Abstract
The seminal discovery of the JAK2V617F mutation, which is highly prevalent in Philadelphia-negative myeloproliferative disorders, now renamed neoplasms, triggered an almost unprecedented explosion of interest and data in the field. Descriptions of additional mutations in exon 12 of JAK2, at position 515 in MPL, and a number of other mutations at low frequency followed these discoveries. These advances in our understanding of molecular pathogenesis of these conditions coincided with the publication of results from two major clinical studies, ECLAP and PT-1, which contributed important clinical insights and facilitated significant correlative data collection. This article, focusing mainly upon essential thrombocythemia and polycythemia vera, reviews four major themes: the impact upon classification of these disorders considering a radical review of current terminology, and then three areas pertinent to clinical management: the indications for cytoreductive therapy in which the key targets are to reduce thrombohemorrhagic complications, relieve disease-related symptoms, and minimize the risk of transformation to secondary myeloid malignancy such as myelodysplasia, leukemia, and secondary myelofibrosis; and second reviewing current and, last, future therapeutic options, in particular interferon and JAK2 inhibitors.
Introduction
There is growing recognition of what has been called “phenotypic mimicry” within the Philadelphia-negative myelodysplastic/myeloproliferative neoplasms (MPNs): essential thrombocythemia (ET), polycythemia vera (PV), and primary myelofibrosis (PMF). Indeed, it was these significant parallels that led William Dameshek in 19511 to group them with chronic myeloid leukemia (CML) first using the term “myeloid proliferative disorders” or “MPDs.” Later the discovery of the Philadelphia chromosome prompted a revision of Dameshek's classification. Might the discovery of JAK2V617F and other more recently identified molecular markers, such as mutations in MPL, exon 12 of JAK2, TET 2, and the 46/1 haplotype in JAK2 herald a similar process for the remaining MPDs? How does one identify the need for treatment in ET and PV? Finally, this article discusses potential roles of novel (JAK2 inhibitors) and not so novel (interferon [IFN]) treatments focusing mainly upon ET and PV.
The Impact of Molecular Markers upon the Current Classification of ET and PV
The current classification of the conventional MPNs as separate clinical entities ET, PV, and PMF2 is based largely upon clinical and pathological descriptions, and has not been significantly revised for some time other than the incorporation of testing for JAK2 mutations. There is an acknowledgement of a difficulty in the application of the World Health Organization (WHO) classification of MPN, perhaps as a consequence of its emphasis upon specific histological features.3 A particular difficulty lies in the proposal that myelofibrosis may occur in a so-called pre-fibrotic form without any clinical evidence of significant fibrosis.4 Furthermore, patients presenting with thrombocytosis and significant (i.e., greater than grade 2/4) reticulin and yet no features of PMF such as anemia or splenomegaly, an abnormal blood film cannot be classified and must retain the label “MPN unclassified.” This is unsatisfactory because clear treatment guidelines and prognostic data are not available for this entity. Furthermore, for individual patients the disease entities may change from one to another or, perhaps even worse, multiple and sometimes confusing terminologies may be used by clinicians: for example, agnogenic myeloid metaplasia, idiopathic or primary or secondary myelofibrosis, spent phase, accelerated phase, etc.
