Abstract
The last decade has produced rapid progress in the management of chronic lymphocytic leukemia (CLL). Fludarabine, bendamustine and two monoclonal antibodies, alemtuzumab and rituximab, have been approved by the European and/or American regulatory agencies. Several, novel monoclonal antibodies targeting CD20, CD23 or CD40, as well as drugs designed to interfere with proteins regulating the cell cycle, apoptotic machinery, or leukemic microenvironment (eg, flavopiridol, oblimersen, or lenalidomide), are currently being tested in clinical trials. Furthermore, the increased experience with reduced-intensity allogeneic progenitor cell transplantation allows offering this option to physically fit patients. In addition, new prognostic markers that may influence therapeutic decisions have been identified. This review attempts to summarize the current use of these different modalities in CLL therapy.
Chronic lymphocytic leukemia (CLL) is a heterogeneous disease with an extremely variable course. Survival after diagnosis can range from months to decades. As the pathogenesis of the disease is increasingly better understood, we begin to unfold the molecular patterns that define the different prognostic subgroups and to develop strategies to predict the clinical course. In addition, a number of more potent treatment modalities have been designed during the last ten years that result in complete remissions (CRs) in almost 50% of patients and a treatment-free time of more than 5 years (Figure 1 ). However, CLL is the classical leukemia of the elderly and the treatment often must be tailored to the patient’s fitness level and ability to tolerate more toxic combination therapies. As a consequence, the therapy of CLL becomes increasingly personalized, requiring a detailed knowledge about the different diagnostic and therapeutic options. This review presents an update of our current understanding in this exciting and rapidly changing field.
Single Agents
Cytostatic Agents
Monotherapy with alkylating agents has served as initial, front-line therapy for CLL for several decades, and chlorambucil has been considered the “gold standard” for several decades.1 Even today, this drug remains an appropriate option, particularly in unfit, elderly patients. The advantages of chlorambucil are its low toxicity, low cost and convenience as an oral drug; its major disadvantages are its low to non-existent CR rate and some side effects that may occur after extended use (prolonged cytopenia, myelodysplasia and secondary acute leukemia).
Three purine analogues are currently used in CLL: fludarabine, pentostatin, and cladribine. Fludarabine remains by far the best studied compound of the three in CLL. Fludarabine monotherapy produces superior overall response (OR) rates compared with other treatment regimens containing alkylating agents or corticosteroids.2–4 Fludarabine induced more remissions and more CRs (7%–40%) than other conventional chemotherapies, such as CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone), CAP (cyclophosphamide, doxorubicin, prednisone), or chlorambucil, but did not improve overall survival when used as single agent.4–7 Similarly, cladribine monotherapy produced a higher CR rate than chlorambucil plus prednisone (47% vs 12%) without resulting in a longer survival.8
More recently, bendamustine, 4-[5-[Bis(2-chloroethyl)amino]-1-methylbenzimidazol-2-yl]butanoic acid, which has been used in Germany for more than 30 years, was compared with chlorambucil in a randomized trial (Table 1 ).9 The OR and median progression-free survival (PFS) rates were 67% and 22 months, respectively, for bendamustine versus 30% and 8 months for chlorambucil (both P < .0001). In 2008, bendamustine was approved by the US Food and Drug Administration (FDA) for the treatment of CLL.
While all these trials (Table 1 ) demonstrate that these drugs when used as single agents are more active than chlorambucil, their precise role in CLL management remains to be determined, since monotherapies have become the domain of elderly or unfit patients in whom drugs that carry more myelotoxicity than chlorambucil may not offer enough benefit to replace the old standard.
Monoclonal Antibodies
In CLL, the anti-CD20 monoclonal antibody rituximab is less active as a single agent than in follicular lymphoma, unless very high doses are used.10–11 In contrast, combinations of rituximab with chemotherapy have proven to be very efficacious therapies for CLL (see below).
Monotherapy with alemtuzumab, a recombinant, fully humanized, monoclonal antibody against the CD52 antigen, has produced response rates of 33% to 53% in patients with advanced or refractory CLL, with a median duration of response ranging from 8.7 to 15.4 months.12–14 Alemtuzumab has also proven efficacy in patients with high-risk genetic markers such as deletions of chromosome 11 or 17 [del(11q) and del(17p)] and p53 mutations.15–16 Therefore, alemtuzumab is a reasonable therapeutic option for patients with poor prognostic features. In a recent prospective randomized study alemtuzumab was tested against chlorambucil (Table 1).17 Alemtuzumab led to a greater rate of OR and CR (P < .0001), superior PFS with a 42% reduction in risk of progression or death (P < .0001) and significantly longer median time to progression (TTP) (P = .0001). Therefore, the drug has been recently approved by the US FDA as front-line therapy for CLL.
