Abstract
The management of factor XI deficiency is not straightforward for three reasons: firstly, the role of this factor in the coagulation pathway is not clearly understood; secondly, the bleeding tendency, although mild, is unpredictable and does not clearly relate to the factor XI level; and thirdly, all treatment products, although available, have some potentially serious side effects. These factors (or enigmas) contribute to the variable management of patients with this coagulation factor deficiency, but recent research is helping to clarify some of these areas.
Factor XI (FXI) deficiency was described in three members a Jewish family in the USA and was called hemophilia C to distinguish it from hemophilias A and B.1 FXI deficiency was distinguished from classical hemophilia by its presence in both men and women, a mild bleeding tendency, and the observation that the laboratory defect could be corrected by mixing tests with hemophilia A and B plasmas. Bleeding occurred after tonsillectomy and dental extractions in two sisters, and their uncle also bled after dental extractions but had a normal circumcision in infancy. Other affected individuals were identified among the 13 individuals in 4 generations.
An assay for FXI was published in 1961, and two subsequent studies of 18 Jewish kindred2,3 demonstrated autoso-mal inheritance with severe deficiency in those with FXI levels less than 20% of normal and partial deficiency in heterozygotes (levels from 20% to 60%, equivalent to the lower limit of the normal range), some of whom had a definite bleeding history.2 Thus, from the early publications there is evidence of bleeding in heterozygotes. Subsequent studies have confirmed this finding.4–6 This disorder is, therefore, not completely recessive and the variability of the bleeding tendency constitutes one enigma of this deficiency.
Even in severe deficiency the bleeding tendency is mild, generally without spontaneous bleeding, and hemarthrosis is not a feature. Indeed, some individuals with severe deficiency do not exhibit excessive bleeding even after tonsillectomy, surgery that is a major challenge to hemostasis. Bleeding is characteristically provoked by surgery and particularly in areas of high fibrinolysis such as the mouth and genitourinary system.
Structure and Function in Coagulation
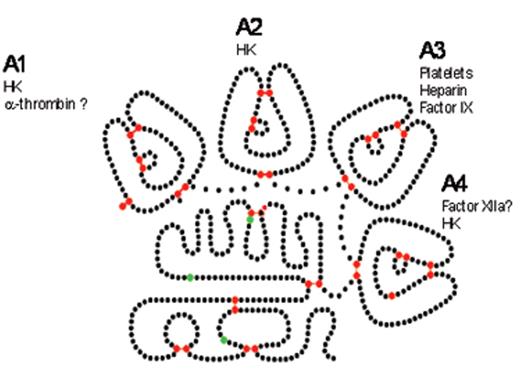
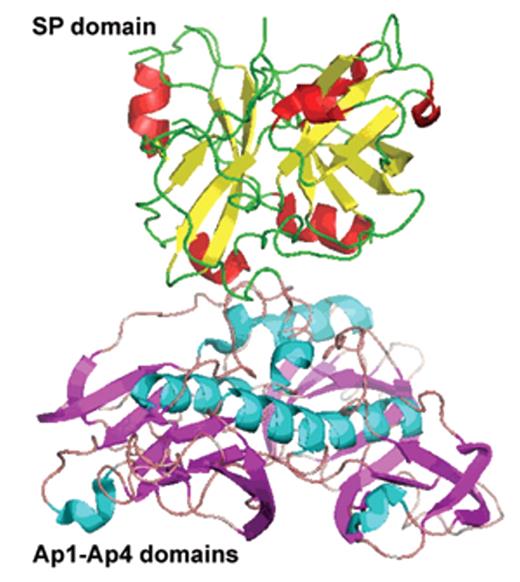
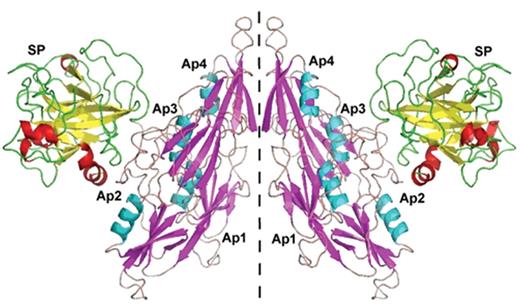
Factor XI is a serine protease unique among coagulation factors as it circulates as a homodimer. Each FXI monomer consists of four apple domains (A1–4) and a serine protease C-terminal light chain (Figure 1A ). The monomers are linked by a disulphide bond—Cys321-Cys321—in the A4 domain, but dimer formation depends upon other important non-covalent interactions. The presence of the Cys321 binding is not necessary for dimerization, but other residues are essential, for example Leu284, Ile290 and Tyr329.7 The apple domains also permit several important protein interactions that are crucial for function. Activation of FXI occurs by cleavage at Arg369-Ile370 by thrombin, FXIIa or FXIa, but it is not clear that this is the important physiological activator. Cleavage results in a conformational change that may bring the two active sites closer together.8 FXI binds via A3 to platelet Gp1b.9 The crystal structure of FXI was reported in 200610 and shows that the apple domains form a circular platform at the base of the catalytic domain, resembling a “cup and saucer” shape (Figure 1B and 1C ). Activation of FXI leads to a major change in shape exposing a FIX binding site promoting its activation by a complex mechanism which is not fully elucidated.