A reasonable starting point for revising the classification of the MPNs might be to subdivide cases into those that are JAK2V617F negative or JAK2V617F positive. The impact of both the less-prevalent MPL and exon 12 JAK2 mutations are less significant and may arise in different clones with differing dynamics from the mutation allele burden.5–7 However, those patients with wild-type JAK2 do have bona fide features of MPN, such as cytogenetic abnormalities, hypercellular bone marrow growth with abnormal megakaryocyte morphology, and clinical complications.8
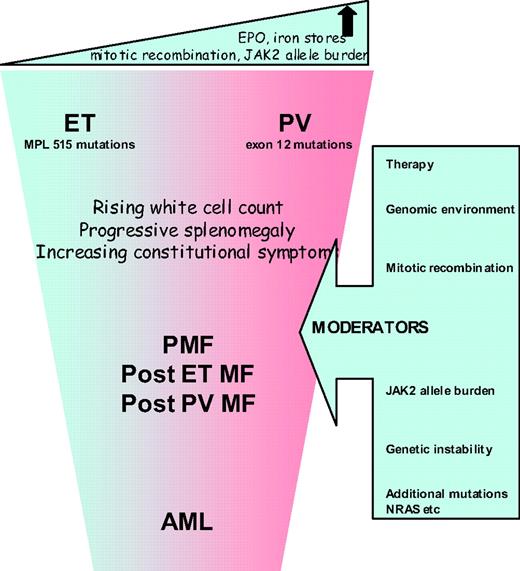
The most attractive proposition is to reconsider whether it is still relevant to subdivide MPNs into the three different entities (ET, PV, and PMF) or to regard them as a continuum of conditions similar to the different phases of CML. High-risk PMF or acute myeloid leukemia (AML) would thus represent the advanced phase and ET with PV the chronic phase (Figure 1). Unlike CML, the rate of progression of ET/PV/PMF patients is slow, not inevitable, and patients may present at any point of the disease spectrum. Progression might occur, for example, when patients become homozygous for JAK2V617F or acquire additional, as-yet-unidentified, mutations or develop refractory splenomegaly or persistently high and uncontrolled leukocytosis or further cytogenetic abnormalities. This so-called “continuum model” proposal for reclassification of MPN remains contentious, but is strengthened by an expanding body of research, as discussed below.
Relationships between ET and PV
Several studies suggest that JAK2V617F positive ET has several features in common with PV: higher hemoglobin levels, neutrophil counts, more venous thrombosis, and a higher incidence of polycythemic transformation.9 JAK2V617F positive ET patients have lower serum erythropoeitin and ferritin levels suggesting that their capacity to generate an erythrocytosis may be constrained. A case series comparing 179 patients with ET and 77 with JAK2V617F positive PV demonstrated a “gradient” in various laboratory values between patients with wild-type ET, JAK2V617F positive ET and PV. Furthermore, the rate of thrombotic complications in JAK2V617F positive ET patients was significantly higher than in wild-type patients but not statistically different from PV patients.10 The suggestion that JAK2V617F-positive compared with wild-type ET is associated with higher thrombotic risk has been confirmed in a meta-analysis but the relative risk is relatively weak.11
The continuum model and a relationship between ET and PV is also consistent with familial predisposition to MPN evidenced by the Swedish Cancer Registry12 and three studies demonstrating that the presence of JAK2V617F disease is strongly associated with inheritance of the 46/1 haplotype in JAK2 (most frequently in cis to the affected allele) and account for approximately half of this inherited predisposition.13–15 Two main models have been proposed to explain the effect of the 46/1 haplotype: “hypermutability,” in which the predisposition allele is particularly prone to acquiring mutations; and “fertile soil,” suggesting that JAK2 mutations are more likely to result in a clonal expansion (i.e., disease) if they are acquired in cis to the predisposition haplotype, perhaps due to the expression or function of the JAK2 protein encoded by this allele. Most recently, an excess of 46/1 in JAK2 exon 12 and MPL mutated cases has been reported, and no difference in sequence, splicing, or expression of JAK2 was found on the 46/1 allele compared with other haplotypes, suggesting that any functional difference of that allele, if it exists, must be relatively subtle.16
Role of the JAK2V617F Allele Burden
While available data support a common clinical phenotype for JAK2V617F-positive ET and PV, a significant underlying biological difference between these conditions was identified by Scott et al.,17 who found that no patients with ET had hemopoietic progenitor cell colonies that were homozygous for JAK2V617F, but such colonies were detected in all PV (p < 0.0001) and two ET patients after polycythemic transformation. This suggests that a fundamental difference between JAK2V617F-positive ET and PV might be the mitotic recombination events that generate daughter cells homozygous for JAK2V617F. There is a significant body of evidence to support the view that the JAK2 allele burden is consistently higher in patients with PV, PMF, or post-PV MF than in those with ET.18 In mouse models, the MPN phenotype induced by the expression of JAK2V617F depends both upon the genetic strain of the recipient animal and the mutant gene-expression level.19 Interestingly, mutations of JAK2 exon 12 are associated with stronger downstream signaling compared with JAK2V617F, and are found in patients with PV but not in those with ET.20 The biological effector for allele burden may be the degree of STAT5 activation, where activation in human CD34+ cells favors erythroid differentiation, and reduced levels favor the megakaryocyte lineage.21
Clinical Correlations with the JAK2 V617F Allele Burden
Clinical correlations with JAK2 V617F allele burden are emerging. Vannucchi22,23 reported data showing that hematocrit, leukocyte count, lactate dehydrogenase, and alkaline phosphatase values were directly related to the relative amount of JAK2V617F RNA, while the MCV and platelet count were inversely related. Patients with the highest ratios were more likely to have splenomegaly, pruritus, and a greater risk of developing myeloid metaplasia or requiring chemotherapy in the follow-up period. This supports the contention that the JAK2V617F-homozygous mutational status is associated with a more symptomatic advanced or accelerated disease across the MPN clinical entities.
