Abstract
Alpha thalassemia disorders are a group of hereditary anemias caused by absent or decreased production of the alpha chain of hemoglobin. Hemoglobin Bart’s hydrops fetalis is usually a fatal in-utero disease caused by absence of the alpha genes. However, the molecular and genotypic expression of hemoglobin Bart’s varies and increasing numbers of births are being reported. Population screening and prenatal diagnosis of at-risk couples is essential but often not performed. Most affected pregnancies are often undetected, resulting in severe fetal and maternal complications. Noninvasive monitoring by Doppler ultrasonagraphy with intrauterine transfusion therapy has changed the prognosis for this disorder. These advances in intrauterine and postnatal therapy have resulted in ethical dilemmas for the family and the provider.
Alpha thalassemia disorders are a group of hereditary anemias caused by absent or decreased production of the α chain of hemoglobin. They are one of the most common single gene disorders, affecting 5% of the world’s population.1–5 The severity of the α-thalassemia disorders is quite variable. At one extreme is a completely asymptomatic condition resulting from one of the four α genes being dysfunctional. In contrast, the deletion of all four α genes results in hemoglobin Bart’s hydrops fetalis, a fatal in-utero disease. In addition to the absence of four α genes, several other mutation combinations result in the phenotype of hydrops fetalis. Identification of at-risk couples by population screening is strongly recommended but largely not successfully implemented. The problem of hydrops fetalis is increasing worldwide, including in North America. As a result of changing demographics, α-thalassemia pregnancies are occurring in North America without adequate community education, prenatal counseling, and well-developed intrauterine management plans.6,7 The recognition of this problem has resulted in the establishment of a statewide newborn screening program for α-thalassemia and increased interest in global approach to this problem.8–10 The diagnosis, management and prognosis of homozygous α-thalassemia/hydrops fetalis is changing; advances in ante-natal diagnosis, intra-uterine intervention, and post-natal therapy have resulted in long-term survival of children previously felt to have an invariably fatal disease.7,11,12 The increasing recognition of this problem and controversies in therapy underscore hydrops fetalis as an important public health problem.
When both α genes on a single chromosome are inactive, the designation α0-thalassemia is used. When there is some production of α globin chains on that chromosome, α+-thalassemia is designated.
Alpha thalassemia is divided into deletional and non-deletional types (Tables 1 and 2 ).13 There are at least 40 different deletions. The size of the deletion is important and affects the clinical phenotype of hydrops fetalis. The α globin gene cluster occurs on the short arm of chromosome 16 and includes the α globin genes as well as the embryonic genes. Common α-thalassemia deletions that spare the embryonic gene allow for the production of functional embryonic hemoglobin early in gestation. In contrast, the large deletions lack the benefit of embryonic hemoglobin. These large deletions are particularly severe. Non-deletional mutations may have a more severe phenotype than most of the deletional mutations. The most common non-deletional α-thalassemia mutation is Hemoglobin Constant Spring; this mutation of the stop codon results in 31 amino acids being added to the α chain. Mutations of a terminal codon to a coding sequence often lead to an elongated α chain that is unstable and produced at a very limited rate.
