Abstract
The myelodysplastic syndromes (MDS) are characterized by cytopenias and risk of transformation to acute myeloid leukemia (AML). Although new treatments are available, a mainstay in MDS remains supportive care, which aims to minimize the impact of cytopenias and transfusion of blood products. Red blood cell (RBC) transfusions place patients at risk of iron overload (IOL). In beta-thalassemia major (BTM), IOL from chronic RBC transfusions inevitably leads to organ dysfunction and death. With iron chelation therapy (ICT), survival in BTM improved from the second decade to near normal and correlated with ICT compliance. Effects of ICT in BTM include reversal of cardiac arrhythmias, improvement in left ventricular ejection fraction, arrest of hepatic fibrosis, and reduction of glucose intolerance.
It is not clear whether these specific outcomes are applicable to MDS. Although retrospective, recent studies in MDS suggest an adverse effect of transfusion dependence and IOL on survival and AML transformation, and that lowering iron minimizes this impact. These data raise important points that warrant further study. ICT is potentially toxic and cumbersome, is costly, and in MDS patients should be initiated only after weighing potential risks against benefits until further data are available to better justify its use. Since most MDS patients eventually require RBC transfusions, the public health implications both of transfusion dependence and ICT in MDS are considerable. This paper summarizes the impact of cytopenias in MDS and treatment approaches to minimize their impact, with a focus on RBC transfusions and their complications, particularly with respect to iron overload.
The myelodysplastic syndromes (MDS) are clonal disorders characterized by ineffective hematopoiesis, cytopenias, and a risk of transformation to acute myeloid leukemia (AML). Survival and AML risk are predicted by the International Prognostic Scoring System (IPSS)1 and the World Health Organization Prognostic Scoring System (WPSS).2 As the median age of MDS diagnosis is in the seventh or eighth decade, most patients are ineligible for hematopoietic stem cell transplantation, the only potentially curative treatment for MDS to date. Other available treatments for MDS include erythropoiesis stimulating agents (ESA) and demethylating and immunomodulatory agents. Strategies to sequence treatment to optimize clinical benefit3 and standardize reporting of outcomes4 have been proposed. Despite these advances, supportive care is the only therapy for many MDS patients; the goal is to minimize the impact of cytopenias on quality of life and comorbidities, and interventions are not generally considered to alter the MDS course.
The predominant feature of most lower-risk MDS is ineffective hematopoiesis; an increased rate of apoptosis leads to peripheral cytopenias. Anemia is the most frequent cytopenia seen in MDS and results in the need for red blood cell (RBC) transfusions, a mainstay of supportive care. This places patients at risk of iron overload (IOL),5 which in turn may lead to cardiac, hepatic and endocrine dysfunction. Recent studies suggest an adverse effect of anemia, RBC transfusion dependence6–8 and IOL9,10 on survival and on progression to AML.10 Based on an incidence of 3.4 per 100,000,11 at least 10,000 new cases of MDS are diagnosed per year in the US. Most patients will eventually require transfusions; therefore, the public health impact is substantial and adds to the already considerable cost of medications to treat this disorder.12 While the benefits of reducing iron with chelation therapy (ICT) are well established in thalassemia,13 more recent data suggest a favorable impact of ICT on survival in MDS in addition.14–16 This paper gives an overview of the ramifications of cytopenias in MDS and treatment approaches to minimize their impact in the usual (non-stem cell transplant) clinical setting, with a focus on RBC transfusion and its complications, particularly iron overload. As recommendations for iron reduction are generally limited to lower-risk MDS, the discussion will largely focus on this group.
Supportive Care in MDS
Neutropenia and Thrombocytopenia
Neutropenia in the IPSS1 was defined as an absolute neutrophil count (ANC) <1.5 × 109/L) and was present in 46% and severe (<0.5 × 109/L) in 6%. Neutropenia had no additive prognostic value for death over and above the IPSS score.8 There are no data supporting the use of prophylactic cytokines or antibiotics for neutropenia in lower-risk MDS, and these interventions are not recommended.17
The impact of thrombocytopenia was examined in 127 patients with refractory anemia with ring sideroblasts (RARS)18; thrombocytopenia (platelet count < 100 × 109/L), was present in 13%, while 98% had anemia (hemoglobin [HGB] < lower limit of normal; HGB < 100 g/L in 60%) and 24% neutropenia (ANC < 1.8 × 109/L). In a multivariate analysis, IPSS score (P = .0001) and platelet count were significantly associated with overall survival (P = .0039); the hazard ratio (HR) was 0.93 [95% confidence interval (CI) 0.88–0.98] for each increase in platelet count of 25 × 109/L.
In another series of 2410 MDS patients, 1605 (67%) presented with thrombocytopenia (platelet count < 100 × 109/L) and 24% with bleeding.19 Of 460 patients in this series whose death was not due to AML, hemorrhage was a contributing factor in 20% and the only factor in 10%.
Transfusion reactions and alloimmunization may occur in up to 85% of multiply transfused patients; therefore, platelet transfusion is generally reserved for bleeding. Cytokine support for thrombocytopenia has lagged behind that of neutropenia and anemia. Serious adverse reactions have limited the use of recombinant interleukin (IL)-11, IL-6 and thrombopoietin. Romiplostim is an Fc peptide fusion protein (peptibody); this agent and eltrombopag promote thrombopoiesis, and clinical trials in MDS are ongoing.