The precise role of FXI in coagulation is debated (a second enigma). Although originally described as part of the “contact system” (describing the proteins involved in coagulation after exposure to artificial surfaces such as glass and silica), the physiological counterpart of this pathway is not fully understood. Factor XII is autoactivated on negatively charged surfaces and then activates FXI. However, in contrast to FXI, individuals with FXII or high-molecular-weight kininogen (HMWK) deficiency have no bleeding symptoms. Because FXI deficiency is associated with bleeding, an alternative activation pathway was proposed. FXI is activated by thrombin, permitting feed-back activation of the intrinsic pathway by this mechanism. Although widely accepted as part of the “revised model of coagulation” this was challenged by work that suggested that thrombin-activation of FXI is caused by contact activation during processing of plasma because it could be abolished by a specific FXIIa inhibitor (corn trypsin inhibitor).11 Further elegant and careful experiments have clearly confirmed that FXI can be activated by an FXII-independent mechanism in a plasma environment.12 Use of recombinant FXI variants in these experiments demonstrated dependence of thrombin generation on a normal FIX-thrombin binding site and on the presence of normal FXI catalytic activity. In addition, substitution of the thrombin binding site in the A1 domain reduced thrombin generation, but activation by FXIIa was normal. The debate continues; it has been suggested that contact activation results in pathological thrombosis but feedback activation promotes hemostasis.13
Another important function of FXI activation is to reduce fibrinolysis by promoting activation of thrombin activatable fibrinolytic inhibitor (TAFI).14 Greater amounts of thrombin are required for this reaction than can be generated by the tissue-factor–FVIIa pathway, so the intrinsic pathway feedback activation via FXI is important for this. Loss or reduction of this normal pathway of inhibition in FXI deficiency fits well with the observation that FXI-deficient individuals are prone to excessive bleeding after surgery or injuries to areas with high levels of fibrinolysis. Conversely, individuals with venous thromboembolism who have high levels of TAFI and FXI have a higher recurrence rate.15 Severe FXI deficiency protects against ischemic stroke16 (but not myocardial infarction), and there is an association between an increased risk of stroke and elevated FXI levels.17
FXI may have other functions. The contact system is linked to inflammatory pathways and is activated in sepsis, inducing alterations in blood flow consequent upon production of bradykinin. Two kinin receptors mediate either acute inflammatory reactions or a chronic response. The coagulation and innate immunity systems have likely co-evolved, and the contact system is activated on bacterial surfaces, resulting in production of antimicrobial peptides, thus protecting against dissemination of bacteria.18
Both FXII- and FXI-knockout mice are protected from thrombosis without evidence of excessive bleeding. FXI deficiency in this model causes thrombus instability that prevents vessel occlusion, which has led to the suggestion that FXI inhibitors may have a role in thrombus treatment or prevention.19 Other knockout mouse studies (combined plasminogen/FXI-knockouts) suggest FXI may have activity in regulation of inflammation and tissue repair.20 FXI-deficient mice also have reduced coagulopathy and increased survival compared to normal mice in a peritoneal sepsis model consistent with a role in sepsis.21
Molecular Genetics
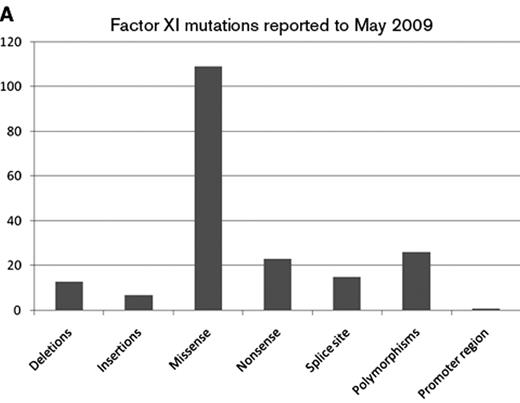
