagnoses of dubious clinical utility. Excluding patients affected by severe deficiency due to recessive inheritance and those with hyper-responsiveness to RIPA, a trial infusion with desmopressin remains the fundamental step to be undertaken in all severe/intermediate cases. VWF/FVIII-containing products remain the mainstay of treatment for patients unresponsive or with contraindication to desmopressin. For these patients, the dosage should always take into account the VWF/FVIII content and ratio in the therapeutic material to avoid the risk of inadequate treatment or over-treatment.
Effects of different single allele mutations on the mature VWF subunit.
| Type of mutation* . | Effect on VWF subunit . | Consequence at the molecular level . |
|---|---|---|
| *Mutations occurring at the propeptide level may act through all of the above-mentioned mechanism, but intrinsic molecular abnormality is not expected. | ||
| †Sequence variations in regulatory regions of the gene may affect the efficiency of transcription. | ||
| Gene deletion or null allele Missense† | No production or unstable protein degraded intracellularly
| Decrease of VWF production
|
| Type of mutation* . | Effect on VWF subunit . | Consequence at the molecular level . |
|---|---|---|
| *Mutations occurring at the propeptide level may act through all of the above-mentioned mechanism, but intrinsic molecular abnormality is not expected. | ||
| †Sequence variations in regulatory regions of the gene may affect the efficiency of transcription. | ||
| Gene deletion or null allele Missense† | No production or unstable protein degraded intracellularly
| Decrease of VWF production
|
Predicted phenotype by type and number of mutations according to mechanisms presented in Table 1.
| Allele 1/Allele 2 . | Phenotype . | Type of VWD . |
|---|---|---|
| Normal/Normal | Normal | – |
| Normal/Null | ~50% VWF reduction in plasma and platelets | Normal (low VWF level); Carriers of type 3 |
| Normal/Missense | Mechanism a) of Table 1: ~75% VWF reduction in plasma (around 25 U/dL normal VWF in plasma) | 1) Type 1 |
| Mechanism b) of Table 1: abnormal multimers or intrinsic functional abnormality | 2) Type 2 VWD | |
| Missense/Missense | As above, increased severity | Type 1 or Type 2 VWD |
| Missense/Null | As above, increased severity | Type 1, 2 or 3 VWD |
| Null/Null | No VWF detectable in plasma and platelets | Type 3 |
| Allele 1/Allele 2 . | Phenotype . | Type of VWD . |
|---|---|---|
| Normal/Normal | Normal | – |
| Normal/Null | ~50% VWF reduction in plasma and platelets | Normal (low VWF level); Carriers of type 3 |
| Normal/Missense | Mechanism a) of Table 1: ~75% VWF reduction in plasma (around 25 U/dL normal VWF in plasma) | 1) Type 1 |
| Mechanism b) of Table 1: abnormal multimers or intrinsic functional abnormality | 2) Type 2 VWD | |
| Missense/Missense | As above, increased severity | Type 1 or Type 2 VWD |
| Missense/Null | As above, increased severity | Type 1, 2 or 3 VWD |
| Null/Null | No VWF detectable in plasma and platelets | Type 3 |
Main characteristics of von Willebrand disease types.
| Type of VWD . | Laboratory . | Multimers . | Mutations associated . | Comments . |
|---|---|---|---|---|
| * Mutation responsible for VWD in the original family described by E von Willebrand. | ||||
| Type 1 | Concurrent reduction of FVIII:C and VWF in plasma; VWF:RCo/VWF:Ag ≥ 0.6 | All multimers present; some minor abnormalities may be evident using sensitive methods | Missense mutations scattered over the entire gene. Possible dominant-negative effect. Y1584C in about10%, causative role uncertain.9,10 Single null allele not associated with bleeding | Usually co-dominant or dominant-negative. Ideal candidates for desmopressin. Short VWF half-life in VWD Vicenza (R1205H)34 and other rare mutations.31,53 |
| Type 2A | Usually VWF:RCo/VWF:Ag < 0.6 | Lack or relative decrease of the high molecular weight (HMW) and of intermediate multimers | Mutations in A2 domain; R1597W or Q or Y and S1506L represent about 60% of cases.51 | Usually co-dominant. Two mechanisms demonstrated by expression experiments. Group I: impaired secretion of HMW multimers, due to defective intracellular transport. Group II: normal synthesis and secretion of a VWF with greater susceptibility to in vivo proteolysis.52 Patients of the latter group may respond to desmopressin.27 |
| Type 2B | Usually VWF:RCo/VWF:Ag < 0.6; RIPA occurs at low ristocetin concentration | Lack of HMW multimers; a normal pattern is present in New York/Malmö variant | Mutations in A1 domain; 90% of cases are due to R1306W, R1308C, V1316M and R1341Q.51 P1266L associated with gene conversion and New York/Malmö phenotype.12 | Usually co-dominant. Enhanced affinity of abnormal VWF for platelet GpIb receptor. Thrombocytopenia after desmopressin and sometimes during pregnancy or stress situations; thrombocytopenia may aggravate bleeding risk conferred by the abnormal VWF.28 |