Anemia
Most patients develop RBC transfusion dependence and many receive transfusions as the only MDS intervention. Most patients have a HGB below normal at diagnosis,18 while in the IPSS, 60% had a HGB < 10 g/dL and 27% < 8 g/dL.1
Anemia may impact significantly on quality of life. Chronic anemia is associated with cardiac complications; in 11 of 12 transfusion dependent patients (mean HGB 8.7 ± 1.4 g/dL), cardiac remodeling was seen, compared with 13 of 27 with a mean HGB of 11.3 ± 2.4 g/dL.20 In a US Medicare population, 522 of 705 (74%) of MDS patients followed over a 3-year period experienced a cardiac event (48% congestive heart failure [CHF], 53% arrhythmia); 79% of transfused patients, and 54% of non-transfused (P < .0001).21 Anemia increases cardiac output, leads to left ventricular hypertrophy, and exacerbates coronary syndromes, and coexistence of renal insufficiency and decreased erythropoietin (EPO) production may further exacerbate anemia.
Transfusion reactions include a 0.2 per 1 million RBC units rate of transmitted bacteremia, and in one study, 80% of patients required special processing or selection of blood products, and 94% had reactions or required premedications.22
Transfusion and Related Costs
The cost of transfusing one unit of RBC is estimated at US $500,12,23 excluding the cost of drug administration, professional fees, laboratory testing, and treatment of complications. Patient-related loss of productivity and expenses are also excluded and estimated at an additional US $450 per unit. The median number of RBC units transfused per patient-year in 50 MDS patients was 11.1 (range 0–91.3) and median cost was $4877 (range $0–$67,050).22 In comparison, according to National Cancer Care Network (NCCN) guidelines,17 the cost of therapeutic agents used to treat MDS was around US $63,577; when transfusion of 2 units of RBC per month and iron chelation were included, this increased to $104,989 annually.12
Medications aimed at decreasing transfusion dependence should theoretically decrease cost. For example, in MDS patients randomized to receive EPO ± granulocyte colony-stimulating factor (G-CSF) or supportive care, transfusion costs during the 1-year study period in cytokine responders were 2085 Euros versus 7579 Euros in the supportive care arm; however, this savings (5495 Euros) was more than offset by the cost of cytokines (19,121 Euros).12,24 Even so, quality of life and other benefits of achieving transfusion independence may be substantial and must be weighed against this cost. The use of erythropoiesis-stimulating agents to improve anemia and possibly improve survival has been multiply reviewed elsewhere.
Transfusion Dependence and Survival in MDS
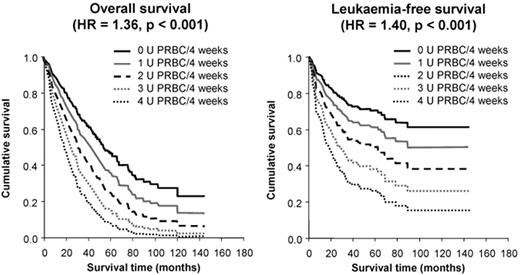
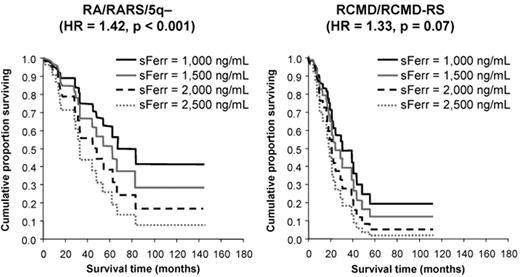
The first demonstration of an adverse effect of RBC transfusion dependence on survival in a sizeable series came from Pavia, Italy.6 In 467 patients, overall survival (OS) and leukemia-free survival (LFS) were inferior in transfusion-dependent patients (requiring ≥ 1 unit of RBC per 4 weeks), with an HR of 1.91 and 1.84, respectively, (P ≤ .001). Moreover, OS and LFS progressively decreased with increasing transfusion dependence, with an HR of 1.36 and 1.40, respectively, for each additional RBC unit transfused per 4 weeks (P < .001; see Figure 1 ).9 The survival effect was mainly seen in lower-risk patients (P < .001), and transfusion dependence increased the probability of non-leukemic death.6 These important observations led to the development of the WPSS,2 which incorporates transfusion dependence into the calculation of patient risk. While it could be argued that these effects are attributable to more advanced bone marrow failure in the transfusion-dependent group, OS also decreased with increasing ferritin level, with an HR of 1.42 for every 500 ng/mL increase in ferritin over 1000 ng/mL (P < .001; see Figure 2 ).9 This suggests that IOL itself may have an adverse effect on survival.