Epidemiology
Alpha thalassemia is particularly common in China and Southeast Asia, with up to 40% of the regional population being carriers.1,2,4,5,13 One of the most frequent α-thalassemia mutations is the --SEA deletion, which deletes both α globin genes but spares the embryonic gene. Homozygosity for this deletion (--SEA) is the most common cause of hydrops fetalis.1,5 The sparing of the embryonic gene allows enough functional embryonic hemoglobin (Hemoglobin Portland 1 and Hemoglobin Portland 2) to allow gestation to continue and the phenotype of hydrops fetalis to develop. In contrast, other common α-thalassemia mutations (--FIL, --THAI) also lack the entire embryonic α globin cluster, and therefore do not produce the functional embryonic Hemoglobin Portland. These embryos may terminate unnoticed early in gestation.1 Over 5% of individuals in the Philippines are carriers for the --SEA or --FIL mutation. Hydrops fetalis, while most common in Southeast Asia, is found worldwide among many ethnic groups; --MED is a common α0-thalassemia mutation in Mediterranean regions, particularly Greece and Cyprus. It has resulted in hydrops fetalis. Non-deletional α-thalas-semia is found throughout the world. Up to 8% of Southeast Asians are carriers of Hemoglobin Constant Spring. In the Middle East, Hemoglobin αTSaudiα is a common α-thalassemia non-deletional mutation. It is a mutation of the polyadenylation signal sequence of the α 2 gene, resulting in decreased expression of structurally normal α chains. Hemoglobin Koya Dora, another structural non-deletional mutation, is found in India. Other structural mutations, such as hemoglobin Quong Sze found in Southeast Asia, are highly unstable and result in defects in the hem pocket.4,13–15
Hydrops Fetalis
The incidence of hydrops fetalis appears to be increasing.1,3,8–10 Advances in perinatal care and recognition of surviving homozygous thalassemia newborns has precipitated studies of long-term survivors with this disorder. Recently, the Newborn Screening Program of California reported 8 surviving α-thalassemia major newborns along with 500 Hemoglobin H babies.9 In southern China, the prevalence of α0-thalassemia trait is 8.5% and 0.23% of births had homozygous α-thalassemia.1,5,16,17 Non-immune hydrops fetalis is most often caused by α-thalassemia in these regions.5,18 In addition to China and Southeast Asia, Bart’s hydrops fetalis is now being recognized in Greece, Turkey, Cyprus, India, Sardinia, and other parts of the world.1,4,5,13,18
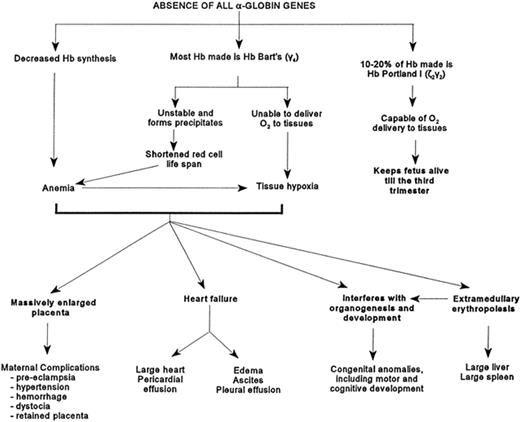
In the first 8 weeks of gestation, functional embryonic hemoglobin is responsible for oxygen delivery. (These embryonic hemoglobins are Hemoglobin Gower 1, Hemoglobin Gower 2, and Hemoglobin Portland). By 8 weeks of gestation, a switch to fetal hemoglobin production (Hemoglobin F α2/γ2) occurs. Since α globin chains are absent, hemoglobin F cannot be synthesized and hemoglobin Bart’s becomes the dominant hemoglobin. Hemoglobin Bart’s is a γ tetromere, which is unable to deliver oxygen to tissues because of its very high oxygen affinity. This results in a progressive severe anemia.19 Mid-trimester intrauterine hemoglobin analysis demonstrates an average hemoglobin (g/dL) of 6.4 in homozygous Bart’s (Table 3 ).19 This anemia underestimates the severity of hypoxia because hemoglobin Bart’s has no hem/hem interaction or Bohr effect and binds oxygen irreversibly tightly. There is severe ineffective erythropoiesis with marked extramedullary hematopoiesis (Figure 1 ). These factors result in massive organomegaly, severe albuminemia, and heart failure. This leads to gross body edema, growth failure, and intrauterine demise.
Most cases of α-thalassemia major, or hydrops fetalis, are consistent with the above description. However, there is a marked variability in the intrauterine clinical course of these mutations. Many of the pregnancies, especially those with large α-thalassemia deletions involving the embryonic gene, terminate unnoticed or early in gestation. Others continue throughout pregnancy and are born as stillbirths or critically ill. Some do not become hydropic and are born spontaneously.19–21
The environmental and genetic factors responsible for the diverse intrauterine course of hydrops fetalis are not all known. The specific α globin mutations are a major factor in the severity of the disease.1,13,22–24 Deletional, non-deletional, hyperunstable, and compound α globin mutations affect red cell survival. Severe hemoglobin H mutations can result in hydrops fetalis even when the hemoglobin Bart’s levels are as low as 40%. Hydrops fetalis from three-gene deletions include Hb H, --MED/αTSaudiα, and Hb H α-serine to proline/αFIL.1,6,13,23–26 In addition, hydrops fetalis has been reported from homozygous hemoglobin Constant Spring and other unexpected α-thalassemia combinations.13,25,27 Non-thalassemia factors may affect the occurrence and severity of hydrops.28 Hydrops fetalis caused by homozygous α-thalassemia, in combination with Rh antigen alloimmunization, has been observed. In some cases, the immune hemolytic anemia may worsen a hemoglobin H disorder so that it demonstrates the phenotype of α-hydrops.
