Abstract
Major progress in understanding the pathogenesis in patients with thrombocytosis has been made by identifying mutations in the key regulators of thrombopoietin: the thrombopoietin receptor MPL and JAK2. Together, these mutations can be found in 50% to 60% of patients with essential thrombocythemia or primary myelofibrosis and in 10% to 20% of hereditary thrombocytosis. A decrease in expression of the Mpl protein can cause thrombocytosis even in the absence of mutations in the coding sequence, due to a shift in the balance between stimulation of signaling in megakaryopoiesis and removal of thrombopoietin by receptor mediated internalization in platelets. When present in a heterozygous state the JAK2-V617F mutation preferentially stimulates megakaryopoiesis and in most cases manifests as essential thrombocythemia (ET), whereas homozygous JAK2-V617F reduces megakaryopoiesis in favor of increased erythropoiesis, resulting in polycythemia vera and/or myelofibrosis. In 30% to 40% of patients with ET or primary myelofibrosis (PMF) and in 80% to 90% of pedigrees with hereditary thrombocytosis the disease-causing gene remains unknown. Ongoing genetic and genomic screens have identified genes that, when mutated, can cause thrombocytosis in mouse models. A more complete picture of the pathways that regulate megakaryopoisis and platelet production will be important for finding new ways of controlling platelet production in patients with thrombocytosis.
Thrombocytosis is defined as a platelet count exceeding 450 × 109/L in adults.1,2 This abnormality is classified as “primary thrombocytosis,” when the increase in platelets is caused by alterations targeting the hematopoietic cells, or as “secondary thrombocytosis” (also called “reactive thrombocytosis”), when the increase in platelets can be traced to an external cause, such as chronic inflammation, cancer, iron deficiency, or rebound after splenectomy. In most cases, thrombocytosis is first detected as an unexpected abnormality upon complete blood count examination. Secondary thrombocytosis is more frequent than primary thrombocytosis and rarely causes complications of its own.3,4 Management of secondary thrombocytosis is usually directed at treating the underlying disease. Primary thrombocytosis accounts for about 10% to 15% of de novo cases and in most instances represent essential thrombocythemia (ET), a subform of myeloproliferative neoplasms (MPN). At first consultation, about 20% of MPN patients already present with a complication, such as thrombosis or bleeding.5 Primary thrombocytosis can be subdivided into familial and sporadic disease. The boundaries of familial and sporadic forms of thrombocytosis are becoming less sharp, since some of the mutations first discovered in familial thrombocytosis can be also found in sporadic cases (eg, MPL-S505N),6 and a hereditary component is increasingly recognized to be contributing in patients with sporadic MPN, eg, increased risk for acquiring MPN in first-degree relatives of patients with MPN and presence of somatic mutations in JAK2-V617F in familial MPN.7 Clonal hematopoiesis is a hallmark of sporadic MPN, but clonality can be also found in familial forms of MPN.8,9 The diagnostic workup of a newly diagnosed MPN should follow the criteria defined by the World Health Organization (WHO).10,11 Although one could expect that thrombocytosis increases the risk of thrombosis, there is little evidence for such a direct link.12 Mouse models with extremely high platelet counts do not appear to have an increased incidence of thrombosis,13–15 and in patients with platelet levels above 1500 × 109/L thrombosis is paradoxically less frequent than bleeding, which occurs as a consequence of an acquired von Willebrand factor deficiency.12 Leukocytosis is suspected to be a contributing factor in the genesis of thrombotic complications in patients with MPN.16 Long duration of thrombocytosis is frequently associated with myelofibrosis, ranging from a mild increase in reticulin or collagen fibers to advanced osteosclerosis. High platelet count, increasing numbers of megakaryocytes in bone marrow and defects in megakaryocyte maturation appear to favor myelofibrosis through the release of pro-fibrotic mediators such as transforming growth factor beta (TGF-β) and platelet-derived growth factor (PDGF).17,18 The physiology of the growth factors, transcription factors, and the hematopoietic microenvironment that regulate platelet production are discussed in detail elsewhere.19 This review will focus on the pathophysiological mechanisms that can contribute to thrombocytosis.