Combination Chemotherapy
Since purine analogs and alkylating agents have different mechanisms of action and partially non-overlapping toxicity profiles, it seemed logical to combine the two modalities to achieve synergistic effects. Preclinical in vitro studies demonstrated that exposure of CLL cells to fludarabine and cyclophosphamide resulted in synergistic cytotoxicity.18 Fludarabine has been evaluated in a variety of combination regimens. The combination of fludarabine with cytarabine appeared to be less effective than fludarabine alone, while the combination of fludarabine with chlorambucil or prednisone increased hematological toxicity without improving the response rate compared with fludarabine alone (response rates 27%–79%).4,19 The most thoroughly studied combination chemotherapy for CLL is fludarabine plus cyclophosphamide (FC) (Table 2 ) (reviewed in Hallek and Eichhorst19).
Three randomized trials have shown that the FC combination clearly improves the CR and OR rate and PFS as compared with fludarabine monotherapy (Table 2 ).20–22 An additional important result of these trials was that FC did not increase the rate of severe infections despite inducing more grade 3 and 4 neutropenias. A recent analysis of the CLL4 trial of the GCLLSG suggests that the first-line treatment of patients with CLL with FC combination may improve the OS of the non-high risk patients with CLL [all patients not exhibiting a del(17p) or p53 mutation].23
The addition of mitoxantrone to FC in 37 patients with relapsed/refractory CLL produced a high CR rate (50%), including 10 cases of minimal residual disease (MRD) negativity, with a median duration of response of 19 months.24 All MRD-negative patients were alive at analysis; the median duration of response had not been reached in the CR patients compared with 25 months in non-CR patients.
Since phase II data seemed to encourage the use of cladribine in combination with cyclophosphamide,25 a Polish study compared cladribine alone with cladribine combined with cyclophosphamide (CC) or to CC plus mitoxantrone (CCM) in 479 patients with untreated progressive CLL.26 The CC combination did not produce any benefit in terms of PFS or response rates when compared with cladribine alone. Compared with cladribine, CCM induced a higher CR rate (36% vs 21%, P = .004), and a trend for a higher CR rate with CC was observed (29% vs 21%, P = .08). The percentage of patients who were in CR and were MRD negative was higher in the CC arm compared with cladribine (23% vs 14%, P = .042). There were no differences in OR, PFS, and overall survival (OS) among treatment groups. Grade 3/4 neutropenia occurred more frequently in CC (32%) and CCM (38%) than with cladribine (20%) (P = .01 and P = .004, respectively). Infections were more frequent with CCM compared with cladribine (40% vs 27%, P = .02). Based on these results, cladribine combination therapies do not seem to offer a major advantage when used as first-line treatment for CLL.
Chemoimmunotherapy
Combinations Using Rtuximab
Since preclinical studies showed evidence for a synergy between rituximab and fludarabine,27 rituximab combinations with fludarabine-based regimens were investigated in several phase II trials. In a trial on 31 previously treated or untreated patients with CLL, the combination of fludarabine with rituximab (FR) showed 27 (87%) responses and 10 (32%) CRs.28 The CALGB 9712 protocol combined rituximab with fludarabine in either a sequential or concurrent regimen in a randomized study. Patients (n = 104) with previously untreated CLL received 6 cycles of fludarabine, with or without rituximab, followed by 4 once-weekly doses of rituximab.29 OR and CR rates were higher in the concurrent group (90% and 47% vs 77% and 28%). All patients of the CALGB 9712 protocol treated with fludarabine and rituximab were compared retrospectively with 178 patients from the previous CALGB 9011 trial, who received only fludarabine.30 The patients receiving fludarabine and rituximab had a better PFS and OS than patients receiving fludarabine alone. Two-year PFS probabilities were 67% versus 45%, and 2-year OS probabilities were 93% versus 81%.
Similarly, the combination of FC with rituximab (FCR) was investigated in a phase II trial on 300 patients with previously untreated CLL. FCR resulted in an OR rate of 95%, with CR in 72%, nodular PR in 10%, PR due to cytopenia in 7%, and PR due to residual disease in 6%.31 Six-year OS and failure-free survival were 77% and 51%, respectively. Median time to progression was 80 months.