Associated Fetal Anomalies
Developmental abnormalities are commonly seen in hydrops fetalis. At least 17% of newborns have one congenital anomaly.1,4,7,13,29 It is likely that fetal hypoxia disturbs organogenesis and fetal development. Most of these anomalies are mild and often affect the genitourinary system.7,30 In particular, hypospadias has been reported. Musculoskeletal defects, such as limb malformations, occur in 8% of fetuses.1,7,13,29 Skeletal malformations may be common because normal limb development occurs around 6 weeks of gestation. Many other anomalies have been reported including microcephaly, hydrocephaly, hypoplasia of lungs, anomalous genitalia, and cardiac defects.13,29,30 Central nervous anomalies are uncommon. A fetus with a malformation consisting of a neuronal migrational defect has been reported.29
Maternal Complications
Serious maternal complications may occur during the pregnancy. In fact, in women with a hydropic fetus who are not receiving adequate medical care, the maternal mortality rate could approach 50%.1,30 Sixty-one percent of women with an affected fetus develop hypertension, 30% develop severe preeclampsia, and 11% develop antepartum hemorrhage.30,31 Several other serious complications may occur including renal failure, premature labor, congestive heart failure, abruptio placenta, and oligohydramios.1,3,13,31 Overall, these are high-risk pregnancies and almost always associated with some obstetrical complication.
Prevention and Screening
Prevention of hydrops fetalis by education, counseling, and screening the at-risk population is the most effective therapy. The approach to screening and counseling is dependent upon community resources. Commonly, microcytosis utilizing an MCV < 82 fL and/or hypochromia (MCH < 27 pg) is often used as a population screening technique.1–4,17 Anemia is unreliable and is often not present in adults with two α globin gene deletions. Once iron deficiency is ruled out or corrected, microcytosis should be further evaluated in the individual and his or her partner in order to determine their risk for hydrops. Hemoglobin electrophoresis and quantitative hemoglobin A2 levels are often used to diagnose β-thalassemia carriers. In the past, diagnosis of α-thalassemia was made if the electrophoresis and hemoglobin A2 levels were normal. However, this is unreliable and will result in missed index cases.13 Many individuals are carriers of both α-thalassemia and β-thalassemia, and an elevated hemoglobin A2 level may mask a couple at risk.1,16 Molecular diagnosis for α globin mutations is essential for at-risk couples. When parents have α-thalassemia trait, DNA analysis on the fetus is required. Fetal tissue obtained by chorionic villus sampling early in the first trimester is indicated. Prenatal diagnosis for hydrops fetalis can also be conducted using fetal blood obtained by cordocentesis, or amniocentesis.
Prenatal Ultrasonography and Intrauterine Fetal Blood Sampling
Anemia increases fetal blood velocities by increasing cardiac output and decreasing blood viscosity.3,15,32–36 Ultrasound measurements can reliably detect fetal anemia. Doppler ultrasonography of the middle cerebral arteries report 88% sensitivity and 87% specificity for the prediction of fetal anemia.35 In addition to increased velocity, other ultrasound abnormalities develop in homozygous alpha thalassemia before hydrops fetalis occurs. Placentomegaly, cardiothoracic ratio abnormalities, and increased placental thickness can be detected as early as 12 to 14 weeks and predict the development of ascites and hepatomegaly of hydrops fetalis.14,15,32,34
Screening of the middle cerebral artery is recommended because it is easily visualized and correlates well to tissue hypoxia and anemia. Middle cerebral artery measurements can be initiated weekly as early as 16 weeks of gestation. The results can be plotted on a normal, validated curve. However, the actual values are most commonly converted to multiples of the median to account for changes in gestational age.33,34 While the peak middle cerebral artery Doppler measurement is highly reliable for anemia, its validity decreases following intrauterine transfusion.36 Therefore, serial empiric monitoring of the fetal hemoglobin after the first transfusion is generally necessary. Although direct measurement of fetal hemoglobin is associated with risks (such as bleeding or fetal bradycardia), the overall procedure-related pregnancy loss is approximately 1%.37
Intrauterine Transfusion Therapy
Most fetuses with homozygous α-thalassemia are not treated, and the majority of cases are most likely not detected. This has resulted in fetal loss and serious maternal complications, especially if the mother is not being monitored. Despite the lack of prenatal treatment, fetal survival is increasingly being recognized. Respiratory distress and severe anemia associated with marked hepatosplenomegaly have been noted in almost all of the births that did not receive intrauterine therapy. Those not transfused died shortly after birth. Long-term surviving patients had major neurologic deficits.1,20,38 In contrast, the outcome of approximately 20 patients receiving intrauterine therapy was markedly better7,12,38–41; long-term neurologic function of those patients transfused in utero appears good. Intrauterine transfusion therapy appears promising in minimizing the morbidity and mortality of homozygous α-thalassemia. However, most of these patients require lifetime transfusion therapy and chelation. Recent advances in stem cell transplantation have resulted in some patients being cured. Successful cases of related, unrelated, and mismatched stem cell transplantation for α-thalassemia major have been reported.40,41
Conclusion
In summary, homozygous α-thalassemia and hydrops fetalis is a complex, usually fatal disease that is devastating to the entire family. There is a diversity in the genotype and phenotype expression of this syndrome that presents challenges in at-risk couple counseling and population screening. Presently, counseling and testing of at-risk populations is inadequate. More cases are being diagnosed unexpectedly. Advances in diagnosis and treatment have raised ethical dilemmas for the patient and provider.
