Abstract
This section reviews the history, pharmacology, administration, efficacy, and toxicity of intravenous iron. Intravenous iron offers advantages over oral iron for the treatment of iron deficiency anemia across a wide range of disease states associated with absolute and functional iron deficiency. However, there remain concerns about the acute safety profiles of the available preparations and the potential for long-term toxicity with their repeated administration. Seven intravenous iron formulations are available. Confusion concerning the relative toxicities of the different formulations abounds. The similarities and differences are discussed. Iron repletion has been associated with adverse outcomes in infections. The relationship, if any, between intravenous iron administration and infections is reviewed. The potential advantages of total dose infusion (TDI), complete repletion in a single setting, are highlighted. A new paradigm for iron replacement therapy in iron deficiency anemia is presented.
Iron has been used to treat anemia for more than 300 years. In Stockman's 1893 review of the treatment of chlorosis, he attributes the first use of oral iron to Sydenham in 1681.1 It was not until the 19th century when Pierre Blaud2 introduced ferrous sulfate and reported cures of chlorosis that oral iron therapy became standard care for what is now recognized as iron deficiency anemia (IDA). Oral iron is a less than ideal treatment, however, with gastrointestinal toxicity occurring in > 35% to 59% of patients,3,4 and a long course needed to resolve anemia and replenish stores. Nonadherence to a prescribed course of oral iron is common, and even in adherent patients, poor intestinal absorption fails to compensate for iron need in the presence of ongoing blood losses.
While intravenous (IV) iron has the capability of bypassing all these issues, there remain concerns about the acute safety profiles of the available products and the potential for long-term harm from repeated iron administration. Although any IV iron can cause acute severe reactions, the incidence and severity of reactions are less than may be appreciated by physicians,5 and the doses commonly administered in clinical practice are insufficient to result in parenchymal organ injury. Similarly, concerns about IV iron therapy potentially increasing the risks for infections and cardiovascular disease have not been confirmed in prospective studies or clinical trials and remain largely unproven hypotheses. This article will review the administration, indications, and safety profiles of the available IV iron preparations in the developed world. However, it is prudent to review the history of injectable iron to better understand the current reluctance to adopt routine use of IV iron therapy.
History of IV Iron
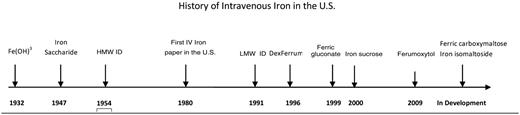
Parenteral iron was first introduced in the early 20th century6 (Figure 1). Ferric hydroxide solutions were injected subcutaneously and intramuscularly into patients with hypochromic anemia, and the observed increments in hemoglobin were proportional to the amount of iron administered. The lack of a carbohydrate shell resulted in immediate iron release and severe toxic reactions that led to use of this therapy being recommended only in extraordinary circumstances.
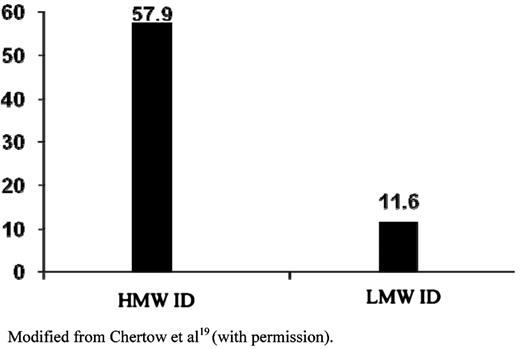
Total reported serious ADEs per million doses of 100 mg. Modified with permission from Chertow et al.19
Total reported serious ADEs per million doses of 100 mg. Modified with permission from Chertow et al.19
In 1947, Nissim7 introduced iron saccharide for IV injection and concluded that this form of iron was safer and more suitable for parenteral administration. In 1954, Baird and Podmore8 introduced Imferon (high molecular weight iron dextran [HMW ID], Fisons plc, Homes Chapel England, Ipswich, UK; marketed by Merrill Pharmaceuticals in the United States) for intramuscular (IM) and IV use. Dextran covered the iron oxide core, reducing release of free iron during infusion that accounted for a low incidence of adverse reactions. Bioavailability of iron occurred via uptake of the iron dextran particles into the reticuloendothelial system with subsequent degradation. Rapid hematologic responses were observed with a low incidence of side effects. However, patients can develop or have preformed antidextran antibodies, and larger dextran molecules, in particular, can cause anaphylaxis, as described with the administration of HMW ID. Although relatively uncommon, these reactions led to the admonition to avoid parenteral iron unless extreme clinical conditions were present and other options unavailable. HMW ID was the only parenteral iron product available until the 1990s.