These results led the GCLLSG to conduct a randomized trial:32 817 patients (median age 61 years) with good physical fitness were randomly assigned to receive 6 courses of FC (n = 409) or FCR (n = 408). Sixty-four percent were at Binet stage B, 32% Binet C and 5% Binet A. FCR induced a higher OR rate than FC (92.8% vs 85.4%) and more CR (44.5 vs 22.9) (P < .001). PFS at 2 years was 76.6% in the FCR arm and 62.3% in the FC arm (P < .01). FCR treatment was more frequently associated with CTC grade 3 and 4 neutropenia (FCR 34%; FC 21%), while other side effects were not increased. Treatment-related mortality occurred in 2.0% in the FCR and 1.5% in the FC arm. A systematic analysis of prognostic factors including molecular cytogenetics showed that the positive effect of FCR applied for most prognostic subgroups. However, FCR did not improve the PFS or OS of patients with a del(17p).
Similar results were obtained in a trial comparing FCR to FC in second-line treatment of CLL33: 272 patients were treated with FC and 274 with FCR. OR rates were 58% and 70% for FC and FCR, respectively, with 13% and 24.3% CR. Time to treatment failure was 20.6 versus 30.6 months. Taken together, these results suggest that the addition of rituximab to fludarabine-based therapies represents a significant advance in treatment of CLL (Table 3 ).
CLL often occurs in elderly patients with relevant comorbidity. Therefore, a dose-modified FCR-Lite regimen to maintain the efficacy but decrease the toxicity of the FCR regimen was investigated in 50 untreated patients with CLL (median age 58 years).34 The FCR-Lite regimen used a reduced dose of the two cytostatic agents (fludarabine to 20 mg/m2 and cyclophosphamide to 150 mg/m2 days 2–4 during cycle 1 and days 1–3 in cycles 2–5) and an increased dose of rituximab (day 1 of cycle 1 at a dose of 375 mg/m2; cycles 2–5 on day 1 at 500 mg/m2 preceding chemotherapy and on day 14 of each cycle). Maintenance rituximab at 500 mg/m2 was given every 3 months until progression. Using the IWCLL 2008 guidelines,35 the CR rate was 77% for 50 previously untreated patients with CLL with an OR rate of 100%. At a median follow-up of 2.4 years all complete responders remained in CR except for one patient who died of a myocardial infarction while still in remission. Five patients with PRs died within 2 years of completing FCR-Lite. Grade 3/4 neutropenia was documented in only 13% of cycles, which is lower than observed with the usual FCR regimen. This regimen requires further testing in larger trials.
Additional variations of the FCR regimen have been tested to further improve its efficacy. In one study, 48 previously untreated patients with high-risk CLL including del(17p) were treated with FCR plus alemtuzumab (CFAR).36 This CFAR regimen produced more MRD eradication than FCR, but at the expense of greater myelosuppression. In another study, mitoxantrone was combined at 6 mg/m2 on day 1 of each cycle with FCR in previously untreated patients with CLL.37 Seventy-two patients were treated, leading to an OR rate of 93%, MRD– CR rate of 46%, MRD+ CRs of 36% and 10% PRs. Major infections were reported in 8% of cycles.
An alternative attempt was the substitution of fludarabine in the FCR regimen by pentostatin (PCR) in order to reduce the myelotoxicity. In a phase III randomized trial of FCR versus PCR in previously untreated and minimally treated patients with CLL there were no statistical differences between treatments in OS or response.38 Infection rate (fever >101°F requiring antibiotics) was the primary endpoint of this study, which also showed no significant difference between the two arms (31% in FCR and 34% in PCR).
Bendamustine has been combined with rituximab (BR) in 81 patients with relapsed CLL.39 Patients received 70 mg/m2 of bendamustine on days 1 and 2 and 375 mg/m2 of rituximab on day 1 of the first cycle and 500 mg/m2 on day 1 of subsequent cycles administered every 28 days for up to 6 cycles. Grade 3/4 neutropenia and thrombocytopenia occurred in 12% and 9% of all courses, respectively. There were 16 episodes (5%) of grade ≥ 3 infections, with treatment-related deaths in 4% of patients. OR rate was 77%, with 15% CRs. These results compare favorably with the FCR regimen in that BR achieves similar response rates, but induces fewer neutropenias than FCR. Therefore, the GCLLSG currently compares BR to FCR in a phase III trial, the CLL10 protocol.