Classification of deletional α-thalassemia.
| Type of deletion . | Phenotype . | Number of examples recognized . | Examples . |
|---|---|---|---|
| *(α) indicates that the gene is present but non-functional. | |||
| †Loss of 16p13.3 may be the result of deletion, inversion plus deletion, formation of a ring chromosome 16 that lacks the a gene cluster or unbalanced inheritance of a derivative (16) lacking 16p13.3 from a parent who had a balanced translocation, eg, t(1;16), t(5;16) or t(16;20). | |||
| ‡(αα) indicates that both α genes are present but non-functional. | |||
| Reprinted with permission from Bain BJ. Haemoglobinopathy Diagnosis. 2nd ed. Malden, Mass.: Blackwell Publishing; 2006. | |||
| Deletion involving one or both α genes | |||
| Deletion of all or part of one α gene | α+ thalassemia | 7 | –α4.2, –α3.71, –α3.7H, –α3.7III, –α3.5, –α5.3*, –α2.7 |
| Deletion of all or part of both α genes, but without deletion of HS-40 | α0 thalassemia | 20 | – –SEA, – –THAI, – –MED, – –FIL, – –BRIT, – –SPAN, –(α)20.5*, –(α)5.2* |
| Deletion of both α genes and of HS-40 (100–250 kb) | α0 thalassemia | 8, without other phenotypic abnormality | – –DUTCH11 |
| Extensive loss of 16p13.3 (1–2 Mb) including both α genes and HS-40† | α0 thalassemia | 17, with mental retardation and dysmorphism | – –BO |
| Deletion of α1 gene and 18–20 kb downstream of α1 gene [17] | α0 thalassemia | 1 | (α)–ZF* |
| Deletion leaving α genes intact | |||
| Deletion of upstream major regulatory element (HS-40) without deletion of genes | α0 or very severe α+ thalassemia | 12 | (αα)RA,‡ (αα)TAT,‡ (αα)MM,‡ (αα)IJ‡ |
| Type of deletion . | Phenotype . | Number of examples recognized . | Examples . |
|---|---|---|---|
| *(α) indicates that the gene is present but non-functional. | |||
| †Loss of 16p13.3 may be the result of deletion, inversion plus deletion, formation of a ring chromosome 16 that lacks the a gene cluster or unbalanced inheritance of a derivative (16) lacking 16p13.3 from a parent who had a balanced translocation, eg, t(1;16), t(5;16) or t(16;20). | |||
| ‡(αα) indicates that both α genes are present but non-functional. | |||
| Reprinted with permission from Bain BJ. Haemoglobinopathy Diagnosis. 2nd ed. Malden, Mass.: Blackwell Publishing; 2006. | |||
| Deletion involving one or both α genes | |||
| Deletion of all or part of one α gene | α+ thalassemia | 7 | –α4.2, –α3.71, –α3.7H, –α3.7III, –α3.5, –α5.3*, –α2.7 |
| Deletion of all or part of both α genes, but without deletion of HS-40 | α0 thalassemia | 20 | – –SEA, – –THAI, – –MED, – –FIL, – –BRIT, – –SPAN, –(α)20.5*, –(α)5.2* |
| Deletion of both α genes and of HS-40 (100–250 kb) | α0 thalassemia | 8, without other phenotypic abnormality | – –DUTCH11 |
| Extensive loss of 16p13.3 (1–2 Mb) including both α genes and HS-40† | α0 thalassemia | 17, with mental retardation and dysmorphism | – –BO |
| Deletion of α1 gene and 18–20 kb downstream of α1 gene [17] | α0 thalassemia | 1 | (α)–ZF* |
| Deletion leaving α genes intact | |||
| Deletion of upstream major regulatory element (HS-40) without deletion of genes | α0 or very severe α+ thalassemia | 12 | (αα)RA,‡ (αα)TAT,‡ (αα)MM,‡ (αα)IJ‡ |
Classification of non-deletional α-thalassemia.