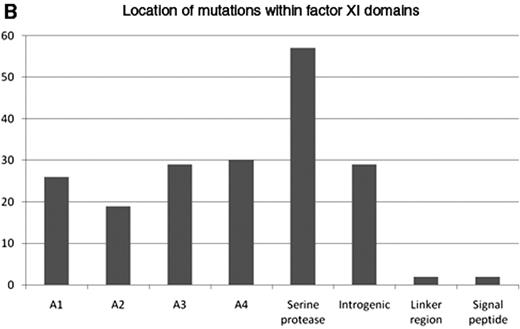
The gene for FXI is located on chromosome 4 and is 23kb in length with 15 exons. Exons 1 and 2 code for an untranslated region and a signal peptide, respectively. Exons 3 to 10 code for the 4 apple domains, and exons 11 to 15 code for the protease domain. Mutations are recorded in FXI gene databases (for example www.factorxi.com updated in May 2009, and www.isth.org/Publications/RegistriesDatabases/MutationsRareBleedingDisorders/FactorXI.aspx, updated in January 2009). Most are missense or nonsense mutations and are spread across all 4 apple domains and the serine protease region (Figures 2A and 2B ). FXI deficiency is common in Ashkenazy Jews with a heterozygote frequency of 8% to 10%. In this population two particular mutations are common, Glu117stop (known as type II) in exon 5, which results in early chain termination and absent protein, and Phe283Leu (known as type III) in exon 9, which results in partial defect in dimerization and retention of monomer within the cell. Individuals homozygous for type II mutation have absent FXI coagulation activity in contrast to those who are homozygous for type III mutations, who have residual activity of about 10 IU/dL. Compound heterozygotes have intermediate levels. In these individuals there is some phenotype-genotype correlation with more severe bleeding symptoms in the type II homozygotes compared to type III homozygotes.
In non-Jewish people the mutations are much more variable although Cys128stop has been described in several families in the UK and Cys38Arg clusters in the French Basques. Most mutations have equally low antigen and activity levels (cross-reacting material [CRM] negative), but 8 very interesting CRM+ variants22 are reported that have given some insight into FXI functioning. Five of these are in the catalytic domain and 3 in the apple domains.
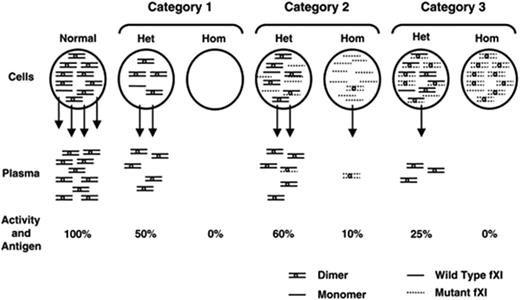
Interestingly, some mutations result in monomers that dimerize normally but are the poorly secreted. This can cause apparent dominant negative effects and a lower plasma FXI level. Individuals who are obligate heterozygous for FXI mutations have variable FXI coagulant activity (FXIC) levels (with a mean of 57 IU/dL, range 43–80 IU/dL in one study5). However, some individuals with FXI levels of less than 20 IU/dL have mutations that have been demonstrated by cotransfection experiments to result in reduction in secretion of the wild-type FXI by about 50%,23 leading to the hypothesis that nonsecretable mutant FXI molecules trap wild type molecules within the cell, resulting in lower FXI levels than in heterozygotes for other FXI gene mutations. A classification for CRM-negative FXI deficiency proposes three mechanisms: mutations that reduce synthesis, mutations that result in molecules that fail to form dimers and are not secreted, and mutations that result in dimers that are not secreted24 (Figure 3 ).
One important observation is that the development of inhibitors to FXI is a significant complication in about one third of individuals who are homozygous for Glu117stop and possibly for other chain termination mutations that result in absent FXI.25 Although most patients who develop inhibitors have been previously exposed to plasma or FXI concentrate, even a minor amount of FXI contaminating anti-D globulin administered for prophylaxis in pregnancy has been convincingly demonstrated to result in inhibitor development.26 While individuals with inhibitors rarely exhibit spontaneous bleeding, they may not respond to therapy with FXI and are at risk of an anamnestic response. There may therefore be benefit in establishing the genotype in severely deficient individuals and in avoiding use of plasma products.
