Abstract
The remarkable progress made in the treatment of chronic myeloid leukemia (CML) over the past decade has been accompanied by steady improvements in our capacity to accurately and sensitively monitor response to therapy. After the initial target of therapy, complete cytogenetic response (CCR), is achieved, peripheral blood BCR-ABL transcript levels measured by real-time quantitative reverse transcriptase PCR (RQ-PCR) define the subsequent response targets, major and complete molecular response (MMR and CMR). The majority of patients on first-line imatinib therapy achieve a “safe haven” defined as a confirmed MMR, but 20% to 30% stop imatinib due to intolerance and/or resistance. Many imatinib-resistant patients can be effectively treated with second generation tyrosine kinase inhibitors (TKIs), but the actual drug selected should be based on the resistance profile of each inhibitor, in addition to issues of tolerance and disease phase. The main purpose of monitoring response with cytogenetics and RQ-PCR is to identify patients likely to achieve better long-term outcome if they are switched early to second-line therapy, either another TKI or an allograft. Mutation screening is most valuable in cases of loss of response to imatinib or a second-line TKI, but there are other settings where a high yield of mutations may justify regular mutation screening.
A decade ago imatinib mesylate, the first tyrosine kinase inhibitor (TKI) received international attention as a promising new “targeted” therapy for disease control in patients with advanced-phase chronic myeloid leukemia (CML) and interferon-refractory chronic-phase disease. Today, imatinib represents the established front-line therapy for nearly all patients with CML and second-generation TKIs are usually the preferred option in cases of imatinib failure. Most hematology practices have a steadily growing number of patients with CML who commenced imatinib soon after diagnosis, have achieved and maintained excellent disease control, and seem destined to enjoy near-normal survival and quality of life. The need for frequent ongoing monitoring for these long-term responders now warrants reassessment. However, for the patient with CML who presents today the initial journey from diagnosis to stable response remains a hazardous one. Around 30% of patients with CML will have to stop imatinib therapy due to intolerance and resistance. Close monitoring still has a vital role.
A risk-adapted monitoring strategy is needed to ensure cost-effective management for all patients. The main aim of monitoring response to TKI therapy is to identify patients who can be reassured that they have achieved a “safe haven” and, perhaps more importantly, those that are likely to achieve better long-term outcome if they are switched to second-line therapy. This may be another TKI or in some specific cases an allogeneic stem cell transplant. Apparent loss of cytogenetic or molecular response can sometimes be attributed to variations in assay values or short-term lapses in compliance. Switching therapy to a second line option should never be based on a single assay result. It is also worth remembering that in some circumstances improved responses may be achieved by escalating the dose of imatinib1,2 or simply achieving better compliance.
Monitoring response to TKI therapy relies on (1) bone marrow cytogenetic analysis in the first 12 to 18 months, as well as (2) regular measurement of the BCR-ABL transcript levels by real-time quantitative PCR (RQ-PCR) assays plus (3) selective testing for BCR-ABL kinase domain (KD) mutations. FISH and drug level testing may also have a role in some circumstances. Monitoring strategies during first-line therapy have been reviewed recently3–5 and reasonable consensus has been achieved. Monitoring strategies during second-line therapy are still being developed.
RQ-PCR for BCR-ABL
In 2006 an international scale (IS) for expressing BCR-ABL RQ-PCR results was proposed.6 This was linked to recommendations for optimizing methodology including a detailed review of the merits of sampling blood versus bone marrow and optimal volume and preparation of blood samples. In-house methods could be continued because local values were converted to the IS using a conversion factor derived from a comparison between the BCR-ABL levels calculated from a set of shared patient samples studied in the local laboratory and a reference laboratory.7 This process has enabled over 50 laboratories to express their results as BCR-ABL level (IS). This allows clinicians to determine whether their patient has achieved specific molecular landmarks (≤ 0.1% = major molecular response [MMR]; 1% is approximately equivalent to a complete cytogenetic response [CCR]). However, reporting BCR-ABL values on the IS does not give an indication of the measurement reliability or the sensitivity of the local RQ-PCR assay, issues that will also impact on the clinical value of RQ-PCR assays.
Monitoring Response over the First 2 Years of TKI Therapy: Need for Vigilance
In the IRIS trial and in other studies of imatinib therapy in chronic-phase CML the incidence of events (including loss of a major cytogenetic response [MCR], loss of a complete hematologic response [CHR] and transformation to accelerated phase or blast crisis and death from any cause) was highest in the first 2 to 3 years of imatinib therapy and dropped off significantly after that. Based on the 6-year update of the IRIS data, the risk of events averaged 5.2% per year for the first 3 years, and only 0.9% per year for the subsequent 3 years.8 This predominance of progression events in the first 3 years could be because some cases of CML, when initially diagnosed, have already advanced beyond the point when kinase inhibition will prevent transformation. Another possible interpretation is that chronic-phase CML, while untreated, is intrinsically unstable and prone to random mutations that lead to progression. This instability is only overcome when TKI therapy has blocked excessive kinase activity that drives genetic instability and substantially reduced the pool of CML progenitors and stem cells capable of clonal progression. The implications of these two possibilities are different. If the latter is true, then rapid achievement of molecular response will lead to a lower risk of early progression. In either case the achievement of MMR appears to be a “safe haven.” Until this is achieved and confirmed, 3 monthly RQ-PCR for BCR-ABL is indicated.