| Type of deletion . | Phenotype . | Number of examples recognized . | Examples . |
|---|---|---|---|
| Reprinted with permission from Bain BJ. Haemoglobinopathy Diagnosis. 2nd ed. Malden, Mass.: Blackwell Publishing; 2006. | |||
| RNA splice site mutation in α1 or α2 gene (donor or acceptor site) | α+ thalassemia | 3 (α2 donor site, α2 acceptor site, α1 acceptor site) | α2 IVS1 (–5nt) donor splice site mutation in Mediterranean area and Middle East |
| RNA polyadenylation signal mutations | α+–α0 thalassemia (ie, severe α +) or α+ | 4 (described only for α2 gene which is likely to account for the severe phenotype) | α2 AATAAA→AATAAG (αPA6A→Gα, αTSaudiα) |
| Imparied RNA translation consequent on initiation codon or intitiation consensus sequence mutation | α+ thalassemia, α+–α0, or when the mutation occurs in association with deletional α thalassemia, α0 thalassemia | 5 (2 in α2 gene, 1 in α1 gene, 2 in single α gene) | α2 ATG→ACG, GTG or A–G; –α3.7 ATG→GTG (mutation in association with deletion gives α0 phenotype) |
| Impaired RNA translation consequent on a frame shift or nonsense mutation | α+ or α0 thalassemia | 5 (4 frame shift plus 1 nonsense) | Codon 30/31 (–4nt) frame shift and α2 CD116 GAG→TAG nonsense mutation |
| Impaired RNA translation consequent on a terminiation codon mutation leading to an elongated mRNA and α globin chain | α+ thalassemia | 5 (all α gene) | Hemoglobin Constant Spring TAA→CAA (αCSα), hemoglobin Icaria TAA→AAA (αIcα), hemoglobin Koya Dora TAA→TCA, hemoglobin Seal Rock TAA→GAA, hemoglobin Paksé TAA→TAT |
| Production of highly unstable α chain as a result of point mutation or a small deletion | α+ thalassemia | At least 18: 14 point mutations, 4 small deletions; 11 affecting α2 gene, 4 affecting α1 gene and 3 affecting a single α gene | Hemoglobin Arginia (αAgrα), hemoglobin Petah Tikvah (αPT), hemoglobin Quong Sze (αQSα), hemoglobin Suan Dok (αSDα) and hemoglobin Evaston (point mutations); hemoglobin Taybe (small deletion) |
| Lack of a transactivating factor encoded by the ATRX gene | α+ thalassemia | ATR-X syndrome | |
| Type of deletion . | Phenotype . | Number of examples recognized . | Examples . |
|---|---|---|---|
| Reprinted with permission from Bain BJ. Haemoglobinopathy Diagnosis. 2nd ed. Malden, Mass.: Blackwell Publishing; 2006. | |||
| RNA splice site mutation in α1 or α2 gene (donor or acceptor site) | α+ thalassemia | 3 (α2 donor site, α2 acceptor site, α1 acceptor site) | α2 IVS1 (–5nt) donor splice site mutation in Mediterranean area and Middle East |
| RNA polyadenylation signal mutations | α+–α0 thalassemia (ie, severe α +) or α+ | 4 (described only for α2 gene which is likely to account for the severe phenotype) | α2 AATAAA→AATAAG (αPA6A→Gα, αTSaudiα) |
| Imparied RNA translation consequent on initiation codon or intitiation consensus sequence mutation | α+ thalassemia, α+–α0, or when the mutation occurs in association with deletional α thalassemia, α0 thalassemia | 5 (2 in α2 gene, 1 in α1 gene, 2 in single α gene) | α2 ATG→ACG, GTG or A–G; –α3.7 ATG→GTG (mutation in association with deletion gives α0 phenotype) |