Several other combinations have been investigated, such as cladribine with rituximab, methylprednisolone plus rituximab followed by alemtuzumab, or rituximab plus alemtuzumab. Their detailed description is beyond the scope of this paper, since none of them resulted in higher efficacy as compared with FCR.
Combinations Using Alemtuzumab
The synergistic activity of fludarabine and alemtuzumab was initially suggested by the induction of responses, including 1 CR, in 5 of 6 patients who were refractory to each agent alone.40 The combination of fludarabine and alemtuzumab (FA) was investigated in a phase II trial enrolling patients with relapsed CLL using a 4-weekly dosing protocol.41 This combination has proven feasible, safe, and very effective. Among the 36 patients, the OR rate was 83% (30 of 36 patients), which included 11 CRs (30%) and 19 PRs (53%) and one stable disease (SD). Sixteen of 31 evaluated patients (52%) achieved MRD negativity in the peripheral blood by 3 months’ follow-up. Resolution of disease was observed in all disease sites, particularly in the blood, bone marrow and spleen. The FA therapy was well tolerated. Infusion reactions (fever, chills, and skin reactions) occurred primarily during the first infusions of alemtuzumab and were mild in the majority of patients. While 80% of patients were cytomegalovirus immunoglobulin G (CMV IgG)–positive before treatment, there were only 2 subclinical CMV reactivations. The primary grade 3/4 hematological events were transient, including leukocytopenia (44%) and thrombocytopenia (30%). Stable CD4+ T-cell counts (> 200/μL) were seen after 1 year.
The combination of FC plus alemtuzumab (FCA) has been studied by the GCLLSG.42 An interim analysis with 24 patients showed an OR rate of 83% with 9 CRs (6 CRu) (38%) and 9 PRs (46%). One herpes zoster and 3 cytomegalovirus reactivations were observed. One patient died due to autoimmune hemolytic anemia, another patient died 2 months after therapy with pancreatitis followed by sepsis. Two phase III trials comparing the activity of alemtuzumab are currently active. The trial of the French study group, which compared FCA with FCR in first-line therapy, was closed prematurely due to the higher toxicity observed in the FCA arm. In the first-line trial of the HOVON group, high-risk patients are randomized to receive either FC or FCA, but efficacy results have to be awaited.
The combination of alemtuzumab with rituximab has also been studied in patients with lymphoid malignancies, including those with refractory/relapsed CLL, producing an OR rate of 52% (8% CR; 4% nodular PR; 40% PR).43 These results need to be confirmed by larger trials.
Combination(s) Using Lumiliximab
Lumiliximab is a primatized anti-CD23 antibody. In a phase I protocol with 46 previously treated and refractory patients with CLL, lumiliximab showed a good safety profile but limited clinical activity.44 Preclinical data demonstrated that lumiliximab enhanced both fludarabine- and rituximab-mediated apoptosis in CLL cells. Therefore, a phase 1/2, open-label, dose-escalation, multicenter study evaluating lumiliximab plus fludarabine, cyclophosphamide, and rituximab (L + FCR) for relapsed CD23+ B-cell CLL on 31 patients was conducted. Patients received either 375 mg/m2 (n = 3) or 500 mg/m2 (n = 28) of lumiliximab in combination with a 28-day cycle of FCR for up to 6 cycles. Preliminary results demonstrated an OR rate of 71% with 48% CR, 10% PR, and 13% unconfirmed PR.45 A comparison with published data from a study of FCR alone in 177 patients with relapsed or refractory CLL46 demonstrated that L + FCR had an acceptable safety profile, did not augment the hematologic toxicity, but increased the CR rate of the FCR regimen. Based upon this data, a multicenter, global, randomized study of L + FCR versus FCR alone was conducted, whose results are expected soon.
Novel Agents in Combination with Chemoimmunotherapy
Oblimersen, an anti-Bcl2 antagonist, was added to the FC regimen and compared to FC in a randomized study on 241 pretreated patients.47 Fludarabine 25 mg/m2/d plus cyclophosphamide 250 mg/m2/d were administered intravenously for 3 days with or without oblimersen 3 mg/kg/d as a 7-day continuous intravenous infusion (beginning 4 days before chemotherapy) for up to 6 cycles. CR/nPR was achieved in 20 (17%) of 120 patients in the oblimersen group and 8 (7%) of 121 patients in the chemotherapy-only group (P = .025) (Table 3 ). Achievement of CR/nPR was correlated with both an extended time to progression and survival (P < .0001). The OS and the PFS were improved by oblimersen in those patients who achieved at least a partial response.