Recent analysis of the first 2 years of the TIDEL I trial where imatinib 600 mg was the initial dose with selective intensification for suboptimal response showed a similar pattern of early events. Eighteen percent of patients lost response to imatinib (loss of CCR, MCR or CHR) during the first 2 years of therapy. In half of these 18 cases, there was no dose reduction or interruption to explain the loss of response. In the 9 patients in whom there was no dose change, 6 had KD mutations detected by direct sequencing. CCR was lost in 7% of patients in the 12 months following its achievement. MMR was only lost in 1 out of 70 patients who achieved it within the first 2 years of imatinib therapy.9
Essential Early Monitoring Tests
Bone marrow morphology and cytogenetics are essential at diagnosis to confirm the diagnosis of chronic-phase CML and establish a baseline regarding the presence of any additional cytogenetic abnormalities. These are particularly significant if acquired while on therapy. After imatinib therapy commences, marrow studies at 3, 6 and 12 months are recommended. Once CCR and MMR are achieved the value of ongoing marrow cytogenetic studies is quite limited unless the BCR-ABL level increases significantly and MMR is lost. The other indication for a repeat marrow study is unexpected cytopenias, which may be an indicator of myelodysplasia or acute myeloid leukemia originating from the Philadelphia chromosome (Ph)–negative hematopoiesis or in rare cases emerging Ph+ blast crisis, although a loss of MMR would usually be a feature. Cytopenia in the first 3 to 6 months is not unexpected, but if it evolves at a later stage, the possibility of underlying marrow abnormalities should be considered.10,11 A baseline PCR test is needed to confirm the type of BCR-ABL transcripts that are expressed to enable proper interpretation of subsequent results. There is no proven prognostic value for the actual baseline BCR-ABL level.
Twice monthly blood count monitoring for the first few months is valuable to document achievement of CHR, which is nearly always achieved in the first 3 months. RQ-PCR can be done monthly for the first 3 months, but its value at this early stage is unproven. The result at 3 months provides an indication of the probability that MMR will eventually be achieved12 but is not yet very predictive of progression-free survival (PFS). In about 10% to 25% of cases cytogenetics will be unsuccessful or yield insufficient metaphases to provide a reliable estimate of the proportion of Ph+ cells. FISH is sometimes used at this stage to determine whether a patient has achieved CCR. This is probably the main value of FISH studies. The RQ-PCR result can also be used to assess the likelihood of CCR where cytogenetics is not available. A BCR-ABL level below 1% is associated with CCR in over 90% of cases.13
Predictive Value of Cytogenetic Response
The European LeukemiaNet (ELN) recommendations are that marrow cytogenetics at 3, 6, 12, and 18 months are still the main determinant of imatinib failure and suboptimal response.14 Imatinib failure is generally an indication to switch to second-line therapy whereas the appropriate action in cases of suboptimal response—including no change in therapy, an increased dose of imatinib, or a switch to a more potent inhibitor—is less clear cut. A study of 224 patients treated at a single institution assessed the outcome for patients who failed or had suboptimal response to imatinib according to the ELN criteria. At 3, 6 and 12 months, suboptimal response and imatinib failure were associated with equally poor 5-year PFS, which was less than 76% for all groups.15
Predictive Value of Molecular Response
In the IRIS trial, molecular monitoring commenced once a patient achieved CCR. Additionally molecular analyses were performed every 3 to 6 months regardless of the cytogenetic response in 2 substudies established for patients enrolled in Germany, Australia and New Zealand. At ASH 2008 the first analysis of the IRIS molecular data incorporating all of the German and Australasian sub-study data as well as the whole of study data was presented.16 This was possible because all of the laboratories involved in the molecular analysis for this trial had undergone standardization and were expressing results on the IS.7 The analysis of event-free survival (EFS) based on molecular response at 6 months showed clearly inferior outcomes for patients with BCR-ABL values greater than 10% IS. Five-year EFS was 56% for this cohort compared with 85% for those between 1% to10% IS. By 12 months the groups who had not achieved 1% IS BCR-ABL had significantly lower EFS (64% for patients 1% to 10% IS and 56% for patients >10% IS). MMR at 18 months was superior to all other response categories with an EFS of 95% compared with 86%, 62% and 58% for patients in the 0.1 to 1% IS;1 to 10% IS and >10% IS categories, respectively. This suggests that for clinicians with access to reliable standardized RQ-PCR analysis, responses at 6, 12 and 18 months could be considered alongside the cytogenetic results when identifying suboptimal and failing patients (Table 1 ).