Thrombocytosis Due to Overproduction of Thrombopoietin
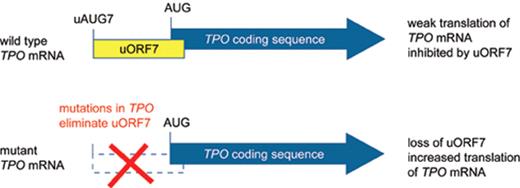
Thrombopoietin (Tpo) is the primary humoral regulator of platelet production.20 Injection of recombinant Tpo protein selectively induced thrombocytosis in vivo21 and forced expression of Tpo in mice by using retroviral vectors, adenoviral vectors or transgenes resulted in thrombocytosis of variable severity, mostly correlating with the Tpo expression levels.22–25 Tpo driven thrombocytosis in mice resulted in variable degrees of myelofibrosis26,27 but did not lead to leukemic transformation. In one report on using retroviral transduction of bone marrow cells a fatal myeloproliferative disease developed, and in two individual mice leukemic transformation was noted.28 It is not clear whether this is attributable to Tpo alone or may be caused by insertional mutagenesis by the retrovirus. The lineage-selective effects of Tpo overexpression in mice suggested that patients with thrombocytosis, polyclonal hematopoiesis and elevated Tpo serum or plasma levels could carry activating mutations in the TPO gene. Four different mutant TPO alleles have been described in families with autosomal dominant thombocytosis (Table 1 ).29–33 All of these mutations act by augmenting the efficiency of translation of the TPO mRNA (Figure 1 ).34 Translation of the TPO mRNA is physiologically inhibited by the presence of a conserved upstream open reading frame (uORF) in the 5′-untranslated region.35 The TPO mutations disrupt the inhibitory uORF and thereby relieve the physiological block that the uORF exerts on TPO translation, resulting in overproduction of Tpo protein. The natural course of the disease is difficult to study due to the low number of cases. The rate of thrombotic and hemorrhagic complications in 23 affected family members from two different kindreds with a G>C transversion in the splice donor of TPO intron 3 was comparable with that of patients with sporadic ET,36 suggesting that the disease is less favorable than generally assumed. However, most of the events in the affected family members were mild and no leukemic transformation occurred among the 23 family members. TPO mutations have so far only been detected in patients with familial thrombocytosis.37
Thrombocytosis Caused by Mutations in the Thrombopoietin Receptor
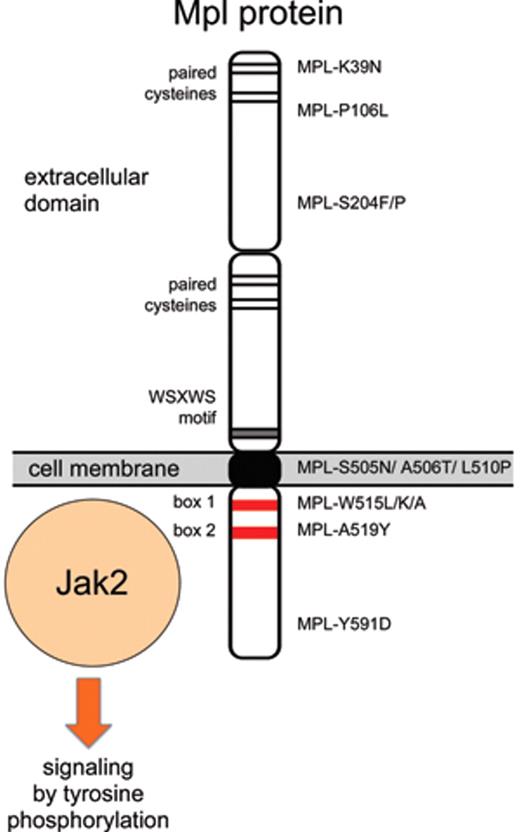
The thrombopoietin receptor (MPL) was discovered as a retroviral oncogene (v-mpl) that caused multilineage myeloproliferative leukemia.38 A mutation designed to dimerize the extracellular domain of mouse Mpl protein (Mpl-S386C) by allowing formation of a disulfide bridge between two receptor molecules induced constitutive activation of signaling in cell lines that were tumorigenic when injected into mice.39 These results suggested that mutations in MPL could be present in patients with MPN. However, initially no alterations were found when small numbers of patients were examined.40 The first MPL mutation, exchanging a serine in position 505 with an asparagine (MPL-S505N) in the transmembrane domain of Mpl protein, was discovered in a family with autosomal dominant thrombocytosis (Figure 2 ).41 Interestingly, the identical mutation was also found in a mutational screening using retroviruses in mice.42 Since then, the same S505N mutation has been detected in other families,43 and recently was found also as a sporadic mutation in ET.6 Mutations in position 515 of Mpl that exchange a tryptophan with leucine (MPL-W515L), lysine (MPL-W515K) or alanine (MPL-W515A) have been found in 3% to 5% of patients with ET and 5% to 8% of patients with primary myelofibrosis (PMF),6,44–46 but so far no familial cases have been