Next Questions
A number of currently ongoing cooperative trials address important questions regarding the management of CLL:
Is bendamustine plus R as efficient as FCR (CLL10 trial of the GCLLSG)?
What is the role of FCA as compared to FCR (trials of the FCLLSG and HOVON group)?
What is the role of lumiliximab when added to FCR (company-sponsored trial)?
Can novel anti-CD20 antibodies (eg, ofatumomab, GA101) replace rituximab (CLL11 trial of the GCLLSG; company-sponsored trials)?
How can we insert other novel, and highly potent agents such as lenalidomide48 and flavopiridol49 into the treatment concepts of CLL patients?
Should we use MRD to monitor the treatment efficacy in our patients?
Eradicating MRD
The assessment of MRD recently has become a very important endpoint with prognostic impact in clinical trials. Detectable MRD after therapy predicts relapse or shorter survival or PFS.24,50–53 Results of a phase III trial showed improved PFS with alemtuzumab consolidation therapy compared with the observation arm (no progression vs 24.7 months, P = .036) when calculated from the start of fludarabine-based treatment.50 In a similar approach, an OR of 53% was achieved by an alemtuzumab consolidation therapy (39% at a 10-mg dose and 65% at a 30-mg dose (P = .066).53 MRD was efficiently cleared from the bone marrow in most patients, with 38% of the patients achieving a molecular remission. Median time to disease progression had not yet been reached for patients who achieved MRD negativity, compared with 15 months for patients who still had residual disease after alemtuzumab consolidation treatment.53 However, this approach may cause considerable myelotoxicity, lymphocytopenia, and sometimes life-threatening infections, in particular if conventional doses of alemtuzumab are administered within 3 to 6 months after the last chemotherapy in patients with a low tumor load.54–55
The quantitative assessment of MRD in 471 patients of the CLL8 trial receiving FC or FCR has provided additional insight into the clinical significance of MRD as assessed by 4-color flow cytometry.56 MRD levels below 10−4 were correlated with longer PFS. The FCR regimen produced lower median MRD levels compared with FC, resulting in longer PFS.
Therefore, MRD assessment is recommended in clinical trials using standardized protocols of either 4-color flow cytometry or allele-specific oligonucleotide PCR (with a sensitivity of one CLL cell per 10,000 leukocytes).57 These trials should assess the benefits and risks (toxicity) of therapies aimed at decreasing the number of CLL patients with detectable MRD levels, before this strategy can be recommended for general practice.35
Conclusion
First-line Treatment
With the increasing potential of newer chemoimmunotherapy combinations, selecting the right treatment for a patient with CLL has become a task that requires a high degree of professional experience. Table 4 proposes an algorithm for the selection of the best treatment option, which is based on three potentially relevant points to consider:
The physical condition (fitness and comorbidity) of the patient, which is independent of calendar age.
The prognostic risk of the leukemia as determined by genetic and other prognostic factors.
The Rai or Binet stage of the disease.
Patients at early stage (Binet A and B, Rai 0-II) without symptoms usually do not require therapy. Early treatment is currently tested in clinical trials for patients at high risk. In patients with advanced (Binet C, Rai III-IV) or active, symptomatic disease, treatment should be initiated. In this situation, patients need to be evaluated for their physical condition (or comorbidity). To patients in good physical condition (“go go”), as defined by a normal creatinine clearance and a low score at the “cumulative illness rating scale” (CIRS),58 an FCR combination therapy should be offered. Patients with relevant comorbidity (“slow go”) may be offered either chlorambucil or a dose-reduced fludarabine containing regimen for symptom control.
Patients with symptomatic disease and with del(17p) or p53 mutations respond poorly to fludarabine or FC, and show a response rate of approximately 50% to alemtuzumab monotherapy or combination therapy, or to FCR, but these responses usually have a short duration of a few months to 1.5 years.17,32,59 Therefore, these patients should be treated within experimental protocols and proposed an allogeneic stem cell transplant whenever possible. Patients with del(17p) may respond to alemtuzumab monotherapy or combination therapy.15
Second-line Treatment
While an extensive review of all treatment options of relapsed or refractory CLL is beyond the scope of this paper, Table 4 summarizes some principles of the management of patients at relapse according to the duration of remission and the physical fitness.