When to Do Mutation Screening
One possible approach to mutation testing is to confine it to patients for whom a switch to a second-line therapy has been decided. There is a strong consensus that mutation testing is indicated in this setting in order to make a rational choice regarding which TKI to choose or whether to consider an allograft. At the other extreme is the suggestion that regular mutation screening on all patients is indicated because even some patients in stable CCR will be identified with mutations.17 The patients with detectable mutations in CCR appear significantly more likely to subsequently lose CCR. In the single center study in which all patients in chronic phase were screened for mutations twice a year, the 5 year cumulative incidence of kinase domain mutations was 7% for early chronic phase and 17% for late chronic phase. The incidence was 29% for patients who did not achieve CCR, compared with 6% for those achieving CCR. Most mutations were detected in the first 2 years. There was a very low incidence of new mutations beyond 3 years, especially in patients in CCR. The median interval between mutation detection and loss of CCR was 21 months. CCR was lost in 14% of patients during follow-up. The study authors concluded that the systematic screening of all patients in CCR might not be cost effective and suggested that a reasonable compromise would be twice yearly mutation screen for all patients who still have BCR-ABL values > 0.3% BCR-ABL.
Another approach being assessed is the use of highly sensitive mutation screening to identify patients with emerging resistant clones at the earliest possible stage. D-HPLC is a sensitive screening method that will detect mutations 6 to 12 months before overt hematological relapse.18 It has been suggested that regular D-HPLC screening in suboptimal responders may be a good strategy for early detection of significant mutations, but the predictive value and specificity of this assay has not been prospectively verified.18 At this stage highly sensitive mutation screening is a research tool and not a recommended part of routine monitoring.
KD mutation screening is recommended by the ELN in cases of imatinib failure, suboptimal response or increasing BCR-ABL transcript level.14 It is our practice and recommendation to also perform mutation analysis for all patients treated with imatinib who have a BCR-ABL value of higher than 10% IS at 6 months, followed by mutation screening every 3 months until the BCR-ABL value falls below 1% IS. BCR-ABL values lower than 10% IS are approximately equivalent to the achievement of an MCR.7,13 We found the highest incidence of mutations occurred in patients failing to achieve an MCR by 6 months.19 Furthermore, patients who have a significant rise in BCR-ABL and have a confirmed loss of an MMR should have mutation screens unless there is a clear association between the rising BCR-ABL level and a dose reduction or interruption. Although loss of MMR in patients treated with first-line imatinib is rare, we have found that loss is frequently associated with KD mutations. In a molecular analysis at our institution of 181 patients treated with 400 or 600 mg of first-line imatinib, mutations were detected in 3 out of 7 patients with loss of MMR that was not associated with imatinib dose change.20
One possible pitfall of mutation screening is the detection of ABL polymorphisms that may be mistaken for mutations.18 There are 6 known polymorphisms in the kinase domain, which are K247R (58778 A>G), T240T (58758 G>A), F311V (68708 T>G), T315T (68722 T>G), Y320C (68736A>G), and E499E (74901 A>G) (GenBank accession number U07563). None have a known effect on TKI binding. It would therefore be prudent in cases where novel point mutations are detected to analyze the normal ABL allele to exclude polymorphisms. Insertions and deletions in the kinase domain have also been reported but their role in TKI resistance has not been clearly defined.21,22,23
Possible Role for Imatinib Trough Plasma Level Testing
The French group originally showed that an adequate imatinib trough plasma level (ITPL) may be critical to optimize cytogenetic and molecular response.24 However, in a similar analysis there was no correlation of mean ITPL and CCR or MMR.25 In a recent analysis of imatinib pharmacokinetic data from the IRIS trial, patients with ITPLs in the lowest quartile (= 644 ng/mL) were more likely to discontinue imatinib than those in quartiles 2 to 4 (41% vs 26%) and less likely to achieve CCR by 12 months (59% vs 72%).26 Loss of CCR by 5 years was also higher for quartile 1 (24% vs 13%).
Whether blood level testing can be used to optimize dosage and subsequently improve response is not resolved. However, there are a number of situations where pharmacokinetic testing may be beneficial27,28:
Patients with excessive toxicity. If the imatinib level is very high it may be more likely that satisfactory response could be maintained on a modified dose.
Patients with suboptimal response, where a low trough level may indicate a greater probability that a dose increase will be beneficial.
Patients who commence another drug that may interfere with or enhance imatinib metabolism, where a trough level before and after starting the new drug may identify a potential problem.
Patients with poor compliance, where it may be used to monitor adherence to therapy, although this is probably only indicative of the compliance in the 24 to 48 hours prior to the blood test. The serial RQ-PCR results may be a better measure of ongoing compliance.
At this stage these indications for ITPL testing are only proposals, and further studies are needed to verify the value of intervention for low or high ITPLs.