Transformation to More Aggressive Disease.
Aggressive disease is reflected in the occurrence of serious vascular events or disabling constitutional symptoms, but most significantly with the development of myelofibrosis or acute leukemia. Diagnostic criteria for these entities are well described in the literature.24 It is important to ensure that a diagnosis of myelofibrosis is only applied to those patients with evidence of characteristic clinical features such as anemia, splenomegaly, constitutional symptoms, or a leukoerythroblastic blood film. Interesting recent data have emerged regarding the routes to development of acute leukemia in 16 patients with JAK2-mutant (7 of 16) or wild-type (9 of 16) AML after a JAK2-mutant MPN. Myelofibrosis preceded all 7 JAK2-mutant but only 1 of 9 JAK2 wild-type AMLs (P = .001), implying that JAK2-mutant AML is preceded by mutation(s) that give rise to a “myelofibrosis” phenotype.25 Mutations in JAK2 may be implicated in the acquisition of additional genetic abnormalities because JAK2V617F is associated with increased genetic instability and has also been shown to co-locate to the nucleus.26,27
Challenges to the “continuum model” include why the majority of ET patients do not transform to PV. In addition, details of the additional genetic hits underlying progressive disease and why some patients progress more rapidly than others are required. The usefulness of this model is that the rigidity of the constraints of the WHO classification do not apply, it accommodates more recent data in relation to clinical and biological heterogeneity of these conditions, and it may rid the field of redundant ambiguous clinical terminology if properly constructed.
The Indications for Cytoreduction
The aim of cytoreductive therapy in ET and PV is to reduce the risk of thrombosis, control disease-related symptoms, and to reduce where possible primary and iatrogenic disease progression. The most common target is to reduce the risk of thrombosis, and a number of risk-stratification schemes have been proposed for this purpose based upon analysis of large numbers of patients from well-conducted studies principally performed in Italy (Table 1 and summarized in Harrison et al.28 ). The intermediate-risk group in ET is controversial, because this classification is variably defined by age 40 to 60 years with no high-risk factors and age less than 60 with cardiovascular risk factors. Data from the ongoing PT-1 trials (http://www.haem.cam.ac.uk/pages/pt1/) will elucidate this and treatment strategies for this group further. High-risk PV is less well-defined than high-risk ET.28 The predominant factors are age, prior thrombosis, concurrent medical conditions that would increase the vascular risk, and the platelet count (although the later is controversial and may be an arbitrary value). In both ET and PV, clinical studies suggest that cytoreductive therapy reduces the incidence of thrombosis, which led to the interpretation that this was secondary to a reduction in the platelet count.29 Paradoxically, current data suggest that platelet number may not be directly correlated with the incidence of thrombosis,30 and data from the PT-1 trial29 suggest that additional factors such as reduction of hematocrit, leukocyte count, or endothelial factors such as nitric oxide production may be important.
Risk stratification of PV and ET patients

*These categories are contentious, and some recommend the use of the terms low- and high-risk only, or to classify individuals with cardiovascular risk factors as intermediate risk.