Although published papers in the world literature9,10 attested to the usefulness of IV iron in treating IDA, it was not until 1980, that the first prospective study on the clinical use of IV iron in the United States appeared.11 Four hundred seventy-one patients with IDA received IV iron at varying doses. All patients responded, but three were considered to have had “anaphylactoid” reactions, with symptoms and signs including respiratory arrest, hypotension, purpura, cyanosis, dyspnea, syncope, wheezing, and hives. There were no deaths in the series. The authors concluded that IV iron should be reserved for those conditions in which oral iron could not be used. HMW ID remained on the pharmacopoeia and was a minor product until the introduction of recombinant human erythropoietin (epoetin alfa), the first erythropoiesis stimulating agent (ESA) in 1989.
Epoetin was first used for the treatment of anemia in renal dialysis patients. Although most patients responded to epoetin, some did not.12 Several explanations for a suboptimal response were found, but commonly the failure was shown to be due to absolute or functional iron deficiency as administration of IV iron restored responsiveness.13 Functional iron deficiency (iron-restricted erythropoiesis) is reviewed elsewhere in this session. Parenteral iron therapy has subsequently become an important adjunct to obtaining and maintaining adequate hemoglobin levels in patients with end-stage renal disease and other disease states.
Although relatively uncommon, severe acute hypersensitivity reactions to HMW ID occurred. A black box warning was placed in the package insert, and a test dose was required. In 1991, a contaminated batch led to the recall of the product worldwide. Serendipitously, at the same time, low molecular weight iron dextran (LMW ID) (INFeD, Schein Pharmaceuticals, now Watson Pharmaceuticals, Inc, Morristown, NJ) was completing registrational trials in the United States and released for clinical use in 1992. Using molecular blueprinting, Fisons determined that this product contained lower molecular weight dextran with less variability among the side chains. The original form of HMW ID was removed from the American market in 1991 and its manufacture ceased in 1992. Use of ESA and LMW ID was common in patients on dialysis and increased even more in 1997, with the adoption of renal clinical practice guidelines promoting routine use of both agents to a target hemoglobin level between 11 and 12 g/dL.
In 1996, another HMW ID (Dexferrum, American Regent Pharmaceuticals, Shirley, NY) was approved by the Food and Drug Administration (FDA), although LMW ID retained the large majority of the dialysis market. Both iron dextrans required a test dose and had black box warnings.
In 1999, ferric gluconate (FG) (Ferrlecit, Schein, then Watson Pharmaceuticals, now Sanofi-Aventis), after having been available in Europe for many years, was introduced into the American market as a safer alternative to iron dextran. An historical review of the use of FG in Europe and iron dextrans in the United States found no deaths attributable to FG, but at least 31 to iron dextran, and concluded that FG was safer, due to the lack of the dextran envelope.14 A double-blind, placebo-controlled crossover study of single dose administration of FG in 2338 hemodialysis patients found only one serious allergic reaction and no fatality.15,16 They further reported that, in patients previously sensitive to iron dextran, reactions to FG were uncommon, but also 7-fold more common than those without prior iron sensitivities.16 Virtually all 2338 patients had received prior iron dextrans. In November 2000, iron sucrose (IS) (Venofer, America Regent Pharmaceuticals) was approved in the United States. It also had long been used in Europe, lacked a dextran coat, and was reported to have a safety profile similar to FG.17 Black box warnings do not appear in the package inserts of either FG or IS, and a test dose was not recommended. Subsequently FG and IS largely replaced the use of iron dextrans in US dialysis patients.