Monitoring Response in the Long Term optimal Responder: Achieving Safe Haven
It is important to consider what level of stability is necessary to determine that a patient has entered a “safe haven” where the intensity of monitoring can perhaps be relaxed. Since the development of second-generation TKIs it is less meaningful to assess the success of first-line therapy based on survival. A first-line therapy that is not optimal may be associated with excellent survival because many patients are efficiently rescued with second-line therapy. Similarly, EFS may be high for these patients because they switch to second-line therapy before an “event” is recorded, because events are currently defined as loss of MCR or CHR or progression to the acute phase. Under current ELN recommendations loss of CCR represents treatment failure, which should be managed by switching to second-line therapy. This means that many patients who lose CCR go on to second-line therapy without actually recording an “event” by current definition. For meaningful comparison of outcomes with different treatment and different subgroups any loss of response that triggers a switch in therapy should be recorded as an event. Thus a confirmed loss of CCR or a confirmed rise in BCR-ABL (IS) above 1% should be included in the analysis of EFS.
Patients who have truly achieved a “safe haven” should have a risk of losing CCR low enough to make frequent RQ-PCR testing unnecessary. A reasonable criterion for this may be a risk of losing CCR of less than 1% per year. The achievement of MMR by 18 months represents a convincing safe haven where the risk of progression including loss of CCR is extremely low and surveillance for resistance could reasonably be relaxed. In a Hammersmith Hospital study of 197 patients treated with first-line imatinib, the 5-year probability of losing CCR was 0% for patients achieving MMR by 18 months, whereas it was 24.6% for patients achieving a CCR but no MMR at 18 months (P = .006).15 The importance of achieving stable MMR in late chronic phase was demonstrated recently in an Italian study of 130 patients followed for a median of 72 months. Among the patients with CCR who achieved a consistent MMR there was a 4% probability of losing CCR over the subsequent 6 years compared with patients with fluctuating BCR-ABL levels and those who never achieved MMR, where the risk was 21% and 33% (P = .03 and .0001), respectively. They concluded that a patient whose CCR is not supported by a stable MMR deserves close monitoring and, in all probability, a different imatinib schedule (dose escalation) or a second-generation TKI treatment.29 Based on these recent studies patients with CML in chronic phase who achieve stable MMR have an annual risk of loss of CCR of < 1%. For this group RQ-PCR monitoring every 6 months may be reasonable.
However, our recent analysis of our own patient series where events were defined as a confirmed loss of MMR as well as loss of CCR, MCR and CHR, transformation to advanced phase or blast crisis and/or the detection of a KD mutation suggests a more cautious approach to ongoing monitoring. In patients receiving imatinib as first-line therapy the extent to which MMR can be regarded as a safe haven may also depend on how quickly MMR was achieved. In our study of 181 patients the probability of an event if MMR was achieved by 6 months was 0%, 6 to 12 months was 8%, and 12 to 18 months was 15%, with similar median follow-up since MMR was achieved in these 3 groups.
Achievement of CCR without MMR cannot be regarded as a safe haven. It is still relatively unstable, especially for patients treated in late chronic phase. In a GIMEMA study of patients in late chronic phase the annual risk of losing CCR once established was 7.5% per year in years 1 and 2 and remained high at 4.5% per year in years 3 and 4.30
Potential Value of Regular RQ-PCR Monitoring
Even where detection of early evidence of resistance in unlikely, regular RQ-PCR for BCR-ABL may serve two other valuable purposes.
Identification of Poor Compliance
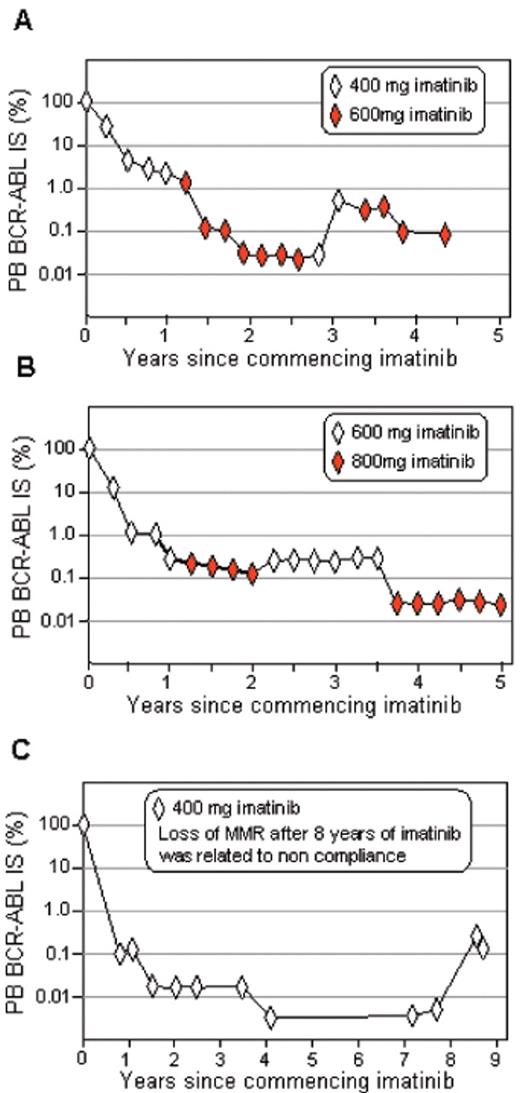
The availability of serial BCR-ABL values when a patient with CML is reviewed in clinic serves to emphasize to the patient that, despite the use of the term “remission,” the battle between normal and CML hematopoiesis in the marrow and blood is ongoing and any premature reduction in treatment intensity will likely lead to regrowth of the CML cells with a consequent rise in the BCR-ABL level. This is best demonstrated by observing the BCR-ABL levels in patients who reduce dose because of toxicity or who are not compliant (Figure 1 ). Regular RQ-PCR tests for BCR-ABL may identify periods of poor compliance and facilitate better long-term compliance.