| Type 2M | Usually VWF:RCo/VWF:Ag < 0.6; | Large multimers present; inner abnormalities may be evident (eg, “smeary pattern”) | A few heterogeneous mutations recurrent (eg, R1315C, G1324S/A, R1374C/H) | Usually co-dominant. Some overlap with Type 2A may occur. Desmopressin may be useful in selected cases. |
| Type 2N | VWF may be normal or only slightly reduced; FVIII:C/VWF:Ag < 0.5; defective FVIII-VWF binding | All multimers present | Mutations in NH2-terminus; R854Q largely the most frequent mutation. | Usually recessive. Bleeding only for homozygosity or compound heterozygosity. Heterozygosity for R854Q in up to 2% of population in Northern Europe.7 Desmopressin may be useful for the majority of minor bleedings |
| Type 3 | Virtual absence of VWF; markedly reduced FVIII:C (< 5 IU/dL) | Lack of multimers | Mutations scattered over the entire gene, but some (eg, 2430delC exon 18* or Arg2535stop) are particularly recurrent in North Europe. High prevalence of null mutations (stop codons, frameshift, gene deletions).32,51 | Recessive. Homozygosity for gene deletion associated with an increased risk of inhibitor, causing anaphylactic reactions to exogenous VWF.33 Desmopressin does not work since cellular storage is devoid of VWF |
| Type of VWD . | Laboratory . | Multimers . | Mutations associated . | Comments . |
|---|---|---|---|---|
| * Mutation responsible for VWD in the original family described by E von Willebrand. | ||||
| Type 1 | Concurrent reduction of FVIII:C and VWF in plasma; VWF:RCo/VWF:Ag ≥ 0.6 | All multimers present; some minor abnormalities may be evident using sensitive methods | Missense mutations scattered over the entire gene. Possible dominant-negative effect. Y1584C in about10%, causative role uncertain.9,10 Single null allele not associated with bleeding | Usually co-dominant or dominant-negative. Ideal candidates for desmopressin. Short VWF half-life in VWD Vicenza (R1205H)34 and other rare mutations.31,53 |
| Type 2A | Usually VWF:RCo/VWF:Ag < 0.6 | Lack or relative decrease of the high molecular weight (HMW) and of intermediate multimers | Mutations in A2 domain; R1597W or Q or Y and S1506L represent about 60% of cases.51 | Usually co-dominant. Two mechanisms demonstrated by expression experiments. Group I: impaired secretion of HMW multimers, due to defective intracellular transport. Group II: normal synthesis and secretion of a VWF with greater susceptibility to in vivo proteolysis.52 Patients of the latter group may respond to desmopressin.27 |
| Type 2B | Usually VWF:RCo/VWF:Ag < 0.6; RIPA occurs at low ristocetin concentration | Lack of HMW multimers; a normal pattern is present in New York/Malmö variant | Mutations in A1 domain; 90% of cases are due to R1306W, R1308C, V1316M and R1341Q.51 P1266L associated with gene conversion and New York/Malmö phenotype.12 | Usually co-dominant. Enhanced affinity of abnormal VWF for platelet GpIb receptor. Thrombocytopenia after desmopressin and sometimes during pregnancy or stress situations; thrombocytopenia may aggravate bleeding risk conferred by the abnormal VWF.28 |
| Type 2M | Usually VWF:RCo/VWF:Ag < 0.6; | Large multimers present; inner abnormalities may be evident (eg, “smeary pattern”) | A few heterogeneous mutations recurrent (eg, R1315C, G1324S/A, R1374C/H) | Usually co-dominant. Some overlap with Type 2A may occur. Desmopressin may be useful in selected cases. |
| Type 2N | VWF may be normal or only slightly reduced; FVIII:C/VWF:Ag < 0.5; defective FVIII-VWF binding | All multimers present | Mutations in NH2-terminus; R854Q largely the most frequent mutation. | Usually recessive. Bleeding only for homozygosity or compound heterozygosity. Heterozygosity for R854Q in up to 2% of population in Northern Europe.7 Desmopressin may be useful for the majority of minor bleedings |
| Type 3 | Virtual absence of VWF; markedly reduced FVIII:C (< 5 IU/dL) | Lack of multimers | Mutations scattered over the entire gene, but some (eg, 2430delC exon 18* or Arg2535stop) are particularly recurrent in North Europe. High prevalence of null mutations (stop codons, frameshift, gene deletions).32,51 | Recessive. Homozygosity for gene deletion associated with an increased risk of inhibitor, causing anaphylactic reactions to exogenous VWF.33 Desmopressin does not work since cellular storage is devoid of VWF |
Clinical severity, VWF:RCo level and response to desmopressin offer preliminary guidance for the optimal management of most typical VWD patients. For particular cases (eg, type 2N or 2B), see text.
Clinical severity, VWF:RCo level and response to desmopressin offer preliminary guidance for the optimal management of most typical VWD patients. For particular cases (eg, type 2N or 2B), see text.
Disclosures Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Acknowledgments
The support of Fondazione Progetto Ematologia (Vicenza, Italy) and of Associazione Veneta per l’Emofilia e le Coagulopatie (Vicenza, Italy) is greatly appreciated.
References
Author notes
Department of Cell Therapy and Hematology, San Bortolo Hospital, Vicenza, Italy