Iron Overload
The Iron Paradox
Iron is vital for survival, but an excess is potentially lethal. Essential functions of iron include cellular respiration, DNA synthesis, translation, proliferation, response to hypoxia, and inflammation. Iron balance is regulated by dietary content and absorption and distribution by hepcidin (reviewed in Cazzola et al25). Adaptive defenses against excess iron include iron-binding proteins, superoxide dismutases and peroxidases. However, if these are overwhelmed, reactive oxygen species are produced, which may damage lipids, proteins and nucleic acids leading to cell death or transformation. Each unit of RBC contains 200 to 250 mg of iron, which the body has no mechanism to excrete. The reticuloendothelial system, a relatively non-toxic storage depot for iron, has a capacity of about 10 to 15 grams, corresponding to about 50 RBC units. When the RES capacity is exceeded, parenchymal deposition and tissue damage occur; in a patient receiving 2 RBC units per month this will occur in just 2 years.25,26
Iron in Cell Growth Regulation
There are suggestions in the literature that iron may be involved in dysregulation of cellular growth and differentiation; the examples provided here are by no means exhaustive. Iron was shown to have a suppressive effect on erythroid progenitors in vitro. Erythroid colony assays were performed on the peripheral blood of 42 patients with MDS; in patients with an elevated ferritin level, the burst-forming units erythroid (BFU-E) were a mean of 2.35; in those with a normal ferritin, 10.1 colonies per culture (P < .004).27 Reducing iron with chelators had antiproliferative activity in vitro and in a murine model induced apoptosis in lung cancer cells.28 The ICT agent deferoxamine upregulated the expression of a gene known to slow tumor growth and inhibit metastases,29 and induced differentiation of the AML cell lines NB4 and U937.30
Reducing iron may reduce the risk of malignancy in the clinical setting. For example, in a prospective study, the use of phlebotomy and a low-iron diet in patients with hepatitis C infection reduced the risk of hepatocellular carcinoma from 39% to 8.6% over 10 years of follow-up (P = .0337).31 Similarly, in a recent provocative study, 1277 patients with peripheral arterial disease were randomized to undergo phlebotomy versus observation.32 There was a reduction in ferritin level in the phlebotomy group, but strikingly, there was also a reduction in the risk of developing a visceral malignancy, seen in 60 of 641 (9.4%) controls and 38 of 636 (5.9%) phlebotomy patients (P = .003).
Thus, excess iron may have deleterious effects, including the suppression of erythropoiesis, and reducing iron may provide benefit, including inhibition of tumor cell proliferation and metastasis, induction of tumor cell differentiation and programmed cell death in preclinical models, and reduction in malignancy in clinical studies. Therapy that improves erythropoiesis should theoretically reduce the iron burden, as iron stores would be mobilized and incorporated into newly formed erythroid cells. However, few data on the effect of MDS treatments on iron burden are available. To our knowledge, there is no published information on the effect of cytokine therapy or immunomodulatory or demethylating agents on modulation of iron parameters in the clinical setting.
Assessing Iron Overload
The simplest and most widely available measure of iron load is the serum ferritin level. Although ferritin can be affected by many variables, trends give important information about iron load and ferritin correlates with transfusion burden.6 Liver iron concentration (LIC) obtained by liver biopsy is the gold-standard for measurement of iron load, and in thalassemia major correlates well with newer non-invasive methods of assessing iron such as T2* MRI. There has been reluctance to assess the liver iron concentration by biopsy in MDS patients because of neutropenia, thrombocytopenia and comorbidities and the risk of complications; however, T2* MRI may provide new insights into iron in relation to organ function in MDS. Interestingly, despite the high incidence of cardiac complications in heavily transfused MDS patients,6,25 the majority of patients failed to demonstrate significant cardiac iron deposition on T2* MRI.33 However, a transfusion burden of 75 to 100 units is required before significant cardiac iron deposition is seen, and the results suggest that the major mechanism of cardiac complications in MDS may be a result of anemia rather than IOL.
Once transferrin saturation exceeds 75%, non-transferrin bound iron (NTBI) may be detected and lead to the formation of labile plasma iron (LPI) and reactive oxygen species (ROS), resulting in damage to cellular components. The use of NTBI, LPI and ROS to inform clinical decision-making is still investigational.
Clinical Adverse Effects of Iron Overload in MDS
The Pavia group demonstrated decreased survival in transfusion-dependent patients with MDS according to ferritin level, suggesting an adverse effect of IOL on survival.9 Several retrospective reviews in MDS have supported these findings. The Japanese reviewed the impact of IOL and ICT in 152 patients with transfusion-dependent MDS. Of deaths in 38 patients with a recorded ferritin level, the ferritin was over 1000 in 37 and over 5000 in 24, suggesting that IOL was marked in most patients who died and could have contributed to mortality.34 A US insurance database was searched for complications potentially attributable to IOL in MDS, such as cardiomyopathy/CHF, conduction/rhythm disorders, diabetes, and liver disease, and identified a higher frequency in patients who received transfusions than in those who did not (odds ratio 2.90, P = .0008).35 A reanalysis of the IPSS found that depth of anemia, implying transfusion dependence, had additive prognostic value on OS over and above the IPSS score in the intermediate-risk groups (P < .0001).8 Finally, in an analysis of 2994 MDS patients, both transfusion dependence (TD) and IOL impacted adversely on OS and, importantly, on LFS. In this study, 835 patients were TD at diagnosis; 526 became TD during follow-up; and 880 were non-TD; the median OS was 19, 60 and 96 months, respectively (P < .0001). A multivariate analysis of 902 patients from this study with complete data showed that IOL (HR 52.4; P < .0001) and TD (HR 8.8; P < .0001) added significant prognostic information on OS to the IPSS and WPSS scores. Moreover, a multivariate analysis of the risk of AML transformation showed IOL (HR, 6.6; P < .0001) and TD (HR, 3.5; P = .003) had a significant prognostic impact on this endpoint in addition.10
Not all data are in agreement as to the impact of transfusion dependence and IOL on clinical outcome in MDS, however. A retrospective review of 126 patients with RARS found that while RBC transfusion requirement at diagnosis predicted survival (P = .001), the number of RBC units transfused and ferritin level did not.7 See Table 1 for a summary of studies assessing an impact of transfusion dependence, IOL and ICT in MDS.