Clinical Presentation and Management
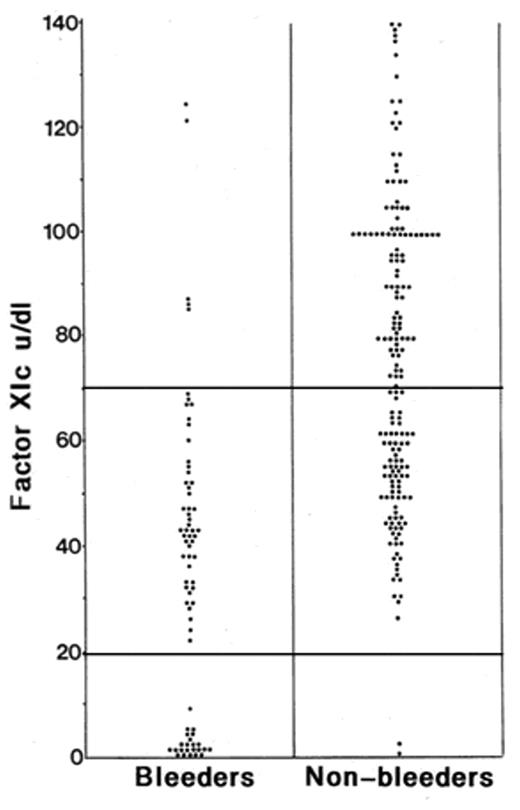
Factor XI deficiency may be diagnosed as a result of bleeding or be found incidentally as part of presurgical work-up for a prolonged activated partial thromboplastin time (APTT). The normal range for FXI should be established (as for all coagulation factor assays) by testing at least 20 normal individuals. The mean will be at about 100 IU/dL, with a range from about 65 to 130. It should be noted that there is considerable variation in the sensitivity of different thromboplastin reagents to FXI so that a normal APTT will not exclude FXI deficiency. Heterozygous individuals with a bleeding history are reported with levels above 50 IU/dL.4 Spontaneous bleeding is rare, and typically bleeding occurs after surgery or injuries to areas of high fibrinolysis such as the mouth, nose and genitourinary system. Individuals with severe deficiency (defined as FXI less than about 10 IU/dL, generally individuals with two abnormal FXI genes) are likely to bleed excessively but do not invariably do so; for example, two brothers, both with severe deficiency, were discordant for bleeding at tonsillectomy in childhood.5 Heterozygotes with partial FXI deficiency may also be at risk of excessive bleeding (Figure 4 ), as discussed above. The risk is not easily predictable and does not relate clearly to the FXI level. It is not related to the genotype (other than in the severely deficient individuals homozygous or compound heterozygous for Cys117stop and Phe283Leu discussed above). The bleeding tendency may not be consistent within an individual or family. There are likely several explanations for this. In some individuals other coagulopathies are present that alter the balance of hemostasis, such as von Willebrand disease or platelet defects. The modifiers may be more subtle, for example a von Willebrand factor level towards the lower end of the normal range is associated with increased bleeding,4 but the factors are still largely undefined. The concept of interacting factors affecting the balance of hemostasis has recently been reviewed27 and is recognized for hemophilia A (for example, co-inheritance of factor V Leiden ameliorates the bleeding phenotype). A survey of healthy teenagers showed that a third of those reporting excessive bleeding symptoms had marginal reductions in von Willebrand factor or mild platelet defects.28 A case-control study of 317 women who developed severe postpartum hemorrhage demonstrated a correlation with low (but not abnormal) levels of fibrinogen, von Willebrand antigen and other factors including FXI.29 In von Willebrand disease the bleeding tendency may be related to the number of collagen receptors on platelets as determined by gene polymorphisms.30 It is likely therefore that the mild bleeding risk in FXI deficiency may be affected by minor changes in other components of the hemostatic pathways. Such factors may be both genetic and acquired.
In recent years there has been increasing recognition of difficulties with interpretation of bleeding histories and a need for standardization by objective methods. Nevertheless, there is convincing data for bleeding in heterozygotes by accepted objective criteria, such as after tonsillectomy and dental extractions.