reported. Both the familial mutation in position 505 and the sporadic mutations in position 515 render the Mpl protein active in the absence of ligand binding. The mechanism of how the S505N mutation, located in the transmembrane domain, activates the Mpl protein is currently unknown. The position W515 is located in the juxtamembrane portion of the cytoplasmic domain and is part of a conserved amphipathic motif (“RWQFP”) that has been shown to be important in maintaining the Mpl protein inactive in the absence of Tpo.47 The MPL-W515L/K/A mutations are therefore thought to interfere with the inhibitory effects of the RWQFP motif. Indeed, the MPL-W515 mutations induce factor-independent growth of BaF3 cells, and transplantation of retrovirally transduced bone marrow cells expressing MPL-W515L resulted in a severe MPN phenotype,44 demonstrating that this mutation is functionally relevant. Ligand-independent signaling by MPL-W515L requires the protein to reach the cell surface, whereas retention in the secretory pathway inactivates the protein, despite being associated with Jak2.48
In addition to the known mutation in positions 505 and 515, four new candidate mutations were identified by sequencing MPL in patients with PMF, resulting in amino acid substitutions S204P, A506T, L510P, and A519Y.49,50 However, expression of these mutant Mpl proteins in BaF3 cells failed to confer IL-3–independent growth or induce tumors in nude mice, suggesting that these sequence alterations alone are not sufficient to constitutively activate the Mpl protein. Since matched control samples were unavailable, these studies could not determine whether the observed sequence alterations were germline and therefore may represent rare polymorphisms. Additional MPL mutations in positions S204F and Y591D were found in MPN patients with uniparental disomy on chromosome 1p (1pUDP),51 but again expression of MPL-S204F or MPL-Y591D in BaF3 cells did not confer IL-3–independent growth,51 leaving the functional significance of these sequence alterations uncertain. As sensitive high-throughput sequencing methodologies are becoming more widely available, it is to be expected that more sequence alterations in MPL and other genes will be discovered in patients with MPN. The distinction between functionally relevant “driver mutations” and functionally irrelevant “passenger mutations” will become increasingly more important.52
A mild co-dominant activating allele was described in the N-terminal region of Mpl protein (MPL-K39N, also called MPL-Baltimore).53 Heterozygotes had mild thrombocytosis or platelet counts at the upper end of the normal range, whereas homozygotes showed thrombocytosis between 600 and 800 × 109/L.53,54 No data on how this mutation may activate signaling by the Mpl protein have been reported. About 7% of African-American individuals have been reported to be heterozygous for MPL-K39N.53 If we assume a Hardy-Weinberg equilibrium, then 0.1% of the African-American population is expected to be homozygous for the MPL-K39N mutation and to have thrombocytosis. Population-based studies are needed to test this assumption. Recently, another mutation in the extracellular domain of MPL (MPL-P106L) with a co-dominant transmission of thrombocytosis was found in families of Arab descent.55 Patients homozygous for MPL-P106L exhibited marked thrombocytosis (> 1000 × 109/L), while the heterozygotes either displayed mild thrombocytosis or normal platelet counts. The patients had elevated Tpo serum levels, suggesting that the MPL-P106L mutation may interfere with the internalization and/or degradation of Tpo, but apparently retains capacity for Tpo binding and activation of the receptor.