Clinical Benefit of ICT in MDS
In the Japanese study, of 126 MDS patients receiving deferoxamine, 8.6% received a dose that is considered optimal, and ferritin levels and liver enzyme abnormalities were reduced from baseline in this group compared to patients receiving suboptimal chelation (P < .05).34
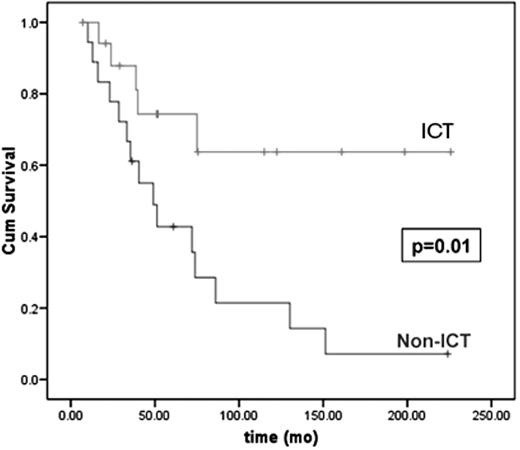
Two retrospective studies in MDS examined the impact of ICT on survival. In a review of 178 MDS patients from Vancouver,14,16 ICT patients received deferoxamine by subcutaneous infusion via portable pump, at least 12 hours per day, at least 5 days per week, and according to ferritin level, as per the product monograph. Supportive care as primary MDS therapy was used in 71%. One hundred and twenty patients (67%) were transfusion dependent, and 18 received ICT for a median of 21.6 (1.3–151) months. The median pre-ICT ferritin level was 4215 (range, 1500–8400 μg/L), and 2659 (567–5228 μg/L; P < .003) over the period of ICT. In a multivariate analysis, factors significant for OS were: IPSS score (P = .008; HR for int-2– or high vs lower risk, 2.2 [95% CI 1.3–3.7]); and ICT (P = .02; HR, 0.1 [0.01–1.0]). For patients with low- or intermediate-1 IPSS, the 4-year OS was 64% in ICT patients and 43% in non-ICT patients (P = .003). In a subgroup analysis in which controls were matched to ICT patients for performance status; MDS subtype; neutrophil, hemoglobin, and platelet counts at MDS diagnosis; number of cytopenias; IPSS karyotype risk group; and total number of RBC units received, the OS benefit of ICT was maintained (P = .01; HR, 0.29 [0.10–0.79]; Figure 3 ).
A retrospective study from the Groupe Francophone des Myelodysplasies (GFM) reported an effect of ICT on survival that appeared to be dose related.15 In this series of 170 MDS patients, 59% had lower-risk disease and 115 received ICT, 76 for at least 6 months. Nineteen patients received deferoxamine by intermittent bolus (“low chelation”), while 57 patients received deferoxamine by infusion ≥ 3 days per week, or deferiprone, deferasirox, or a combination of agents (“standard chelation”). The median OS was 115 and 51 months in ICT and non-ICT groups, respectively, with statistical significance maintained after adjustment for prognostic factors unequally distributed between groups (P = .0001), and the median OS was 120 and 69 months in the standard- and low-chelation groups, respectively (P < .001).15
Within the limitations of retrospective analyses, the data suggest that in lower-risk MDS, ICT reduces ferritin levels, improves liver enzyme abnormalities, is associated with improved survival, and a survival effect may correlate with the degree of chelation. Whether the apparent survival impact in these studies was truly a result of reducing iron burden, or was a question of confounding factors or bias will hopefully be settled by prospective, controlled trials in the near future.
Improvement of Cytopenias with Iron Chelation; Possible Relation to Oxidative Stress
Some observations suggest that cytopenias in iron-overloaded patients with MDS could be mitigated by ICT, possibly by a decrease in reactive oxygen species-mediated damage to hematopoietic cells. There are case reports of 10 MDS patients who became RBC TI after beginning ICT (reviewed in Leitch et al36). Nine additional patients had decreased RBC transfusion requirements, and some showed improvement in trilineage myelopoiesis. In addition, in the US03 trial of deferasirox in MDS, hematologic improvement was seen in 5 of 53 patients (9.4%).37 In this trial, labile plasma iron normalized in all patients over 12 months of treatment; whether this is related to the mitigation of cytopenias remains to be determined. Further, in another study of 15 iron-overloaded patients with MDS, there was a significant decrease over a mean of 3 months of ICT in measures of oxidative stress in RBC including reactive oxygen species (28%, P = .006), lipid peroxidation (138%, P = .008) and labile iron (23%, P = .004), and a concomitant increase in glutathione levels (scavengers of ROS; 123%, P = .001).38 The potential for mitigating suppression of and ineffective erythropoiesis by reducing iron and/or oxidative stress is an intriguing area worthy of further investigation.