Management of bleeding episodes and prevention of bleeding in relation to surgery is not straightforward and needs to be tailored to individual circumstances. Options are shown in Table 1 . As indicated above, the first decision is whether any treatment is necessary or whether management can be expectant, that is, observation only. Additional coagulation problems such as von Willebrand disease or platelet disorders should be excluded. Exposure to FXI should be avoided if possible in individuals with severe deficiency and Glu117stop homozygosity because of the risk of inhibitor development.25 Careful consideration should be given to the need for replacement therapy, which is not always mandatory (Table 2 ), and low-dose recombinant activated factor VIIa (rVIIa) may be considered as an alternative, although further work is required. Factors influencing management include the site and nature of surgery and the presence of any risk factors for thrombosis. In those without inhibitors the factor XI level can be raised by infusion of fresh frozen plasma (FFP), preferably pathogen-inactivated, but large volumes may be required, or by plasma-derived factor XI concentrates. The hemostatic level of FXIC is debated, but for those with severe deficiency a level of 30 to 45 IU/dL is probably sufficient. For individuals with partial deficiency who have a bleeding history, higher levels may be required (because their baseline exceeds this). Two FXI concentrates are available (Table 1 ), but both have been associated with thrombotic events in some patients, particularly those with pre-existing vascular disease. Concentrates provide safe and effective treatment with advantages over FFP. However, because of the thrombotic risk plasma FXI levels of 70 IU/dL should probably not be exceeded, and some experience suggests that a dose of 10 IU/kg may be sufficient. Moreover, if FFP is used it may be that other factors are being adjusted that contribute to hemostasis so that the absolute level of FXI may not be the only requirement. It is important to decide which patients need treatment. Dental extractions can be managed with oral antifibrinolytic agents alone.31 The type of surgery will affect the bleeding risk,32 for example, surgery in areas without fibrinolysis is much less likely to be complicated by bleeding. A review32 of 120 surgical procedures in 165 unrelated people with severe deficiency who did not receive prophylaxis demonstrated that the rate of bleeding in areas of fibrinolysis (tonsillectomy, dental extractions and prostatectomy) was 49% to 67%, but there was no excessive bleeding after 9 orthopedic procedures. Individuals who have developed inhibitors may be successfully treated for surgery with rVIIa together with tranexamic acid.33–35 Experimental work has shown that a lower amount of rVIIa is required for thrombin generation in FXI-deficient plasma than in severe hemophilia A or B plasmas.36 Use of lower doses of rVIIa (15–20 μg/kg) may reduce the risk of thrombosis observed in a series of patients treated for surgery where the conventional dose of 90 μg/kg was used.33,37
Women with FXI deficiency are likely to have menorrhagia; conversely, women with menorrhagia should be screened for both FXI deficiency and von Willebrand disease.38 Women with mild as well as severe FXI deficiency may bleed excessively at childbirth. Women with mild deficiency may be managed expectantly; some consider that prophylactic treatment is not always necessary for labor in women with severe deficiency39 but others advocate an active policy of replacement.40 A previous bleeding history predicts for postpartum hemorrhage.41
Circumcision is carried out in infancy in Jewish boys. UK guidelines recommend postponing surgery for 6 months if the FXI at birth is below 10 IU/dL, and active management with FFP or concentrate if it remains at this level; oral tranexamic acid is recommended for all infants with low FXI levels.42 In Israel, however, bleeding at circumcision is not often seen, and there is no pre-emptive treatment, but the operation is carried out under medical supervision (Table 2 ).
The management of surgery and other bleeding problems in FXI deficiency continues to be variable because of the risks of currently available therapies and the variability of bleeding risk. In 2006 a group of international hemophilia treaters with an interest in FXI deficiency discussed a series of case scenarios (based on real cases) and gave variable recommendations related to their own previous experience and the availability of FXI concentrates in their different countries.
Conclusion
Factor XI deficiency remains challenging because the factors influencing bleeding risk are still not fully understood. The enigmas—the variable bleeding tendency, the precise role of FXI in coagulation and difficulty choosing treatment modalities—remain challenging. However, advances in structural biochemistry and molecular genetics together with the increasing recognition of subtle additional influences on the balance of hemostasis are contributing to our understanding. Recent work with thrombin generation studies and rVIIa has provided evidence to support a role for this agent in management.
Treatment options in factor XI (FXI) deficiency.