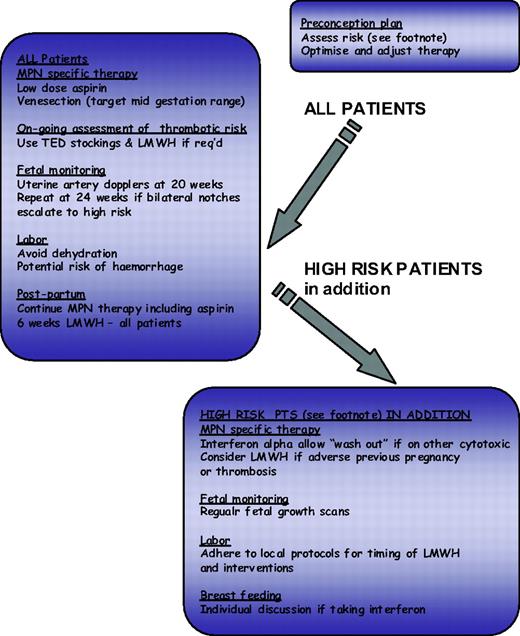
The impact of conventional risk factors for atherosclerosis have been assessed in MPN with varying results, and whether these risk factors should contribute to risk group allocation for ET and PV patients is unclear. Recent recommendations for the management of atherosclerosis suggest that this patient group may benefit from aggressive risk management with the use of antihypertensives and a statin where appropriate. The identification of risk markers for thrombosis is an evolving field; however, any such novel markers should be robust and easily measurable. Current candidates include the leukocyte count, reticulin grade, and JAK2V617F allele burden, all of which require prospective evaluation.28 The choice of cytoreductive therapy is relatively limited, and a better evidence base is probably desirable; current published recommendations are summarized in Table 2. In addition, the European Leukaemia Net has produced by consensus response criteria for patients with ET and PV.31 These criteria are designed primarily for clinical trials and also include reference to bone marrow morphology and to molecular markers. The same group also produced criteria for resistance or intolerance to hydroxycarbamide (formally known as hydroxyurea),32 which provide useful guidance but omit to mention pneumonitis as a side effect and refer to low leukocyte count rather than neutrophil count where considering hematological intolerance. Options for management in the face of hydroxyurea resistance would include adjusting therapeutic targets (e.g., to a platelet count of 600 × 109/L) or switching to an alternative agent either alone or in combination. In this regard, it is important to consider that hydroxyurea, when used with or succeeded by agents such as busulfan with leukemogenic potential, will significantly potentiate leukemogenicity of either agent alone. For this reason non-leukemogenic agents such as IFN-α or anagrelide are more appropriate in this setting.28 Pregnancy, although not relevant for the majority of MPN patients, may be challenging for the small proportion of young women with these diseases, and a proposed algorithm for its management is shown in Figure 2.
Algorithm for ET/PV in pregnancy. Footnote Risk factors for complications in pregnancy: Previous hemorrhage or venous or arterial thrombosis in mother (whether pregnant or not); Previous pregnancy complication that may have been caused by ET or PV: Unexplained recurrent first trimester loss (three unexplained first trimester losses); Intrauterine growth restriction (birthweight <5th centile for gestation); Intrauterine death or still birth (with no obvious other cause, evidence of placental dysfunction, and growth-restricted fetus); Severe preeclampsia (necessitating preterm delivery <34 weeks) or development of any such complication in the index pregnancy; Placental abruption; Significant antepartum or postpartum hemorrhage (requiring red-cell transfusion); Marked sustained rise in platelet count rising to above 1500 × 109/L
Algorithm for ET/PV in pregnancy. Footnote Risk factors for complications in pregnancy: Previous hemorrhage or venous or arterial thrombosis in mother (whether pregnant or not); Previous pregnancy complication that may have been caused by ET or PV: Unexplained recurrent first trimester loss (three unexplained first trimester losses); Intrauterine growth restriction (birthweight <5th centile for gestation); Intrauterine death or still birth (with no obvious other cause, evidence of placental dysfunction, and growth-restricted fetus); Severe preeclampsia (necessitating preterm delivery <34 weeks) or development of any such complication in the index pregnancy; Placental abruption; Significant antepartum or postpartum hemorrhage (requiring red-cell transfusion); Marked sustained rise in platelet count rising to above 1500 × 109/L
The Potential Role of Interferon as a Disease-Modifying Therapy
Despite advances in our understanding of the pathogenesis of MPNs, processes for risk stratification and therefore indications for cytoreduction lack better refinement. Furthermore, the MPN field has probably lagged behind others in the identification of novel therapies and has yielded some disappointing results (e.g., with anagrelide in PT1).29 Debate continues over the role of venesection versus cytoreduction as a first-line therapy in PV, and whether hydroxyurea, which is associated with better thrombotic prophylaxis, may be linked to a higher rate of leukemic transformation. Furthermore, while hydroxyurea is regarded as a first-choice therapy in most high-risk patients with ET and PV; up to 10% of the patients do not attain the desired reduction of platelet number or hematocrit with the recommended dose of the drug either immediately or after a period of adequate control. Such patients are then clinically resistant, whereas some will develop unacceptable side effects, thus manifesting clinical intolerance.