In a retrospective review of more than 30 million doses of IV iron, Chertow et al18 showed that exposure to HMW ID was associated with significantly higher risk of adverse events (AEs), compared with LMW ID (Figure 218 ). In a follow-up analysis, the authors concluded that AEs were relatively low overall, with lower rates among LMW ID, FG, and IS.19 Two small prospective studies and one meta-analysis comparing the efficacy and safety of LMW ID to IS, however, showed no difference.20–22
Total reported serious ADEs per million doses of 100 mg of iron dextran.
In the past 18 months, three new IV iron compounds have been released for use in patients with anemia of chronic kidney disease (CKD). Two are currently approved for use in Europe (ferric carboxymaltose [FC], Ferrinject, ViforPharma, St. Gallen, Switzerland, and iron isomaltoside [II], Monofer, Pharmacosmos, Holbaek, Denmark) and one in the United States (ferumoxytol, Feraheme, AMAG Pharmaceuticals, Inc, Lexington, MA). All three compounds show promise to offer complete replacement doses of iron in 15 minutes or less.
Administration and Pharmacology
The earliest iron preparations were associated with unacceptable acute toxicity resulting from the release of bioactive free iron. Today, all IV iron products are iron-carbohydrate complexes or colloids based on small spheroidal iron-carbohydrate particles. Each particle consists of a core made of an iron-oxyhydroxy gel surrounded by a shell of carbohydrate that stabilizes the gel, slows the release of iron, and maintains the resulting particles in colloidal suspension.23 The currently approved IV irons all share this structure, but differ from each other by the size of the core and the identity and density of the surrounding carbohydrate. The characteristics of the seven iron compounds are shown in Table 1. The strengths of the iron complex affect pharmacokinetic characteristics of the IV irons relevant to therapeutic use. The rate of release of bioactive iron is inversely related to the strengths of the complex—the stronger the complex, the slower the release of the iron.24 The toxicologic implication of the complex size-release timing relation is that stronger complexes have a lower potential to saturate transferrin with subsequent free iron toxicity, compared with the weaker complexes.25
IV iron preparations
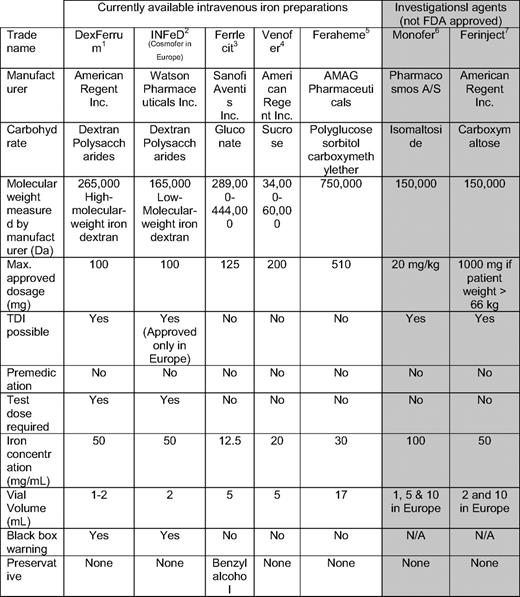
NA indicates not available; Max., maximum; TDI, total dose infusion. Injectafer is marketed outside the United States under the brandname Ferinject.
*DexFerrum Prescribing Information. Shirley, NY: American Regent, Inc, 2010 http://www.americanregent.com/AllProducts.aspx?ProductID=16 Letter and PI.pdf.
†INFeD Prescribing Information. Morristown, NJ: Watson Pharmaceuticals, Inc, 2010. http://www.infed.com/ information.html.
‡Ferrlecit Prescribing Information. Bridgewater, NJ: Sanofi-aventis, Inc, 2010. http://products.sanofi-aventis.us/ferrlecit/ferrlecit.html.
§Venofer Prescribing Information. Shirley, NY: American Regent, Inc, 2010. http://www.venofer.com/pdfs/Venofer%20IN2340%20Rev%2010–08.pdf.
¶Feraheme Prescribing Information. Lexington, MA: AMAG, Inc, 2010. http://www.feraheme.com/documents/Feraheme%20PI.pdf.
#Monofer data on file. Holbaek, Denmark: Pharmacosmos A/S, 2010. http://www.pharmacosmos.com/.
**FDA Advisory Committee Briefing Document, Drug Safety and Risk Management Committee, February 1, 2008. http://www.fda.gov/ohrms/dockets/AC/08/briefing/2008–4337b1–01-FDA.pdf. Accessed March 1st., 2010.