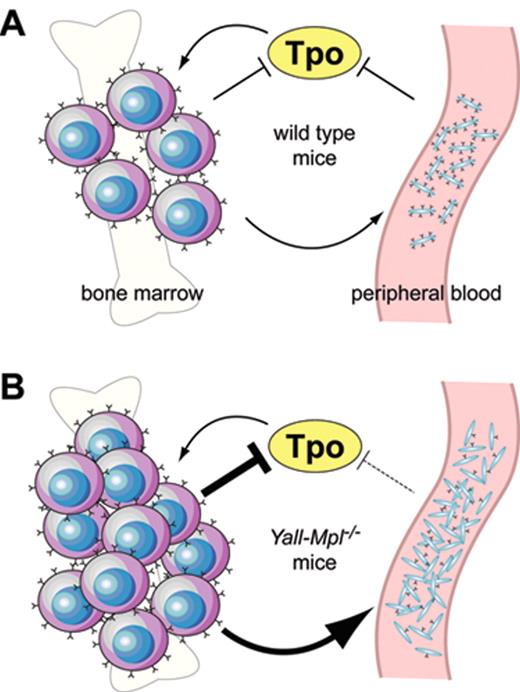
Ectopic overexpression of wild type murine Mpl cDNA by retrovirally mediated gene transfer suppressed megakaryopoiesis, but enhanced erythropoiesis in mice,23,44,48,56 presumably due to sequestering of Tpo by erythroid precursor cells expressing the Mpl protein. Conversely, decreased expression of the Mpl protein in platelets in the absence of a mutation in the MPL gene is a frequent finding in patients with MPN57,58 and appears to be due to impaired posttranslational processing of the Mpl protein in mega-karyocytes.59 The decrease in Mpl protein levels is inversely correlated to the JAK2-V617F mutant allele burden.60 However, decreased Mpl protein in platelets can also be found in patients with familial TPO mutation in the absence of mutated JAK2.61 The functional effect of decreased Mpl protein expression for thrombopoiesis in patients with MPN remains uncertain. Recently, two studies in transgenic mice showed that reduced expression of the wild type Mpl mRNA and protein during the terminal stages of megakaryopoiesis and on platelets can result in thrombocytosis even in the absence of a mutation in the Mpl coding sequence.62,63 To correct thrombocytopenia and decreased numbers of stem cells of Mpl knockout mice, both studies introduced a full-length Mpl cDNA as a transgene under the control of a 2 kb Mpl promoter into Mpl−/− mice. Unexpectedly, these mice exhibited thrombocytosis with platelet levels two- to fivefold higher than wild type controls, but only partial correction of the stem cell defect. The reintroduction of one wild type Mpl allele restored normal platelet counts, indicating that the transgene was not a dominant gain-of-function mutation. Instead, both groups found that the transgene expression driven by the 2 kb Mpl promoter fragment was decreased during late megakaryopoiesis, resulting in strongly diminished Mpl protein expression in large polyploid megakaryocytes and in platelets. The severe reduction of Mpl protein in platelets in these mice interferes with the auto-regulatory mechanism of Tpo that is exerted by normal platelets and thereby causes thrombocytosis (Figure 3 ). Decreased expression of Mpl protein was also observed in megakaryocytes from patients with MPN,58,59,64 but it is currently unclear whether this specifically affects late megakaryocytes, as observed in the mouse model.