Pioneering studies with IFN-α were reported by Silver in 1988,33 and were followed by many studies showing the clinical efficacy of IFN in controlling myeloproliferation and relieving pruritus and other constitutional symptoms in PV. Similar efficacy was at the same time shown in ET. This drug is non-leukemogenic and may have a preferential activity on the malignant clone in PV, as suggested by cytogenetic remissions obtained in patients treated with rIFNα-2b. Several investigators recently reported that patients with PV treated with rIFNα-2b had lower JAK2V617F allele burdens compared with a control group that included patients treated with phlebotomy, hydroxyurea, or anagrelide, or who remained untreated.34 However, these studies included only limited numbers of patients treated with rIFNα-2b at variable dosages (from 1 mega unit [MU] 3 times weekly to 3 MU daily) and for variable periods (13–132 months), and reduction of JAK2V617F allele has also been reported after treatment with hydroxyurea. IFN-α is associated with significant side effects that are particularly worrying for patients undergoing prolonged exposure. For this reason, different formulations of IFN have been explored; to date, reports of the use of peg-IFN-α-2b in patients with ET have shown good efficacy but no clear advantage in terms of toxicity compared with standard IFN-α35
Recently two phase II studies have evaluated the role of the pegylated IFN-α2a isoform of IFN (Pegasys, Roche). In the first (PVN1), a multicenter phase II trial in PV patients, Kiladjian et al.36 prospectively evaluated both clinical/hematological and molecular responses. At 12 months, all 37 evaluable patients had a hematologic response, including 94.6% complete responses (CRs). Only 3 patients (8%) had stopped treatment. After the first year, 35 patients remained in hematologic CR, including 5 who had stopped Pegasys. Sequential samples for %V617F monitoring, available in 29 patients, showed a %V617F decrease in 26 (89.6%). Median %V617F decreased from 45% to 22.5%, 17.5%, 5%, and 3% after 12, 18, 24, and 36 months, respectively. Molecular CR (JAK2V617F undetectable) was achieved in 7 patients, lasting from 6 to 18 months and persisting after Pegasys discontinuation in 5. No vascular event was recorded. The results confirm the hypothesis that IFN-α preferentially targets the malignant clone in PV, and show that JAK2V617F allele burden maybe useful in monitoring minimal residual disease in PV patients.
The second study was conducted by the M.D. Anderson group,37 in which both PV (n = 40) and ET patients (n = 39) were included. Here the median time that had elapsed from diagnosis was longer, and these patients had previously received at least one other cytoreductive drug and therefore represented a different clinical and biological cohort. Despite this, excellent hematological response rates were observed: 81% (including 78% CR), and 92% (including 86% CR) in PV and ET, respectively. Major molecular responses were achieved in 30% and 13% of PV and ET patients, respectively, with 13% molecular CRs for both diseases. Tolerance was also better than reported with other forms of IFN, since only 10% of treatment discontinuation was due to Pegasys toxicity. No patients suffered grade 4 toxicity, and grade 3 toxicities were reported in a small number of patients only and in the following categories: pain, fatigue, dyspnea, and pruritis.