Iron Chelation Agents
The options for ICT include deferoxamine, which, because of its short half-life, must be given by continuous infusion to result in effective chelation. Deferiprone, an oral agent that is not available in North America, is administered three times daily. Deferasirox, another oral agent, has once daily dosing. The medications and their characteristics are summarized in Table 2 .
Guidelines for Iron Chelation
A number of guidelines on the use of ICT in patients with MDS have been reviewed and a consensus statement published.39 The aim of ICT is to prevent or reverse established IOL and end organ dysfunction. Other possible benefits include a potential impact on transfusion requirements, AML transformation and survival. Recommendations for initiating and monitoring ICT are summarized in Table 3 .
Conclusions
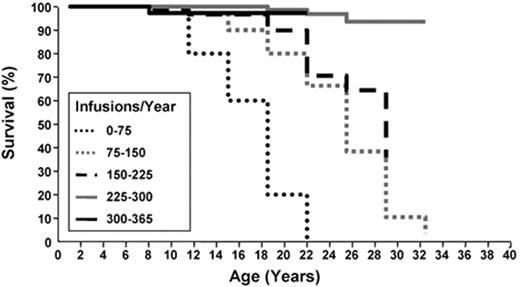
It is well documented that IOL inevitably leads to progressive organ dysfunction and death in beta-thalassemia major (BTM).13,26 Prior to the routine use of ICT, few patients with BTM survived beyond the second decade. The use of deferoxamine reduced iron burden, morbidity (cardiac, hepatic and endocrine dysfunction) and mortality, and the survival of patients not only approached normal but correlated to the degree of compliance with deferoxamine (Figure 4 ).26 Whether the outcomes related to organs apply to the MDS population is not well studied. On the one hand, the older age of MDS patients places them at risk for organ dysfunction from comorbidities, which could increase the risk of iron-related complications and make patients amenable to a proportionally greater benefit of iron reduction. On the other hand, the shorter remaining life expectancy of patients with MDS than with BTM could render a beneficial effect of ICT less clinically relevant. The data showing a beneficial survival impact of ICT in MDS are retrospective, not all data or opinions are in agreement as to the importance of IOL and ICT in MDS,7,40 and there are few data available as to the impact of IOL and ICT on end-organ function in this population. Still, there are few interventions in medicine that have had the positive impact on survival that ICT has had in BTM, there are preclinical and clinical data showing biological plausibility to a beneficial effect of iron reduction, and important points have been raised in MDS that warrant further study and should be analyzed in prospective trials. Such trials would ideally include objective measurements of iron burden and oxidative stress as well as measurements of organ function at standardized intervals, and measure impact on quality of life and cost/benefit ratio. As ICT is costly, it should be undertaken only after carefully weighing the potential risks, including financial, against potential benefits. In view of the preliminary studies suggesting survival and other benefits to ICT, however, and in view of the availability of oral iron chelators, further study of this intervention in patients with MDS is needed.
Summary of studies assessing the impact of transfusion dependence, iron overload and iron chelation therapy on clinical parameters in MDS patients in the usual (non-transplant) clinical setting.
| . | . | . | Transfusion dependence HR . | Iron overload HR . | Iron chelation therapy HR . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Study . | n . | Reference population . | OS . | LFS . | OS . | LFS . | OS . | LFS . | Comments . | Ref . |
| 1Comparison group non-transfusion dependent patients (0 RBCU/4 wks) without | ||||||||||
| 2Comparison group low/int-1 IPSS risk not receiving iron chelation therapy | ||||||||||
| 3Comparison group, patients who did not receive chelation | ||||||||||
| 4Comparison group RARS, for TD, non-TD; for IOL, ferritin <1000 compared to ≥ 1000, and compared to 1000–5000 and >5000 | ||||||||||
| 5Comparison group for transfusion dependence, requiring <1 RBCU/4 wk; for IOL, ferritin ≤ 1000 ng/mL | ||||||||||
| 6Comparison group Hb>10 g/dL | ||||||||||
| 7Comparison group, intermittent ICT patients; of deaths, 24% were from CHF and 6.7% excess blasts from liver-related causes | ||||||||||
| 8Japanese units are smaller than North American units, usually derived from 200 mL of whole blood | ||||||||||
| 9Comparison group, controls were randomly selected from MDS non-cases (see footnote 10) | ||||||||||
| 10Complications of IOL, cases; cardiomyopathy/CHF, conduction/rhythm disorders, diabetes, liver disease | ||||||||||
| ALT indicates alanine aminotransferase; AN, anemia; aprx, approximately; AST, aspartate aminotransferase; CHF, congestive heart failure; int, intermediate; DFO, deferoxamine; DM, diabetes mellitus; inter, intermittent; GFM, Groupe Francophone des Myelodysplasies; Hb, hemoglobin; HR, hazard ratio; ICT, iron chelation therapy; IMRAW, International MDS Risk Analysis Workshop; IOL, iron overload; IPSS, International Prognostic Scoring System; LFS, leukemia-free survival; MDS, myelodysplastic syndrome; Med, median; ng, nanogram; NS, not significant; OR, odds ratio; OS, overall survival; RARS, refractory anemia with ring sideroblasts; RBC, red blood cell; STD, standard; TD, transfusion dependence; U, unit(s); US, United States | ||||||||||