Thrombocytosis Associated with Mutations in JAK2
The erythropoietin receptor (Epo-R), Mpl and to a large extent also the granulocyte colony-stimulating factor receptor (Gcsf-R) signal by associating with the Jak2 tyrosine kinase. It is therefore not surprising that a gain-of-function mutation in JAK2, such as JAK2-V617F, can cause hyperproliferation of the three corresponding lineages and frequently manifests as polycythemia vera (PV) with erythrocytosis, thrombocytosis and neutrophilia. What needs explanation is why some MPN patients lack the involvement of the erythroid lineage and display the phenotypes of ET or PMF. JAK2-V617F–positive ET was proposed to be a “forme fruste” of PV, based on the observations that ET patients with the JAK2-V617F mutation display platelet counts lower and hemoglobin levels higher than ET patients with normal JAK2.65 However, this concept has created some confusion, since two separate issues need to be looked at independently: First, it is not surprising that patients with ET may differ regarding the phenotype depending on whether ET is caused by JAK2-V617F or a mutation in an as yet unknown gene. ET patients with a mutation in MPL have clearly lower hemoglobin and higher platelet levels than patients with JAK2-V617F, and this is easily understandable knowing the functions of JAK2 and MPL. Second, the key question is what makes an ET patient with JAK2-V617F different from a PV patient with JAK2-V617F. Currently, the best answer is that ET patients with JAK2-V617F almost never progress to homozygosity, whereas PV patients with JAK2-V617F almost always have a subclone that is homozygous for JAK2-V617F, which can only be detected when single colonies are analyzed.66 Thus, there is a fundamental molecular difference between ET and PV patients rather than a “continuum,” as suggested by the “forme fruste” model. The currently available data are best compatible with a model in which erythropoiesis is only marginally stimulated, whereas megakaryopoiesis is strongly enhanced when the progenitors are heterozygous for JAK2-V617F. Conversely, erythropoiesis is strongly stimulated at the expense of megakaryopoiesis when the progenitors are homozygous for JAK2-V617F. It is currently unknown whether the “strength” or the quality of the signal is different between cells heterozygous or homozygous for JAK2-V617F. Another postulate of this hypothesis is that the homozygous subclone, which in some PV patients can be a minor fraction of the progenitors, most likely secretes a soluble or paracrine factor(s) that sensitizes the erythroid progenitors to produce more red blood cells. Expansion of the megakaryocytic lineage in response to JAK2-V617F has also been observed in serial samples from patients with refractory anemia with ring sideroblasts and thrombocytosis (RARS-T) that showed increasing thrombocytosis as the JAK2-V617F mutant allele burden increased over time.67 Further support for a model of differential responsiveness of the megakaryocytic versus erythroid lineages to the JAK2-V617F signals comes from studies with transgenic mice that conditionally express the human JAK2-V617F at different expression levels.13 Mice with a low expression of JAK2-V617F showed an ET phenotype with in some cases extremely high platelet levels (up to 15× normal), whereas mice that expressed JAK2-V617F at levels comparable to the endogenous wild type mouse Jak2 displayed a PV phenotype.13 Effects of genetic background can be excluded, as these mice were in an inbred C57/BL6 background. Again, it remains to be determined whether the absolute expression levels or the ratio of the mutant versus wild type Jak2 determine the presence or absence of an erythroid phenotype. Interestingly, JAK2 exon 12 mutations are sufficient to cause erythrocytosis when heterozygous. Target proteins, such as Stat5, appeared to be more strongly phosphorylated in cells expressing JAK2 exon 12 mutations than in cells transfected with JAK2-V617F.68
Other Regulatory Pathways Controlling Platelet Production
In 30% to 40% of patients with ET or PMF and in 80% to 90% of pedigrees with hereditary thrombocytosis the disease-causing gene remains unknown.9,69–71 It is likely that the genes mutated in these patients may modulate or bypass the effects of the key players TPO, MPL and JAK2. Patients with JAK2-V617F–positive ET showed detectable phosphorylation of Stat3 in granulocytes and elevated expression of Stat3 mRNAs, whereas patients with JAK2-V617F–negative ET lacked Stat3 phosphorylation and displayed a different expression profile, suggesting that the pathogenetic events in JAK2-V617F–negative ET do not involve activation of the Jak/Stat signaling pathway.72 In contrast, staining of bone marrow biopsies with antibodies specific for phosphorylated Stat3 and Stat5 identified subgroups of MPN with activated Stat3 and/or Stat5, but failed to detect a dependence on the presence or absence of JAK2-V617F.73 Thus, the subgroup of ET and PMF patients with as yet unknown mutations may turn out to be heterogeneous and could be at least in part caused by mutations in pathways other than Jak/Stat. The Myb/p300 pathway of transcriptional regulators was found in a recessive genetic suppressor screen to rescue thrombocytopenia of Mpl−/− mice.74 Loss of function mutations in the mouse Myb gene caused a myeloproliferative syndrome with increased megakaryocyte and platelet production in the absence of thrombopoietin signaling.74 Other mutations capable of suppressing thrombocytopenia in Mpl−/− mice were located in the transcriptional co-activator p300 that forms a complex with Myb,75–77 and in the pro-survival protein Bcl-xL that was shown to increase the platelet half-life.78 So far, mutations in these genes have not been detected in patients with thrombocytosis or ET. Also, genes encoding microRNAs may play a role in determining lineage choice between the erythroid and megakaryocytic lineage.79 The ongoing studies of inherited forms of thrombocytosis and the genomic screens will undoubtedly draw a more complete picture of how platelet production is regulated and which genes play a role in this process. This information will be important for finding new ways of controlling platelet production in patients with thrombocytosis.