In general, the first-line treatment may be repeated, if the duration of the first remission exceeds 12 months (or with the modern chemoimmunotherapies 24 months). The choice becomes more difficult and limited in treatment-refractory CLL (as defined by an early relapse within 6 months after the last treatment) or in cases with the chromosomal aberration del(17p). In principle, the initial regimen should be changed. The following treatment options exist:
The choice of one of these options strongly depends on the fitness of the patient. According to recent recommendations of an EBMT consensus group, physically fit patients with refractory CLL or with a del(17p) should be offered an allogeneic transplantation, since their prognosis has remained extremely poor with conventional therapies.60 Finally, it is important to emphasize that patients with refractory disease should be treated within clinical trials whenever possible.
Monotherapy: randomized trials comparing novel agents with chlorambucil.
| Reference . | Regimen . | N . | Median age, y . | Advanced stage, %* . | ANC, toxicity grade 3–4, % . | CR, % . | OR, % . | PFS, mo . |
|---|---|---|---|---|---|---|---|---|
| *Rai III-IV | ||||||||
| **Binet C | ||||||||
| †Study included only patients older than 65 years. | ||||||||
| F indicates fludarabine; Clb, chlorambucil; A, alemtuzumab; B, bendamustine; ANC, absolute neutrophil count. | ||||||||
| Rai4 | F | 179 | 64 | 39 | 27 | 20 | 63 | 20 |
| Clb | 193 | 62 | 41 | 19 | 4 | 37 | 14 | |
| Eichhorst61 | F | 93 | 71† | 36** | 12 | 7 | 72 | 19 |
| Clb | 100 | 70† | 40** | 12 | 0 | 51 | 18 | |
| Hillmen17 | A | 149 | 59 | 34 | 41 | 24 | 83 | 14.6 |
| Clb | 148 | 60 | 33 | 25 | 2 | 55 | 11.7 | |
| Knauf9 (updated) | B | 162 | 63 | 28** | 23 | 31 | 68 | 22 |
| Clb | 152 | 66 | 29** | 11 | 2 | 31 | 8 | |
| Reference . | Regimen . | N . | Median age, y . | Advanced stage, %* . | ANC, toxicity grade 3–4, % . | CR, % . | OR, % . | PFS, mo . |
|---|---|---|---|---|---|---|---|---|
| *Rai III-IV | ||||||||
| **Binet C | ||||||||
| †Study included only patients older than 65 years. | ||||||||
| F indicates fludarabine; Clb, chlorambucil; A, alemtuzumab; B, bendamustine; ANC, absolute neutrophil count. | ||||||||
| Rai4 | F | 179 | 64 | 39 | 27 | 20 | 63 | 20 |
| Clb | 193 | 62 | 41 | 19 | 4 | 37 | 14 | |
| Eichhorst61 | F | 93 | 71† | 36** | 12 | 7 | 72 | 19 |
| Clb | 100 | 70† | 40** | 12 | 0 | 51 | 18 | |
| Hillmen17 | A | 149 | 59 | 34 | 41 | 24 | 83 | 14.6 |
| Clb | 148 | 60 | 33 | 25 | 2 | 55 | 11.7 | |
| Knauf9 (updated) | B | 162 | 63 | 28** | 23 | 31 | 68 | 22 |
| Clb | 152 | 66 | 29** | 11 | 2 | 31 | 8 | |
Combination chemotherapy—recent randomized trials comparing fludarabine plus cyclophosphamide versus fludarabine.