| Pavia1 | 467 | Without excess blasts | 1.91 1.36/RBCU/4 wks P < .001 | 1.84 1.40/RBCU/4 wks P < .001 | 1.42 per 500 ng/mL P < .001 | – | – | – | IOL, ferritin ≥ 1000 ng/mL | 6,9 |
| Vancouver2 | 178 | IPSS low/int-1 ICT, n = 18 | – | – | – | – | 0.1 P = .02 | – | 4y OS 64% vs 43% | 14, 16 |
| GFM3 | 170 | IPSS low/int-1 ICT, n = 115 | – | – | – | – | P = .003 | – | Median OS (mo) STD ICT, 120 Low ICT, 69 No ICT, 50–69 | 15 |
| Mayo4 | 126 | RARS | P = .001 | – | NS | – | – | – | ICT, n = 15 | 7 |
| Sanz5 | 2884 | n = 902 | 8.8 | 3.5 | 52.4 | 6.6 | – | – | 10 | |
| IMRAW/IPSS6 | 816 | Int-1/Int-2 Hb ≤ 10.0 g/dL | P = .0001 | NS | - | - | - | - | MedOS aprx 1/2 AN vs TD vs IOL effect? | 8 |
| Japanese7 | 292 MDS, n = 152 | DFO, n = 126 8.6% continuous (91.4% intermittent) | Median NRBCU8 Died, n = 289.2 Alive, n = 160.7 P = .0033 | – | Died, n = 38 Ferritin >1000, n = 37/38 Ferritin >5000, n = 24 | – | – | – | Ferritin, AST, ALT decreased in continuous ICT pts P ≤ .005 | 34 |
| US insurance database9 | 4546 | 511 cases 511 controls | OR 2.90, P = .0008 For developing complications of IOL10 | – | – | – | – | – | Arrhythmia OR 4.18, P = .0005 DM OR 5.06, P = .0025 Liver OR 3.31, P = .0008 | 35 |
| . | . | . | Transfusion dependence HR . | Iron overload HR . | Iron chelation therapy HR . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Study . | n . | Reference population . | OS . | LFS . | OS . | LFS . | OS . | LFS . | Comments . | Ref . |
| 1Comparison group non-transfusion dependent patients (0 RBCU/4 wks) without | ||||||||||
| 2Comparison group low/int-1 IPSS risk not receiving iron chelation therapy | ||||||||||
| 3Comparison group, patients who did not receive chelation | ||||||||||
| 4Comparison group RARS, for TD, non-TD; for IOL, ferritin <1000 compared to ≥ 1000, and compared to 1000–5000 and >5000 | ||||||||||
| 5Comparison group for transfusion dependence, requiring <1 RBCU/4 wk; for IOL, ferritin ≤ 1000 ng/mL | ||||||||||
| 6Comparison group Hb>10 g/dL | ||||||||||
| 7Comparison group, intermittent ICT patients; of deaths, 24% were from CHF and 6.7% excess blasts from liver-related causes | ||||||||||
| 8Japanese units are smaller than North American units, usually derived from 200 mL of whole blood | ||||||||||
| 9Comparison group, controls were randomly selected from MDS non-cases (see footnote 10) | ||||||||||
| 10Complications of IOL, cases; cardiomyopathy/CHF, conduction/rhythm disorders, diabetes, liver disease | ||||||||||
| ALT indicates alanine aminotransferase; AN, anemia; aprx, approximately; AST, aspartate aminotransferase; CHF, congestive heart failure; int, intermediate; DFO, deferoxamine; DM, diabetes mellitus; inter, intermittent; GFM, Groupe Francophone des Myelodysplasies; Hb, hemoglobin; HR, hazard ratio; ICT, iron chelation therapy; IMRAW, International MDS Risk Analysis Workshop; IOL, iron overload; IPSS, International Prognostic Scoring System; LFS, leukemia-free survival; MDS, myelodysplastic syndrome; Med, median; ng, nanogram; NS, not significant; OR, odds ratio; OS, overall survival; RARS, refractory anemia with ring sideroblasts; RBC, red blood cell; STD, standard; TD, transfusion dependence; U, unit(s); US, United States | ||||||||||
| Pavia1 | 467 | Without excess blasts | 1.91 1.36/RBCU/4 wks P < .001 | 1.84 1.40/RBCU/4 wks P < .001 | 1.42 per 500 ng/mL P < .001 | – | – | – | IOL, ferritin ≥ 1000 ng/mL | 6,9 |
| Vancouver2 | 178 | IPSS low/int-1 ICT, n = 18 | – | – | – | – | 0.1 P = .02 | – | 4y OS 64% vs 43% | 14, 16 |
| GFM3 | 170 | IPSS low/int-1 ICT, n = 115 | – | – | – | – | P = .003 | – | Median OS (mo) STD ICT, 120 Low ICT, 69 No ICT, 50–69 | 15 |
| Mayo4 | 126 | RARS | P = .001 | – | NS | – | – | – | ICT, n = 15 | 7 |
| Sanz5 | 2884 | n = 902 | 8.8 | 3.5 | 52.4 | 6.6 | – | – | 10 | |
| IMRAW/IPSS6 | 816 | Int-1/Int-2 Hb ≤ 10.0 g/dL | P = .0001 | NS | - | - | - | - | MedOS aprx 1/2 AN vs TD vs IOL effect? | 8 |
| Japanese7 | 292 MDS, n = 152 | DFO, n = 126 8.6% continuous (91.4% intermittent) | Median NRBCU8 Died, n = 289.2 Alive, n = 160.7 P = .0033 | – | Died, n = 38 Ferritin >1000, n = 37/38 Ferritin >5000, n = 24 | – | – | – | Ferritin, AST, ALT decreased in continuous ICT pts P ≤ .005 | 34 |
| US insurance database9 | 4546 | 511 cases 511 controls | OR 2.90, P = .0008 For developing complications of IOL10 | – | – | – | – | – | Arrhythmia OR 4.18, P = .0005 DM OR 5.06, P = .0025 Liver OR 3.31, P = .0008 | 35 |
Iron chelation agents currently available for clinical use; properties and indications.