| Impaired RNA translation consequent on a frame shift or nonsense mutation | α+ or α0 thalassemia | 5 (4 frame shift plus 1 nonsense) | Codon 30/31 (–4nt) frame shift and α2 CD116 GAG→TAG nonsense mutation |
| Impaired RNA translation consequent on a terminiation codon mutation leading to an elongated mRNA and α globin chain | α+ thalassemia | 5 (all α gene) | Hemoglobin Constant Spring TAA→CAA (αCSα), hemoglobin Icaria TAA→AAA (αIcα), hemoglobin Koya Dora TAA→TCA, hemoglobin Seal Rock TAA→GAA, hemoglobin Paksé TAA→TAT |
| Production of highly unstable α chain as a result of point mutation or a small deletion | α+ thalassemia | At least 18: 14 point mutations, 4 small deletions; 11 affecting α2 gene, 4 affecting α1 gene and 3 affecting a single α gene | Hemoglobin Arginia (αAgrα), hemoglobin Petah Tikvah (αPT), hemoglobin Quong Sze (αQSα), hemoglobin Suan Dok (αSDα) and hemoglobin Evaston (point mutations); hemoglobin Taybe (small deletion) |
| Lack of a transactivating factor encoded by the ATRX gene | α+ thalassemia | ATR-X syndrome | |
Hematology of red blood cell indices in mid-pregnancy fetuses (anova test before posthoc multiple comparisons).
| . | Fetuses (n = 86) . | |||
|---|---|---|---|---|
| Parameters (mean ± SD) . | Normal (n = 22) . | Alpha-1 trait (n = 40) . | Hb Bart’s disease (n = 26) . | P . |
| Reprinted with permission from Srisupundit K, Piyamongkol W, Tongsong T. Comparison of red blood cell hematology among normal, alpha-thalassemia-1 trait, and hemoglobin Bart’s fetuses at mid-pregnancy. Am J Hematol. 2008;83:908–910.19 | ||||
| Gestational age, weeks | 18.9 + 9.5 | 18.6 + 7.9 | 18.8 + 8.8 | .422 |
| Hb level, g/dL | 11.34 ± 0.83 | 10.42 ± 0.91 | 6.4 ± 1.64 | <.001 |
| MCV, fL | 123.40 ± 7.76 | 105.95 ± 7.35 | 96.26 ± 8.19 | <.001 |
| MCH, pg | 41.39 ± 3.35 | 33.71 ± 2.74 | 26.23 ± 2.6 | <.001 |
| MCHC, g/dL | 33.44 ± 1.3 | 31.81 ± 1.09 | 27.29 ±1.78 | .001 |
| . | Fetuses (n = 86) . | |||
|---|---|---|---|---|
| Parameters (mean ± SD) . | Normal (n = 22) . | Alpha-1 trait (n = 40) . | Hb Bart’s disease (n = 26) . | P . |
| Reprinted with permission from Srisupundit K, Piyamongkol W, Tongsong T. Comparison of red blood cell hematology among normal, alpha-thalassemia-1 trait, and hemoglobin Bart’s fetuses at mid-pregnancy. Am J Hematol. 2008;83:908–910.19 | ||||
| Gestational age, weeks | 18.9 + 9.5 | 18.6 + 7.9 | 18.8 + 8.8 | .422 |
| Hb level, g/dL | 11.34 ± 0.83 | 10.42 ± 0.91 | 6.4 ± 1.64 | <.001 |
| MCV, fL | 123.40 ± 7.76 | 105.95 ± 7.35 | 96.26 ± 8.19 | <.001 |
| MCH, pg | 41.39 ± 3.35 | 33.71 ± 2.74 | 26.23 ± 2.6 | <.001 |
| MCHC, g/dL | 33.44 ± 1.3 | 31.81 ± 1.09 | 27.29 ±1.78 | .001 |
Pathophysiology caused by the absence of the α-globin genes.1 Reprinted with permission from Chui DH, Waye JS. Hydrops fetalis caused by alpha-thalassemia: an emerging health care problem. Blood. 1998;91:2213–2222.
Pathophysiology caused by the absence of the α-globin genes.1 Reprinted with permission from Chui DH, Waye JS. Hydrops fetalis caused by alpha-thalassemia: an emerging health care problem. Blood. 1998;91:2213–2222.
Disclosures Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
References
Author notes
Children’s Hospital & Research Center Oakland, Oakland, CA