| Reference . | Regimen . | N . | Median age, y . | Advanced stage, %* . | ANC, toxicity grade 3–4, % . | CR, % . | OR, % . | PFS, mo . |
|---|---|---|---|---|---|---|---|---|
| *Rai III-IV | ||||||||
| **Binet C | ||||||||
| F indicates fludarabine; Clb, chlorambucil; C, cyclophosphamide; ANC, absolute neutrophil count. | ||||||||
| Catovsky22 | Clb | 387 | 65 | 31 | 28 | 7 | 72 | 20 |
| F | 194 | 64 | 29 | 41 | 15 | 80 | 23 | |
| FC | 196 | 65 | 30 | 56 | 38 | 94 | 43 | |
| Flinn21 | F | 137 | 61 | 42 | 63 | 5 | 59 | 19 |
| FC | 141 | 61 | 44 | 69 | 23 | 74 | 32 | |
| Eichhorst62 | F | 182 | 59 | 41 | 26 | 7 | 83 | 20 |
| FC | 180 | 58 | 39 | 56 | 24 | 95 | 48 | |
| Reference . | Regimen . | N . | Median age, y . | Advanced stage, %* . | ANC, toxicity grade 3–4, % . | CR, % . | OR, % . | PFS, mo . |
|---|---|---|---|---|---|---|---|---|
| *Rai III-IV | ||||||||
| **Binet C | ||||||||
| F indicates fludarabine; Clb, chlorambucil; C, cyclophosphamide; ANC, absolute neutrophil count. | ||||||||
| Catovsky22 | Clb | 387 | 65 | 31 | 28 | 7 | 72 | 20 |
| F | 194 | 64 | 29 | 41 | 15 | 80 | 23 | |
| FC | 196 | 65 | 30 | 56 | 38 | 94 | 43 | |
| Flinn21 | F | 137 | 61 | 42 | 63 | 5 | 59 | 19 |
| FC | 141 | 61 | 44 | 69 | 23 | 74 | 32 | |
| Eichhorst62 | F | 182 | 59 | 41 | 26 | 7 | 83 | 20 |
| FC | 180 | 58 | 39 | 56 | 24 | 95 | 48 | |
Therapies combining fludarabine plus cyclophosphamide with rituximab or oblimersen.
| Reference . | Regimen . | N . | Median age, y . | Advanced stage, %* . | ANC, toxicity grade 3–4, % . | CR, % . | OR, % . | PFS, mo . |
|---|---|---|---|---|---|---|---|---|
| *Rai III-IV | ||||||||
| **Binet C | ||||||||
| †Only CTC grade 4 | ||||||||
| ‡Time to treatment failure | ||||||||
| FC indicates fludarabine and cyclophosphamide; FCR, FC plus rituximab; FCO, FC plus oblimersen; ANC, absolute neutrophil count. | ||||||||
| First line | ||||||||
| Hallek32 | FC | 409 | 61 | 31 | 21 | 23 | 85 | 32 |
| FCR | 408 | 61 | 31 | 34 | 44 | 92 | 43 | |
| Second line | ||||||||
| O’Brien47 | FC | 121 | 63 | 50 | 11† | 3 | 45 | 9 |
| FCO | 120 | 63 | 45 | 7† | 9 | 41 | 6 | |
| Robak33 | FC | 272 | 62 | NA | 40 | 13 | 58 | 21‡ |
| FCR | 274 | 62 | NA | 42 | 24 | 70 | 31‡ | |
| Reference . | Regimen . | N . | Median age, y . | Advanced stage, %* . | ANC, toxicity grade 3–4, % . | CR, % . | OR, % . | PFS, mo . |
|---|---|---|---|---|---|---|---|---|
| *Rai III-IV | ||||||||
| **Binet C | ||||||||
| †Only CTC grade 4 | ||||||||
| ‡Time to treatment failure | ||||||||
| FC indicates fludarabine and cyclophosphamide; FCR, FC plus rituximab; FCO, FC plus oblimersen; ANC, absolute neutrophil count. | ||||||||
| First line | ||||||||
| Hallek32 | FC | 409 | 61 | 31 | 21 | 23 | 85 | 32 |
| FCR | 408 | 61 | 31 | 34 | 44 | 92 | 43 | |
| Second line | ||||||||
| O’Brien47 | FC | 121 | 63 | 50 | 11† | 3 | 45 | 9 |
| FCO | 120 | 63 | 45 | 7† | 9 | 41 | 6 | |
| Robak33 | FC | 272 | 62 | NA | 40 | 13 | 58 | 21‡ |
| FCR | 274 | 62 | NA | 42 | 24 | 70 | 31‡ | |
Proposal of an algorithm for first- and second-line therapy of CLL.