| Property . | Deferoxamine . | Deferiprone . | Deferasirox . |
|---|---|---|---|
| *Monitoring as per product monograph for all agents | |||
| †Yearly monitoring recommended for all | |||
| ‡Usually reversible or non-progressive | |||
| BTM indicates beta-thalassemia major; DFO, deferoxamine; GI, gastrointestinal; h, hours; IOL, iron overload; iv, intravenous; min, minutes; po, orally; RBC, red blood cell; rxn, reaction; sc, subcutaneous; TD, transfusion-dependent. | |||
| Usual dose | 20–60 mg/kg/d | 75–100 mg/kg/d | 20–30 mg/kg/d |
| Route | sc, iv ≥ 8–12 h, ≥ 5 d/wk | po 3 times daily | po once daily |
| Half-life | 20–30 min | 3–4 h | 8–16 h |
| Excretion | Urinary, fecal | Urinary | Fecal |
| Side effects* | Injection site rxn Potential ocular† and/or otic toxicity† | (rare) agranulocytosis | Renal insufficiency in up to one third‡ GI disturbance |
| Indications | Acute iron intoxication Chronic IOL from TD-anemias | IOL in BTM when DFO contraindicated or inadequate | BTM ≥ 6 y with IOL from frequent RBC transfusion IOL when DFO contraindicated or inadequate in: Other anemias Age 2–5 y BTM with IOL from infrequent RBC transfusion |
| Property . | Deferoxamine . | Deferiprone . | Deferasirox . |
|---|---|---|---|
| *Monitoring as per product monograph for all agents | |||
| †Yearly monitoring recommended for all | |||
| ‡Usually reversible or non-progressive | |||
| BTM indicates beta-thalassemia major; DFO, deferoxamine; GI, gastrointestinal; h, hours; IOL, iron overload; iv, intravenous; min, minutes; po, orally; RBC, red blood cell; rxn, reaction; sc, subcutaneous; TD, transfusion-dependent. | |||
| Usual dose | 20–60 mg/kg/d | 75–100 mg/kg/d | 20–30 mg/kg/d |
| Route | sc, iv ≥ 8–12 h, ≥ 5 d/wk | po 3 times daily | po once daily |
| Half-life | 20–30 min | 3–4 h | 8–16 h |
| Excretion | Urinary, fecal | Urinary | Fecal |
| Side effects* | Injection site rxn Potential ocular† and/or otic toxicity† | (rare) agranulocytosis | Renal insufficiency in up to one third‡ GI disturbance |
| Indications | Acute iron intoxication Chronic IOL from TD-anemias | IOL in BTM when DFO contraindicated or inadequate | BTM ≥ 6 y with IOL from frequent RBC transfusion IOL when DFO contraindicated or inadequate in: Other anemias Age 2–5 y BTM with IOL from infrequent RBC transfusion |
Recommendations for initiating and monitoring iron chelation therapy in myelodysplastic syndromes.1
| 1Adapted from Bennett JM et al. Am J Hematol. 2008;83(11):858–861. Copyright 2008, Wiley-Liss Inc. Reprinted with permission of John Wiley & Sons Inc., Hoboken, USA. |
| ICT indicates iron chelation therapy; IPSS, International Prognostic Scoring System; LPI, labile plasma iron; MDS, myelodysplastic syndrome; NTBI, non-transferrin bound iron; RA, refractory anemia; RARS, refractory anemia with ring sideroblasts; ROS, reactive oxygen species; WHO, World Health Organization. |
| MDS patients who would benefit most from treatment of iron overload |
| Requiring transfusion of ≥ 2 RBC units/month for ≥ 1 year |
| Ferritin level >1000 ng/mL |
Low-risk MDS
|
| Life expectancy >1 year |
| Without comorbidities that would limit prognosis |
| Candidate for allograft |
| In whom there is a need to preserve organ function |
| Unresponsive to or ineligible for primary therapy such as immunomodulatory or hypomethylating agents |
| Monitoring Iron Overload |
| Serum ferritin |
| Transferrin saturation |
| MRI where available |
| Investigational parameters (NTBI, LPI, ROS) where available |
| Monitoring of organ function (cardiac, hepatic, endocrine) where indicated |
| At least every 3 months in patients receiving transfusions, following recommendations for individual ICT agents |
| Duration of ICT |
| As long as transfusion therapy continues |
| As long as IOL remains clinically relevant |
| 1Adapted from Bennett JM et al. Am J Hematol. 2008;83(11):858–861. Copyright 2008, Wiley-Liss Inc. Reprinted with permission of John Wiley & Sons Inc., Hoboken, USA. |
| ICT indicates iron chelation therapy; IPSS, International Prognostic Scoring System; LPI, labile plasma iron; MDS, myelodysplastic syndrome; NTBI, non-transferrin bound iron; RA, refractory anemia; RARS, refractory anemia with ring sideroblasts; ROS, reactive oxygen species; WHO, World Health Organization. |