Summary of TPO and MPL mutations associated with thrombocytosis.
| Authors . | Gene mutation . | Consequence . |
|---|---|---|
| TPO indicates thrombopoietin gene; uORF, upstream open reading frame; IVS, intron; UTR, untranslated region; MPL, thrombopoietin receptor (“myeloproliferative leukemia”); ET, essential thrombocythemia; PMF, primary myelofibrosis. | ||
| Wiestner29 | TPO, G>C in intron3 position +1 | Loss of uORF-mediated repression29 |
| Kondo,30 Ghilardi32 | TPO, deletion of G in 5′-UTR | Loss of uORF-mediated repression32 |
| Ghilardi31 | TPO, G>T in 5′-UTR | Loss of uORF-mediated repression31 |
| Jorgensen33 | TPO, A>G in intron3 position +5 | Not studied |
| Ding80 | MPL, G>A in exon 10 resulting in S505N in Mpl protein | Constitutively active Mpl protein |
| Moliterno53 | MPL-K39N | Co-dominant, mild thrombocytosis in homozygotes, function uncertain |
| El-Harith55 | MPL-P106L | Co-dominant, elevated Tpo serum levels |
| Kawamata51 | MPL-S204F | Found in uniparental disomy 1p, function uncertain |
| Williams 49 | MPL-S204P | Function uncertain |
| Komatsu41 | MPL-S505N | Constitutive activation of Mpl protein, autosomal dominant thrombocytosis |
| Chaligne50 | MPL-A506T | Function uncertain |
| Chaligne50 | MPL-L510P | Function uncertain |
| Pikman44 | MPL-W515L | Constitutive activation of Mpl protein, sporadic ET or PMF |
| Pardanani44 | MPL-W515K | Constitutive activation of Mpl protein, sporadic ET or PMF |
| Vannucchi46 | MPL-W515A | Constitutive activation of Mpl protein, sporadic ET or PMF |
| Chaligne50 | MPL-A519Y | Function uncertain |
| Kawamata51 | MPL-Y591D | Found in uniparental disomy 1p, function uncertain |
| Authors . | Gene mutation . | Consequence . |
|---|---|---|
| TPO indicates thrombopoietin gene; uORF, upstream open reading frame; IVS, intron; UTR, untranslated region; MPL, thrombopoietin receptor (“myeloproliferative leukemia”); ET, essential thrombocythemia; PMF, primary myelofibrosis. | ||
| Wiestner29 | TPO, G>C in intron3 position +1 | Loss of uORF-mediated repression29 |
| Kondo,30 Ghilardi32 | TPO, deletion of G in 5′-UTR | Loss of uORF-mediated repression32 |
| Ghilardi31 | TPO, G>T in 5′-UTR | Loss of uORF-mediated repression31 |
| Jorgensen33 | TPO, A>G in intron3 position +5 | Not studied |
| Ding80 | MPL, G>A in exon 10 resulting in S505N in Mpl protein | Constitutively active Mpl protein |
| Moliterno53 | MPL-K39N | Co-dominant, mild thrombocytosis in homozygotes, function uncertain |
| El-Harith55 | MPL-P106L | Co-dominant, elevated Tpo serum levels |
| Kawamata51 | MPL-S204F | Found in uniparental disomy 1p, function uncertain |
| Williams 49 | MPL-S204P | Function uncertain |
| Komatsu41 | MPL-S505N | Constitutive activation of Mpl protein, autosomal dominant thrombocytosis |
| Chaligne50 | MPL-A506T | Function uncertain |
| Chaligne50 | MPL-L510P | Function uncertain |
| Pikman44 | MPL-W515L | Constitutive activation of Mpl protein, sporadic ET or PMF |
| Pardanani44 | MPL-W515K | Constitutive activation of Mpl protein, sporadic ET or PMF |
| Vannucchi46 | MPL-W515A | Constitutive activation of Mpl protein, sporadic ET or PMF |
| Chaligne50 | MPL-A519Y | Function uncertain |
| Kawamata51 | MPL-Y591D | Found in uniparental disomy 1p, function uncertain |