| Treatment . | . | Advantages . | Disadvantages . |
|---|---|---|---|
| Watch and wait | Suitable for many individuals with partial deficiency | No exposure to blood products. | Hemorrhage may occur. |
| Fibrinolytic inhibitors | eg, Tranexamic acid | Effective as single agent for dental extractions even in severe deficiency. Good for menorrhagia. | Not available in some countries. |
| Fresh frozen plasma (FFP) | Standard FFP | Readily available. | Large volumes may be required Infection risk. |
| Pathogen-inactivated FFP | Safer than standard FFP. | Large volumes may be required. | |
| a) Solvent-detergent | Pooled donations. | ||
| b) Methylene blue | Single donor units. | Variable FXI activity. | |
| Factor XI concentrates | Factor XI BPL Hemoleven LFB | Predictable rise in FXI level, small volume of infusion. | Not licensed or available in many countries. Thrombotic risk especially in those with predisposition. |
| rVIIa | Small volume. | Not licensed for this indication. | |
| Instant action. Effective for patients with inhibitors. | Thrombotic risk, the safe and effective dose yet to be decided. | ||
| Desmopressin | Non-blood product. Easy to administer. | No clear evidence of benefit in reported series. Need for fluid restriction for 24 h. |
| Treatment . | . | Advantages . | Disadvantages . |
|---|---|---|---|
| Watch and wait | Suitable for many individuals with partial deficiency | No exposure to blood products. | Hemorrhage may occur. |
| Fibrinolytic inhibitors | eg, Tranexamic acid | Effective as single agent for dental extractions even in severe deficiency. Good for menorrhagia. | Not available in some countries. |
| Fresh frozen plasma (FFP) | Standard FFP | Readily available. | Large volumes may be required Infection risk. |
| Pathogen-inactivated FFP | Safer than standard FFP. | Large volumes may be required. | |
| a) Solvent-detergent | Pooled donations. | ||
| b) Methylene blue | Single donor units. | Variable FXI activity. | |
| Factor XI concentrates | Factor XI BPL Hemoleven LFB | Predictable rise in FXI level, small volume of infusion. | Not licensed or available in many countries. Thrombotic risk especially in those with predisposition. |
| rVIIa | Small volume. | Not licensed for this indication. | |
| Instant action. Effective for patients with inhibitors. | Thrombotic risk, the safe and effective dose yet to be decided. | ||
| Desmopressin | Non-blood product. Easy to administer. | No clear evidence of benefit in reported series. Need for fluid restriction for 24 h. |
Clinical management of different scenarios in factor XI (FXI) deficiency.
| Clinical scenario . | Management of severe deficiency . | Management of partial deficiency . | Cautions and comments . |
|---|---|---|---|
| Notes: Assuming that other causes of bleeding have been investigated and excluded (eg, von Willebrand disease, platelet disorders). All patients should avoid aspirin or other non-steroidal anti-inflammatory agents within the 7 days prior to and following surgery. | |||
| *Replacement therapy means either FXI concentrate or FFP with the cautions noted in Table 1. | |||
| Circumcision | If circumcision is required for religious reasons in the first few days of life, male children at risk should have a level done at birth. Bleeding is uncommon (1.5%) and Israeli experience indicates that replacement is not mandatory.32 An alternative is to delay surgery or to use replacement therapy.*42 | No indication for replacement therapy.* | The level at birth is lower than the adult level, so children with apparent partial deficiency should be retested at >6 months of age to establish true level. |
| Labor and delivery39,40 | Many women with severe deficiency experience no excessive bleeding. Oral antifibrinolytic agents are advisable to reduce blood loss post-partum. There is an increased risk of hemorrhage if treatment to raise FXI level is not used. Spinal and epidural anesthesia is contraindicated unless FXI level is corrected to normal (and documented). | Expectant management unless previous bleeding history. In the absence of a bleeding history with adequate challenge, spinal anesthesia may be considered. Oral antifibrinolytics will reduce postpartum bleeding and may be useful for 2 wks. | Neonatal bleeding is very rare even in severe deficiency, but obstetricians should manage delivery of the ‘at risk’ infant with caution, avoiding instrumentation as far as possible, and the FXI level measured from cord blood in males who may require circumcision. |
| Major surgery in areas of high fibrinolytic activity, including tonsillectomy or prostatectomy | Replacement therapy* to achieve trough levels of 45 IU/dL for 5 to 7 days together with oral antifibrinolytic agents. Regular monitoring of FXI level is required to determine dosing. | Replacement therapy* for those with previous bleeding history together with antifibrinolytic agents. | |
| Major surgery not associated with increased fibrinolysis, eg, orthopedic surgery, appendectomy | Replacement therapy* usually required as above, but even without this bleeding is uncommon.32 | Expectant management with close observation and attention to hemostasis. | |
| Minor surgery including skin biopsies | No replacement therapy.* | No replacement therapy.* | |
| Dental extractions | Oral antifibrinolytic agent alone starting the night before and continuing for 7 days. | If previous bleeding history as for severe deficiency. If no bleeding history despite challenge, expectant management only. | |
| Menorrhagia | Oral antifibrinolytic agents for the days with heaviest bleeding; if unsuccessful move to hormonal control with oral contraceptives. | As for severe deficiency. | |