Confirmation that Complete Molecular Response Has Been Achieved and Maintained
The term complete molecular response (CMR) does not always indicate eradication of leukemia. Rather, it is used to describe the absence of detectable BCR-ABL transcripts. The ability to detect BCR-ABL is highly dependent on the quality of the RNA and the efficiency of the reverse transcription reaction. Therefore, the limit of detection by RQ-PCR will vary for each sample, which may explain why some patients have undetectable BCR-ABL on some occasions but not others. Up to 107 leukemic cells could still be present in the absence of measurable BCR-ABL. For some studies CMR is defined as confirmed undetectable BCR-ABL where the sample quality ensures that a specific sensitivity level is achieved.31–33 Does a confirmed CMR have any special significance and is it worth identifying and following? Some studies suggest that patients treated with imatinib with a CMR have the most favorable relapse-free survival. In a study of 90 patients with a CCR, only 1 out of 28 with a CMR subsequently lost their CCR whereas 11 out of 48 with an MMR subsequently lost their CCR, P =.005.34 In a study of 276 patients treated at the M.D. Anderson Cancer Center, 100 patients treated with imatinib achieved CMR. There were no treatment failures if CMR was sustained for 6 or more months.35 These studies included patients with prior therapy. Whether the achievement of CMR confers the most favorable prognosis for patients treated with first-line imatinib is not confirmed. In our most recent analysis of 144 patients treated with first-line imatinib with an MMR, the lowest frequency of events occurred in the 55 patients with a CMR (1.8%) compared with the 89 patients with MMR but no CMR (7.9%). Earlier achievement of an MMR resulted in a greater probability of a CMR and greater stability of response.20
The other possible significance of achieving a stable CMR is the potential to cease active therapy without losing CMR. This was first recognized in the interferon era, but has been confirmed on a larger scale in patients who achieve stable CMR on imatinib. Both French and Australian studies show that about half of the patients who achieve and maintain CMR for 2 years on imatinib therapy using strict RQ-PCR sensitivity criteria remain in CMR after imatinib is ceased. Many of these patients have remained in CMR off therapy for 2 to 4 years. The vast majority of relapses seen so far occurred within 6 months of cessation.36,37 Despite these encouraging results cessation of imatinib cannot be recommended outside the clinical trial setting.
Molecular Monitoring for Patients Treated with More Potent Inhibitors after Imatinib Failure
Molecular monitoring is likely to be highly relevant for patients on second-line therapy where the risk of primary and secondary resistance is higher. The most widely studied inhibitors, nilotinib and dasatinib, have demonstrated variable efficacy in patients treated in chronic phase after imatinib failure.38,39 In molecular studies of 1067 patients in chronic phase treated with dasatinib during phase II/III trials, achieving an MMR conferred an advantage. After 12 months of dasatinib therapy, MMR was achieved in 35%.40 This compares favorably with the 40% MMR rate at 12 months for the patients treated with first-line imatinib in the IRIS trial.32 In a 12-month landmark analysis, PFS at 24 months was higher for patients with an MMR compared with those without an MMR, 96% versus 82%, P < .0001. The overall survival was similar, 98% versus 97%. Among the patients with a CCR, there was no significant difference in the PFS or overall survival for those with an MMR. However, the achievement of an MMR was significantly associated with a longer duration of CCR. At 24 months, 86% of patients with an MMR at 12 months remained in CCR, compared with only 64% of those who did not achieve an MMR, P = .0006.41
What Is the Value of Molecular Monitoring for the Early Prediction of Response to Nilotinib or Dasatinib?
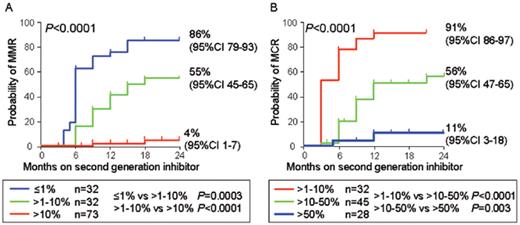
For patients treated with imatinib the early reduction of BCR-ABL can predict the longer term cytogenetic and molecular response.12,42 To determine whether response to the second generation inhibitors can similarly be predicted, we monitored 155 patients in chronic phase who were treated with nilotinib (n = 73; 400 mg twice daily) or dasatinib (n = 82; all treated with 100 mg or more per day; 76 of these received 70 mg twice daily) after imatinib failure for a median of 18 months (range 3 to 36 months).43 The BCR-ABL level measured at 3 months was highly predictive of MMR by 24 months. Patients with a BCR-ABL value of ≤ 1.0% IS had an 86% probability of achieving an MMR by 24 months. In contrast, those with values of 1.0% to 10% IS or >10% IS had a significantly lower probability of MMR at 55% and 4%, respectively, P < .0001 (Figure 2A ). It has recently been suggested that failure to achieve an MCR by 12 months of nilotinib or dasatinib defines inadequate response and these patients should be considered for alternative therapies.44 Patients in minor cytogenetic response or complete hematologic response at 12 months had a projected 1-year progression rate of 17% compared with 3% for those with MCR at 12 months. We found that the initial molecular response was predictive of an MCR, P < .0001 (Figure 2B ). Patients with BCR-ABL values of > 1.0% to 10% had a 91% probability of MCR, whereas for those with values of > 50% IS the probability was only 11%.