Effect ofTPOgene mutations on the translational inhibition by upstream open reading frame (uORF) in the 5′-untranslated region of the TPO mRNA. The TPO coding region is shown as a thick blue arrow. A) Translation of normal TPO mRNA is physiologically almost completely inhibited by the presence of uORF7 in the 5′-UTR, which extends beyond the physiological start site. B) Mutations found in patients with hereditary thrombocytosis eliminate uORF7 in the 5′-untranslated region of the TPO mRNA and thereby allow the ribosome to efficiently initiate translation from the physiological translational start site (AUG).
Effect ofTPOgene mutations on the translational inhibition by upstream open reading frame (uORF) in the 5′-untranslated region of the TPO mRNA. The TPO coding region is shown as a thick blue arrow. A) Translation of normal TPO mRNA is physiologically almost completely inhibited by the presence of uORF7 in the 5′-UTR, which extends beyond the physiological start site. B) Mutations found in patients with hereditary thrombocytosis eliminate uORF7 in the 5′-untranslated region of the TPO mRNA and thereby allow the ribosome to efficiently initiate translation from the physiological translational start site (AUG).
Summary of established and putative MPL mutations found in patients with thrombocytosis.
Summary of established and putative MPL mutations found in patients with thrombocytosis.
Model illustrating a hypothetical shift of the Mpl-Tpo equilibrium inYall-Mpl−/− mice. (A) In wild type mice, both platelets in the periphery and megakaryocytes in the bone marrow act as negative regulators of Tpo through absorption via surface Mpl, restricting the expansion of the megakaryocytic lineage. (B) In Yall-Mpl−/− mice, platelets are almost devoid of surface Mpl, thus having a reduced capacity to absorb Tpo (dashed blunt arrow). Consequently, the megakaryocytic lineage expands until the combined amount of Mpl on megakaryocytes and platelets is sufficiently high to reduce Tpo concentration to normal levels. In this new equilibrium, the increased megakaryocyte mass has a more pronounced role in Tpo absorption than in the wild type equilibrium (large blunt arrow). Figure reprinted with permission from Tiedt et al. Blood. 2009;113:1768-1777 © the American Society of Hematology
Model illustrating a hypothetical shift of the Mpl-Tpo equilibrium inYall-Mpl−/− mice. (A) In wild type mice, both platelets in the periphery and megakaryocytes in the bone marrow act as negative regulators of Tpo through absorption via surface Mpl, restricting the expansion of the megakaryocytic lineage. (B) In Yall-Mpl−/− mice, platelets are almost devoid of surface Mpl, thus having a reduced capacity to absorb Tpo (dashed blunt arrow). Consequently, the megakaryocytic lineage expands until the combined amount of Mpl on megakaryocytes and platelets is sufficiently high to reduce Tpo concentration to normal levels. In this new equilibrium, the increased megakaryocyte mass has a more pronounced role in Tpo absorption than in the wild type equilibrium (large blunt arrow). Figure reprinted with permission from Tiedt et al. Blood. 2009;113:1768-1777 © the American Society of Hematology
Disclosures Conflict-of-interest disclosure: The author is a consultant for Genentech Inc. Off-label drug use: None disclosed.
References
Author notes
Experimental Hematology, Department of Biomedicine, University Hospital Basel, Basel, Switzerland