| . | . | . | First-line treatment . | |
|---|---|---|---|---|
| Stage . | Fitness . | Molecular cytogenetics . | Standard . | Alternatives* . |
| Asymptomatic Binet A–B or Rai 0–II | Irrelevant | Irrelevant | None | Only in trials: treat high-risk patients |
| Binet C or Rai III–IV, or symptomatic disease (any stage) | Go Go | No del(17p) Del(17p) | FCR FCR, A or FA → Allo SCT | BR, FR, FA, FCA |
| Slow Go | No del(17p) Del(17p) | CLB A? | CLB+R, CLB+GA101, B, dose reduced F or FC or FCR | |
| . | . | . | First-line treatment . | |
|---|---|---|---|---|
| Stage . | Fitness . | Molecular cytogenetics . | Standard . | Alternatives* . |
| Asymptomatic Binet A–B or Rai 0–II | Irrelevant | Irrelevant | None | Only in trials: treat high-risk patients |
| Binet C or Rai III–IV, or symptomatic disease (any stage) | Go Go | No del(17p) Del(17p) | FCR FCR, A or FA → Allo SCT | BR, FR, FA, FCA |
| Slow Go | No del(17p) Del(17p) | CLB A? | CLB+R, CLB+GA101, B, dose reduced F or FC or FCR | |
| . | . | . | Relapse therapy . | |
|---|---|---|---|---|
| Relapse . | Fitness . | Molecular cytogenetics . | Standard . | Alternatives . |
| *In part tested in Phase III trials. | ||||
| Clb indicates chlorambucil; F, fludarabine; C, cyclophosphamide; A, alemtuzumab; R, rituximab; B, bendamustine; Allo SCT, allogeneic stem cell transplantation; GA101, novel anti-CD20 antibody. | ||||
| Early (< 1 year) = refractory disease | Go Go | No del(17p) Del(17p) | A or FA → Allo SCT A or FA → Allo SCT | BR, flavopiridol, lenalidomide Flavopiridol, lenalidomide |
| Slow Go | No del(17p) Del(17p) | A A | BR, B, lenalidomide Lenalidomide | |
| Late (> 1 year) | Go Go & Slow Go | Repeat first line | ||
| . | . | . | Relapse therapy . | |
|---|---|---|---|---|
| Relapse . | Fitness . | Molecular cytogenetics . | Standard . | Alternatives . |
| *In part tested in Phase III trials. | ||||
| Clb indicates chlorambucil; F, fludarabine; C, cyclophosphamide; A, alemtuzumab; R, rituximab; B, bendamustine; Allo SCT, allogeneic stem cell transplantation; GA101, novel anti-CD20 antibody. | ||||
| Early (< 1 year) = refractory disease | Go Go | No del(17p) Del(17p) | A or FA → Allo SCT A or FA → Allo SCT | BR, flavopiridol, lenalidomide Flavopiridol, lenalidomide |
| Slow Go | No del(17p) Del(17p) | A A | BR, B, lenalidomide Lenalidomide | |
| Late (> 1 year) | Go Go & Slow Go | Repeat first line | ||
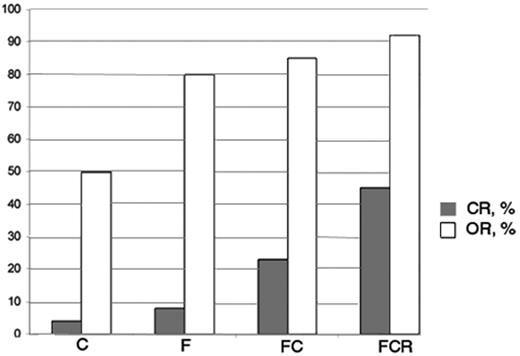
Progress in CLL therapy during the last decade by combining different agents. The figure shows approximate rates of complete remissions and overall responses in CLL first line treatment as reported by recent randomized trials (see Tables 1–3Table 2,Table 3). F indicates fludarabine; C, cyclophosphamide; R, rituximab.
Progress in CLL therapy during the last decade by combining different agents. The figure shows approximate rates of complete remissions and overall responses in CLL first line treatment as reported by recent randomized trials (see Tables 1–3Table 2,Table 3). F indicates fludarabine; C, cyclophosphamide; R, rituximab.
Disclosures Conflict-of-interest: The author is on the speakers bureau and receives research funding from Roche and Mundipharma. Off-label drug use: None disclosed.
Acknowledgments
I wish to thank all patients and physicians participating at the studies of the German CLL Study Group for their continuing support and excellent cooperation. I also wish to thank Drs Eichhorst, Goede, and Elter for critically reading this manuscript. This work is supported by the Deutsche Krebshilfe (German Cancer Aid) and the Kompetenznetz Maligne Lymphome (Competence Network Malignant Lymphoma).
References
Author notes
University of Cologne, Cologne, Germany