Correlating Clinical Response with BCR-ABL Mutation Status
Approximately half of the patients with imatinib resistance who commence second-generation inhibitor therapy have BCR-ABL KD mutations. These mutations are often referred to as “baseline mutations.” Do these mutations have an impact on response? It is known that T315I confers resistance to both nilotinib and dasatinib. Recent reports of patients in chronic phase treated in phase II/III trials demonstrated clinical efficacy for both the mutant and non-mutant groups, although the response rates were slightly lower for the patients with baseline mutations.45,46 However, distinct differences in response were evident when individual mutations were examined according to their in vitro sensitivity to either nilotinib or dasatinib.
In an examination of 281 patients in chronic phase enrolled and evaluated for efficacy in the phase II nilotinib registration study, 41% had detectable mutations at baseline.45 Of the patients with imatinib resistance, 106 of 192 (55%) had baseline mutations. Responses at 12 months for patients with baseline mutations with high in vitro sensitivity to nilotinib (as assessed by IC50 values of ≤ 150 nM according to Weisberg et al47), or mutations with unknown nilotinib sensitivity, were equivalent to the responses for patients without mutations. Conversely, less favorable responses were observed in the 26 patients with baseline mutations less sensitive to nilotinib in vitro (IC50 > 150 nM) (T315I was excluded). These mutations were Y253H, E255V/K and F359V/C. The responses for patients with these mutations were 19%, 0% and 4% for MCR, CCR and MMR, respectively, compared with 59%, 43% and 29% for patients with the other mutations (both known and unknown sensitivity) and 60%, 40% and 29% for those without baseline mutations. Patients with the less sensitive baseline mutations also had the highest rate of progression at 69%. The progression rates for patients with other mutations or no baseline mutation were 38% and 26%, respectively.
Mutations with a nilotinib IC50 > 150 nM were also the most common to emerge during nilotinib therapy. New mutations that were not present at baseline were detected in 53 of the 281 patients (19%). Of the new mutations, 72% had an IC50 > 150 nM and 28% were other mutations. A higher incidence of new mutations occurred in patients with baseline mutations (34 of 114, 30%) compared with those without mutations (19 of 167, 11%). Progression was associated with the detection of mutations in 70% of assessable patients. These mutations were either new mutations (39%) or the same mutation that was detected at baseline (31%). The study concluded that nilotinib therapy is unlikely to be effective and responses are unlikely to be durable in patients with the less sensitive mutations at baseline.
The baseline mutation status also influences the response to dasatinib therapy. In a study of 1043 patients in chronic phase treated in phase II/III trials of dasatinib, 39% had baseline mutations.46 Of the patients with imatinib resistance or suboptimal response, 48% had baseline mutations (386 out of 805). After a minimum follow up of 24 months the best responses for patients without baseline mutations were 60%, 44% and 33% for MCR, CCR and MMR, respectively. Responses for patients with baseline mutations with high in vitro sensitivity to dasatinib (as assessed by IC50 values of ≤ 3 nM according to O’Hare et al48), or mutations with unknown sensitivity, were 62%, 49% and 35% for MCR, CCR and MMR, respectively. Conversely, in the 47 patients with baseline mutations classified as having intermediate sensitivity to dasatinib in vitro (IC50 > 3 nM) less favorable responses were observed (T315I was excluded). These mutations were F317L, E255K/V and Q252H and the responses were 34%, 25% and 18% for MCR, CCR and MMR, respectively. However, when mutations in this group were examined individually, a relatively favorable response rate was achieved in the 28 patients with E255K/V mutations (MCR in 56%/36%, and CCR in 38%/36%). In contrast, the 14 patients with F317L mutations had inferior responses (MCR in 14% and CCR in 7%). This indicates that the IC50 cut-off of 3 nM, as derived from in vitro studies, does not accurately correlate with a decreased level of clinical response to dasatinib and that dasatinib therapy is unlikely to be effective for patients with F317L at baseline. Only 6 patients with the other intermediate sensitivity mutation Q252H, were evaluable and 1 out of 6 achieved an MCR and CCR. F317L was among the most common new mutations detected at the time of progression or discontinuation (19% of all new mutations). Mutation analysis was performed in 174 patients at the time of progression or discontinuation, and 54 mutations were detected in 47 patients. T315I emerged in 46% of these patients and V299L in 13%. Patients with baseline mutations had a higher incidence of new mutations, 70% versus 37%.