| Surgical management of patients with FXI inhibitors33 | rVIIa is effective at low dose of 15 μg/kg for major surgery used with oral tranexamic acid. | Inhibitors not described in partial deficiency. | Higher doses of rVIIa (90 μg/kg have been associated with increased thrombotic risk |
| Clinical scenario . | Management of severe deficiency . | Management of partial deficiency . | Cautions and comments . |
|---|---|---|---|
| Notes: Assuming that other causes of bleeding have been investigated and excluded (eg, von Willebrand disease, platelet disorders). All patients should avoid aspirin or other non-steroidal anti-inflammatory agents within the 7 days prior to and following surgery. | |||
| *Replacement therapy means either FXI concentrate or FFP with the cautions noted in Table 1. | |||
| Circumcision | If circumcision is required for religious reasons in the first few days of life, male children at risk should have a level done at birth. Bleeding is uncommon (1.5%) and Israeli experience indicates that replacement is not mandatory.32 An alternative is to delay surgery or to use replacement therapy.*42 | No indication for replacement therapy.* | The level at birth is lower than the adult level, so children with apparent partial deficiency should be retested at >6 months of age to establish true level. |
| Labor and delivery39,40 | Many women with severe deficiency experience no excessive bleeding. Oral antifibrinolytic agents are advisable to reduce blood loss post-partum. There is an increased risk of hemorrhage if treatment to raise FXI level is not used. Spinal and epidural anesthesia is contraindicated unless FXI level is corrected to normal (and documented). | Expectant management unless previous bleeding history. In the absence of a bleeding history with adequate challenge, spinal anesthesia may be considered. Oral antifibrinolytics will reduce postpartum bleeding and may be useful for 2 wks. | Neonatal bleeding is very rare even in severe deficiency, but obstetricians should manage delivery of the ‘at risk’ infant with caution, avoiding instrumentation as far as possible, and the FXI level measured from cord blood in males who may require circumcision. |
| Major surgery in areas of high fibrinolytic activity, including tonsillectomy or prostatectomy | Replacement therapy* to achieve trough levels of 45 IU/dL for 5 to 7 days together with oral antifibrinolytic agents. Regular monitoring of FXI level is required to determine dosing. | Replacement therapy* for those with previous bleeding history together with antifibrinolytic agents. | |
| Major surgery not associated with increased fibrinolysis, eg, orthopedic surgery, appendectomy | Replacement therapy* usually required as above, but even without this bleeding is uncommon.32 | Expectant management with close observation and attention to hemostasis. | |
| Minor surgery including skin biopsies | No replacement therapy.* | No replacement therapy.* | |
| Dental extractions | Oral antifibrinolytic agent alone starting the night before and continuing for 7 days. | If previous bleeding history as for severe deficiency. If no bleeding history despite challenge, expectant management only. | |
| Menorrhagia | Oral antifibrinolytic agents for the days with heaviest bleeding; if unsuccessful move to hormonal control with oral contraceptives. | As for severe deficiency. | |
| Surgical management of patients with FXI inhibitors33 | rVIIa is effective at low dose of 15 μg/kg for major surgery used with oral tranexamic acid. | Inhibitors not described in partial deficiency. | Higher doses of rVIIa (90 μg/kg have been associated with increased thrombotic risk |
Factors to be considered in preparation for surgery.45
|
|
Structure of factor XI (FXI). A) Cartoon structure of FXI. Filled circles represent amino acids, the catalytic triad is in green and cys-cys bonds in red. A1–4 are the apple domains. (Figure provided by David Gailani and modeled after an image in McMullen et al.43) B) Crystal structure of FXI. (Figures provided by S.L. Perkins, UCL, adapted by permission from Macmillan Publishers Ltd10 copyright 2006.) The FXI monomer is displayed in the “cup and saucer” view. The serine protease domain (SP) is shown at the top with red α-helices and yellow β-strands. The four apple (Ap1–Ap4) domains are shown at the bottom with cyan α-helices and violet β-strands. The artwork was prepared using PYMOL from the Protein Data Base entry code 2F83. C) Crystal structure of the FXI dimer is displayed to show the interaction of the two Ap4 domains with each other. The SP and Ap1–Ap4 domains follow the same coloring as in Figure 1B. The two monomers are shown on either side of the dashed line.
Structure of factor XI (FXI). A) Cartoon structure of FXI. Filled circles represent amino acids, the catalytic triad is in green and cys-cys bonds in red. A1–4 are the apple domains. (Figure provided by David Gailani and modeled after an image in McMullen et al.43) B) Crystal structure of FXI. (Figures provided by S.L. Perkins, UCL, adapted by permission from Macmillan Publishers Ltd10 copyright 2006.) The FXI monomer is displayed in the “cup and saucer” view. The serine protease domain (SP) is shown at the top with red α-helices and yellow β-strands. The four apple (Ap1–Ap4) domains are shown at the bottom with cyan α-helices and violet β-strands. The artwork was prepared using PYMOL from the Protein Data Base entry code 2F83. C) Crystal structure of the FXI dimer is displayed to show the interaction of the two Ap4 domains with each other. The SP and Ap1–Ap4 domains follow the same coloring as in Figure 1B. The two monomers are shown on either side of the dashed line.