The studies of Pegasys in PV and ET described above both report high levels of hematologic CR rates; good tolerance of the drug, particularly when started at a low dose; and, most significantly, a clear selective effect on the disease clone in a significant proportion of patients, including molecular CRs. This suggests that Pegasys may be effective when other therapies have failed or not been tolerated, and that it may challenge the position of hydroxyurea as a first-line therapy. However, this will require large randomized trials with comprehensive evaluation for long-term side effects. Two such trials are currently being launched internationally: a phase III randomized study of hydroxyurea versus Pegasys in newly diagnosed high-risk ET and PV patients, and a phase II study of Pegasys in hydroxyurea-resistant or refractory patients to include a cohort of patients with abdominal vein thrombosis.
What Impact Are JAK2 Inhibitors Having in These Disorders?
A significant number of JAK2 inhibitors are now at varying stages of clinical evaluation, with the most data available for patients with myelofibrosis (see review by Mesa in this issue). Most of these agents are well tolerated and have clinically measurable benefit for these patients, particularly in reducing symptomatic splenomegaly and constitutional symptoms such as pruritis and fatigue. There are data supporting the ability of JAK2 inhibitors to control myeloproliferation in patients with PV and ET, but no data are available in terms of their ability to prevent thrombosis or affect the probability of accelerated/more aggressive disease such as post-ET or post-PV MF or leukemia. Moliterno et al.38 treated 39 patients (12 ET, 27 PV) with CEP 701 (Cephalon). All patients had high-risk disease and many prior exposures to cytoreductive therapy. Marked gastrointestinal side effects occurred and, while there were responses in splenomegaly, pruritis, and phlebotomy requirements (after 6 months), treatment was not associated with a reduction in either leukocytosis or thrombosis, and five thrombotic events occurred during treatment. In a study using INC18424 in 39 ET and 34 PV patients,39 there were similar rates of reduction of splenomegaly and symptom scores, but all patients had leukocyte counts below 10 × 109/L and 41% achieved a CR with platelets less than 400 × 109/L. No thrombotic events have been reported thus far. Meanwhile, interest in histone deacetylase (HDAC) inhibitors such as vorinostat and givinostat40,41 in patients with ET and PV is growing, and the possibility of combination therapies should also be considered. Further studies of sufficient duration to permit the evaluation of the safety and efficacy of these novel agents as alternatives to current therapeutic strategies are certainly needed. Furthermore, the burden of constitutional symptoms and concern about long-term side effects of drugs such as hydroxyurea are significant for this patient population, and their evaluation is an essential component of future studies.
Conclusions
The management of ET and PV has previously been problematic from the perspectives of achieving a timely certain diagnosis and the absence of robust evidence-based clinical management guidelines. Recent advances in our understanding of the molecular pathogenesis of these disorders and data from clinical trials have improved this. Evidence is now growing to support a reconsideration of the classification of these disorders from essentially those based upon clinical and morphological descriptions to a molecular and clinical classification based upon prospective correlative analyses from large numbers of patients. There have been further refinements and better agreement upon what the indications for cytoreductive therapy are. However, we still lack agents that achieve our therapeutic goals and robust data about the long-term safety (particularly leukemogenicity) of commonly used agents such as hydroxycarbamide. Interest in IFNs has been rejuvenated with data suggesting that pegylated IFNs may induce high levels of clinical and hematologic remission and, in some patients, a molecular CR. Provisional data with JAK2 inhibitors is emerging, but is less promising than early expectations, and novel therapeutics such as HDAC inhibitors are also currently being explored.
Disclosure
Conflict-of-interest disclosure: The author has received honoraria from Novartis and Incyte.
Off-label drug use: Experimental use of JAK2 inhibitors and IFN in the management of PV and ET.
Correspondence
Claire Harrison, Guy's and St Thomas' NHS Foundation Trust, Great Maze Pond, London SE1 9RT, UK; Phone: 44–2071882742; Fax: 44–2071882728; e-mail Claire.Harrison@gstt.nhs.uk