It is clear from the clinical studies that the mutation status at baseline may influence response and progression for patients treated with nilotinib or dasatinib after imatinib failure. Therefore mutation status, along with disease phase and tolerability concerns, should be considered when determining optimal therapy. There is a limited spectrum of resistant mutations where careful selection of second- or third-line inhibitor is warranted (Table 2 ). Dasatinib has demonstrated clinical efficacy for the mutations that are less sensitive to nilotinib. The best rates of CCR after a minimum follow up of 24 months of dasatinib were Y253H 64%, E255K/V 38%/36% and F359V/C 52%/60%.46 This compares favorably with the CCR response rate of 56% for patients without baseline mutations. The dasatinib-resistant mutation F317L is detected at a frequency of less than 4% of all mutations that arise during imatinib therapy.49 It is among the mutations with high in vitro sensitivity to nilotinib. The group of patients with these high sensitivity mutations had a CCR rate of 40% at 12 months of nilotinib therapy, which was the same as the patients without mutations.45 Patients treated with dasatinib who acquired the novel dasatinib-resistant mutations T315A and V299L have responded to retreatment with imatinib or with nilotinib therapy.50 F317I, a novel mutation that confers clinical resistance to dasatinib, is also described as imatinib sensitive.50 Less is known of the mutations that will arise with clinical resistance to bosutinib, although T315I and V299L confer high level resistance in vitro.51In vitro resistance, however, does not always correlate with the patterns of clinical resistance. In addition to the example above, F317V is more than 3-fold more resistant to dasatinib than F317L in vitro,52 yet has rarely been reported with clinical dasatinib resistance. No doubt, the issue of resistance due to KD mutations is complex and is complicated by the presence of multiple mutations within a patient that may alter oncogenic potency.50 Nevertheless, mutation analysis remains an essential assessment for patients treated with more potent inhibitors.
When Should Patients Be Assessed for Mutations?
The emergence of new mutations and their association with resistance during nilotinib and dasatinib therapy indicates a role for mutation screening during second-line TKI therapy. We detected new mutations in 33 of 155 chronic phase patients treated with nilotinib or dasatinib therapy after imatinib failure, which emerged at a median of 6 months (range 1 to 24 months).43 There was a significantly higher incidence of new mutations among patients where the BCR-ABL value never fell below 10% IS (40% vs 14%, P = .0008). This suggests that at least every 3 months a mutation analysis in patients whose BCR-ABL remains above 10% may be warranted. A rise in BCR-ABL was associated with new mutations. Of the 65 patients whose BCR-ABL value fell below 10% and, following that, there was no significant rise in BCR-ABL, none had a new mutation. However, 35% of the 45 patients with a significant rise after BCR-ABL fell below 10% had a new mutation, P < .0001. We do not know how many of these patients with a significant rise had a dose reduction or cessation. Nevertheless, a significant rise in BCR-ABL should trigger mutation analysis for patients treated with nilotinib or dasatinib after imatinib failure.
Conclusions
Increased understanding of the dynamics of leukemic response to TKI therapy has led to definitions of molecular responses that are optimal and those that are suboptimal. Suboptimal responses are associated with a significantly higher risk of mutations and loss of response. Regular molecular monitoring will allow loss of response to be recognized at an early stage in most cases and may also facilitate better compliance. The resistance profile of each TKI to specific mutations has been better defined so that clinical recommendations based on these findings are now being established. An emerging challenge will be to determine molecular criteria for the safe cessation of TKI therapy, since it is now clear that many patients who achieve long-term CMR on imatinib will remain in a CMR off therapy for 2 to 4 years and possibly longer.
European LeukemiaNet (ELN) criteria for suboptimal response and failure at 3, 6, 12 and 18 months incorporating molecular responses at 6, 12 and 18 months based on analysis of the IRIS PCR data.
| . | Failure . | Suboptimal response . | Warning . |
|---|---|---|---|
| 3 months | No HR | No CHR, | |
| 6 months | No CHR | No MCR and/or >10% BCR-ABL (IS) | |
| 12 months | No MCR and/or >10% BCR-ABL (IS) | No CCR and/or >1% BCR-ABL (IS) | No MMR |
| 18 months | No CCR and/or >1% BCR-ABL (IS) | No MMR |
| . | Failure . | Suboptimal response . | Warning . |
|---|---|---|---|
| 3 months | No HR | No CHR, | |
| 6 months | No CHR | No MCR and/or >10% BCR-ABL (IS) | |
| 12 months | No MCR and/or >10% BCR-ABL (IS) | No CCR and/or >1% BCR-ABL (IS) | No MMR |
| 18 months | No CCR and/or >1% BCR-ABL (IS) | No MMR |
Proposal for the selection of either nilotinib or dasatinib therapy according to the baseline BCR-ABL mutation status. The proposal is based on the current understanding from clinical studies. Less is known of the response to bosutinib from clinical studies; however, T315I and V299L have low sensitivity to bosutinib in vitro. Choice of therapy should be considered in conjunction with tolerability and disease phase.