Types of factor XI gene mutations reported to May 2009 at www.factorxi.com (site accessed June 26, 2009).44 2B. Location of factor XI gene mutations—A1–4—apple domains, the linker regions are short peptide segments that join the domains together to make the intact factor XI molecule.
Types of factor XI gene mutations reported to May 2009 at www.factorxi.com (site accessed June 26, 2009).44 2B. Location of factor XI gene mutations—A1–4—apple domains, the linker regions are short peptide segments that join the domains together to make the intact factor XI molecule.
Mechanisms for cross-reactive material (CRM)–negative FXI deficiency.24 FXI dimers and monomers within circles at the top of the figure represent intracellular protein, arrows indicate secretion, and FXI dimers below the arrows represent plasma FXI. The percentage of activity and antigen values shown are what would be expected, on average, for different mechanisms. Mutations in category 1 prevent or reduce synthesis of FXI polypeptide, and heterozygosity for such a mutation would not affect the product of the normal allele. Glu117Stop is the most common example from this group. Category 2 contains polypeptides that are synthesized but dimerize poorly, resulting in intracellular retention of monomers. Heterozygosity for this type of mutant would have little effect on secretion of product from the normal allele, because the mutant would dimerize poorly with the wild-type polypeptide, and heterodimers that do form are secreted. The diagram shows the expected result of the most common mutation in this group, Phe283Leu, which has a partial defect in dimerization. Category 3 includes polypeptides that are synthesized and form dimers, but that are secreted poorly in homodimeric or heterodimeric forms. This category includes Ser225Phe and Cys398Tyr. The mutant polypeptide reduces wild-type FXI polypeptide secretion through formation of nonsecretable heterodimers (dominant-negative effect). This research was originally published in Kravtsov DV, Monahan PE, Gailani D. A Classification system for cross-reactive material-negative factor XI deficiency. Blood. 2005;105:4671–3, © the American Society of Hematology and is reproduced here with permission. Het indicates heterozygote; Hom, homozygote for fFXI gene mutation.
Mechanisms for cross-reactive material (CRM)–negative FXI deficiency.24 FXI dimers and monomers within circles at the top of the figure represent intracellular protein, arrows indicate secretion, and FXI dimers below the arrows represent plasma FXI. The percentage of activity and antigen values shown are what would be expected, on average, for different mechanisms. Mutations in category 1 prevent or reduce synthesis of FXI polypeptide, and heterozygosity for such a mutation would not affect the product of the normal allele. Glu117Stop is the most common example from this group. Category 2 contains polypeptides that are synthesized but dimerize poorly, resulting in intracellular retention of monomers. Heterozygosity for this type of mutant would have little effect on secretion of product from the normal allele, because the mutant would dimerize poorly with the wild-type polypeptide, and heterodimers that do form are secreted. The diagram shows the expected result of the most common mutation in this group, Phe283Leu, which has a partial defect in dimerization. Category 3 includes polypeptides that are synthesized and form dimers, but that are secreted poorly in homodimeric or heterodimeric forms. This category includes Ser225Phe and Cys398Tyr. The mutant polypeptide reduces wild-type FXI polypeptide secretion through formation of nonsecretable heterodimers (dominant-negative effect). This research was originally published in Kravtsov DV, Monahan PE, Gailani D. A Classification system for cross-reactive material-negative factor XI deficiency. Blood. 2005;105:4671–3, © the American Society of Hematology and is reproduced here with permission. Het indicates heterozygote; Hom, homozygote for fFXI gene mutation.
Bleeding history in factor XI (FXI) deficiency in relation to FXI level—a summary of data from two UK studies involving 54 families. The bleeding histories were classified by at least two hemophilia treaters who did not know the FXIC levels.
Bleeding history in factor XI (FXI) deficiency in relation to FXI level—a summary of data from two UK studies involving 54 families. The bleeding histories were classified by at least two hemophilia treaters who did not know the FXIC levels.
Disclosures Conflict-of-interest disclosure: The author is a consultant for LFB. Off-label drug use: FXI concentrates are available from two companies, LFB (France) and BPL (Bio Products Laboratory; UK), and neither of these are licensed in the US although used in Europe and elsewhere. They are designed for use in FXI deficiency to raise the level prior to surgery or in the treatment of bleeding. Recombinant activated FVII has also been used in FXI deficiency to prevent bleeding and which is not licensed for this use.
References
Author notes
Department of Clinical Haematology, Manchester Royal Infirmary, Manchester, United Kingdom