| MDS patients who would benefit most from treatment of iron overload |
| Requiring transfusion of ≥ 2 RBC units/month for ≥ 1 year |
| Ferritin level >1000 ng/mL |
Low-risk MDS
|
| Life expectancy >1 year |
| Without comorbidities that would limit prognosis |
| Candidate for allograft |
| In whom there is a need to preserve organ function |
| Unresponsive to or ineligible for primary therapy such as immunomodulatory or hypomethylating agents |
| Monitoring Iron Overload |
| Serum ferritin |
| Transferrin saturation |
| MRI where available |
| Investigational parameters (NTBI, LPI, ROS) where available |
| Monitoring of organ function (cardiac, hepatic, endocrine) where indicated |
| At least every 3 months in patients receiving transfusions, following recommendations for individual ICT agents |
| Duration of ICT |
| As long as transfusion therapy continues |
| As long as IOL remains clinically relevant |
Survival of patients with myelodysplastic syndrome (MDS) according to the severity of transfusion requirement; overall survival is shown in the left panel and leukemia-free survival on the right. Adapted from Malcovati L, et al. Haematologica. 2006;91:1588–90, with permission from the Ferrata Storti Foundation, Pavia, Italy.
Survival of patients with myelodysplastic syndrome (MDS) according to the severity of transfusion requirement; overall survival is shown in the left panel and leukemia-free survival on the right. Adapted from Malcovati L, et al. Haematologica. 2006;91:1588–90, with permission from the Ferrata Storti Foundation, Pavia, Italy.
Overall survival of transfusion-dependent patients with myelodysplastic syndrome (MDS) according to ferritin level. The survival effect is mainly seen in lower risk patients, RA, RARS and 5q-, shown on the left as compared with higher risk, RCMD and RCMD-RS, shown on the right. Adapted from Malcovati L, et al. Haematologica. 2006;91:1588–90, with permission from the Ferrata Storti Foundation, Pavia, Italy.
Overall survival of transfusion-dependent patients with myelodysplastic syndrome (MDS) according to ferritin level. The survival effect is mainly seen in lower risk patients, RA, RARS and 5q-, shown on the left as compared with higher risk, RCMD and RCMD-RS, shown on the right. Adapted from Malcovati L, et al. Haematologica. 2006;91:1588–90, with permission from the Ferrata Storti Foundation, Pavia, Italy.
Overall survival in patients with myelodysplastic syndromes (MDS) according to receipt of ICT in a subgroup analysis. In this study, 18 patients receiving ICT were matched for baseline features to 18 controls with low or intermediate-1 IPSS risk MDS. Adapted from Leitch H, et al. Clinical Leukemia 2008;2:205–211, with permission from CIG Media Group, Danvers, USA.
Overall survival in patients with myelodysplastic syndromes (MDS) according to receipt of ICT in a subgroup analysis. In this study, 18 patients receiving ICT were matched for baseline features to 18 controls with low or intermediate-1 IPSS risk MDS. Adapted from Leitch H, et al. Clinical Leukemia 2008;2:205–211, with permission from CIG Media Group, Danvers, USA.
Compliance with deferoxamine infusions is related to survival in beta-thalassemia major. Kaplan-Meier analysis of survival in 257 consecutive thalassemic patients by the number of days deferoxamine infusions were administered per year. Adapted from Gabutti V and Piga A. Acta Haematol. 1996;95:26–36, with permission from S. Karger AG, Basel.
Compliance with deferoxamine infusions is related to survival in beta-thalassemia major. Kaplan-Meier analysis of survival in 257 consecutive thalassemic patients by the number of days deferoxamine infusions were administered per year. Adapted from Gabutti V and Piga A. Acta Haematol. 1996;95:26–36, with permission from S. Karger AG, Basel.
Disclosures Conflict-of-interest disclosure: HL and LV have received research funding and honoraria from Novartis Canada. Off-label drug use: Off-label use of deferiprone and deferasirox are discussed.
References
Author notes
Division of Hematology, St. Paul’s Hospital and the University of British Columbia, Vancouver, BC, Canada