| Mutation status . | Dasatinib . | Nilotinib . |
|---|---|---|
| *Unclear whether current TKIs may be beneficial where a minority population of T315I is present | ||
| †Clinical efficacy was demonstrated for E255K/V in CP-CML with dasatinib therapy, although the response rates were lower than for Y253H and F359V/C.46 | ||
| ‡Clinical efficacy to nilotinib was demonstrated in 5 CP patients with G250E at baseline; however, it was among the most frequent newly detectable mutations in nilotinib treated patients after imatinib failure and was detected at the time of progression.45 Further clinical studies are required to establish long-term response. | ||
| §Q252H is classed as an intermediate sensitivity mutation to dasatinib in in vitro studies, and 1 of 6 CP patients treated with dasatinib after imatinib failure achieved an MCR and CCR.46 Further clinical studies are required to establish clinical efficacy. | ||
| No mutation | Yes | Yes |
| T315I* | No | No |
| F317L/I/V | No | Yes |
| Y253H E255K/V† F359V/C | Yes | No |
| T315A | No | Yes |
| V299L | No | Yes |
| G250E | Yes | Yes‡ |
| Q252H | ?§ | Yes |
| Other mutations | Yes | Yes |
| Mutation status . | Dasatinib . | Nilotinib . |
|---|---|---|
| *Unclear whether current TKIs may be beneficial where a minority population of T315I is present | ||
| †Clinical efficacy was demonstrated for E255K/V in CP-CML with dasatinib therapy, although the response rates were lower than for Y253H and F359V/C.46 | ||
| ‡Clinical efficacy to nilotinib was demonstrated in 5 CP patients with G250E at baseline; however, it was among the most frequent newly detectable mutations in nilotinib treated patients after imatinib failure and was detected at the time of progression.45 Further clinical studies are required to establish long-term response. | ||
| §Q252H is classed as an intermediate sensitivity mutation to dasatinib in in vitro studies, and 1 of 6 CP patients treated with dasatinib after imatinib failure achieved an MCR and CCR.46 Further clinical studies are required to establish clinical efficacy. | ||
| No mutation | Yes | Yes |
| T315I* | No | No |
| F317L/I/V | No | Yes |
| Y253H E255K/V† F359V/C | Yes | No |
| T315A | No | Yes |
| V299L | No | Yes |
| G250E | Yes | Yes‡ |
| Q252H | ?§ | Yes |
| Other mutations | Yes | Yes |
Rise and fall of BCR-ABL levels related to imatinib dosage. Marked variation in BCR-ABL levels was observed in patient A when the dosage was changed from 400 to 600 mg, whereas the changes were more subtle for patient B when the dosage was changed from 600 to 800 mg imatinib. Patient C had a confirmed loss of MMR more than 8 years after commencing 400 mg imatinib. The patient subsequently acknowledged that imatinib was ceased 6 weeks prior. All patients were followed in Adelaide.
Rise and fall of BCR-ABL levels related to imatinib dosage. Marked variation in BCR-ABL levels was observed in patient A when the dosage was changed from 400 to 600 mg, whereas the changes were more subtle for patient B when the dosage was changed from 600 to 800 mg imatinib. Patient C had a confirmed loss of MMR more than 8 years after commencing 400 mg imatinib. The patient subsequently acknowledged that imatinib was ceased 6 weeks prior. All patients were followed in Adelaide.
Predictive value of molecular response on second-line therapy. For chronic-phase patients treated with nilotinib or dasatinib after imatinib failure the 3-month BCR-ABL value was predictive of a major molecular response (MMR). (A) Eighteen of the 155 patients included in the analysis already had an MMR at 3 months of therapy and were excluded. The 3-month BCR-ABL value was also predictive of a major cytogenetic response (MCR). (B) The 50 patients with values of ≤ 1.0% International Scale (IS) at 3 months already had an MCR.
Predictive value of molecular response on second-line therapy. For chronic-phase patients treated with nilotinib or dasatinib after imatinib failure the 3-month BCR-ABL value was predictive of a major molecular response (MMR). (A) Eighteen of the 155 patients included in the analysis already had an MMR at 3 months of therapy and were excluded. The 3-month BCR-ABL value was also predictive of a major cytogenetic response (MCR). (B) The 50 patients with values of ≤ 1.0% International Scale (IS) at 3 months already had an MCR.
Disclosures Conflict-of-interest disclosures: TPH receives honoraria and research funding from Novartis and Bristol-Myers Squibb; he is on the speakers’ bureaus of Novartis and BMS. SB receives research funding and honoraria from Novartis and Bristol-Myers Squibb. Off-label drug use: None disclosed.
References
Author notes
Centre for Cancer Biology, Departments of Molecular Pathology and Haematology, SA Pathology, Adelaide, Australia