After injection, the different preparations all share a similar metabolic fate. The iron-carbohydrate complexes mix with plasma and are phagocytosed within the reticuloendothelial system, wherein the carbohydrate shell is degraded and iron is stored as ferritin or transported out of the cell by the only known iron export protein, ferroportin. Exported iron is bound to the extracellular binding protein, transferrin, which delivers iron to the transferrin receptors on the surface of erythroid precursors, supporting hemoglobinization.
The IM route has been considered a safer alternative to IV. No published data supports this conclusion, because the incidence of reported AEs is similar with both routes of administration. Additionally, IM injections are painful, cause permanent discoloration of the skin, and have been associated with gluteal sarcomas.26,27 The use of IM injections should be abandoned.5
The administration of any of the available preparations can be associated with acute chest and back tightness, without accompanying hypotension, wheezing, stridor, or periorbital edema. With the iron dextrans, this usually is seen after the test dose.5 This infrequent event rarely recurs with rechallenge. These events are more likely in patients with an allergic diathesis.28 It is important not to overreact to these minor AEs. In patients at higher risk for AEs, pretreatment with corticosteroids may be beneficial.29
In a review of LMW ID therapy in patients with iron deficiency and normal renal function who were intolerant of oral iron, Barton et al3 suggested that pretreatment with antihistamines was responsible for the majority of perceived reactions to iron dextran. Because there is no published evidence that antihistamines are beneficial in decreasing acute hypersensitivity reactions to IV iron, it is recommended that pretreatment not be routinely given.
The Products
High Molecular Weight Iron Dextran
Several publications have cited safety concerns with this preparation.18,19,30–35 During a brief period in 1997, when LMW ID became unavailable, HMW ID was the only formulation available. During this period, spontaneous reports to the FDA of AEs from IV iron increased 11-fold (obtained by the author through the Freedom of Information). Numerous publications warned against the use of HMW ID.30 The National Comprehensive Cancer Network31 and the nursing journal of the American Society of Nephrology32 proscribed its use. It has been removed from the formularies of all Veterans' Administration hospitals in the United States and its protectorates, and is not available in Europe. Despite this, some hospital pharmacists continue to view all iron dextran products as equals and many physicians lack awareness of the differences between the two iron dextrans. The Centers for Medicare and Medicaid Services currently assign identical J-codes for billing purposes, further confounding prescribers.
In 2002, Mamula et al33 published their experience with total dose infusion (TDI) in children with inflammatory bowel disease and reported a 9-fold increase in the incidence of AEs with HMW ID, compared with LMW ID. In a review of suspected iron dextran AEs, Fletes et al34 noted that AEs were 8.1 times more likely to occur with HMW ID, and concluded that serious AEs related to IV iron dextran were rare and appeared to depend on the specific formulation of IV iron dextran. Similar results were observed by McCarthy et al35 in a study examining AEs in chronic hemodialysis patients receiving IV iron dextran. In the first study to demonstrate the benefit of IV iron in synergizing with ESAs in the management of chemotherapy-induced anemia, 81 of 157 patients received iron dextran either as a bolus or TDI. Of the 79 who received LMW ID, no significant AEs were seen, whereas one of two patients who received HMW ID, during a short period when LMW ID was unavailable, developed anaphylaxis.36 Four months after recovery, this patient received LMW ID uneventfully, similar to previously published experience.32,33 In a recent study of 25 patients with restless legs syndrome who received 1000 mg of HMW ID over several hours, Ondo37 reported a high rate of improvement of symptoms; however, two patients developed anaphylaxis. However, in a publication of a retrospective analysis of 48,059 dialysis patients, of whom 20,213 were iron-naive, from the Gambril US Healthcare Database, Walters and Van Wyck38 found that patients who received HMW ID were no more likely to experience severe AEs than those who received LMW ID.
Recently, the FDA mandated a warning to note “the differences in the chemical characteristics and clinical effects of iron dextran preparations to minimize potential confusion between the available products on formulary.” The increased incidence of reported serious AEs with HMW ID suggests caution should be used when considering this formulation.
Low Molecular Weight Iron Dextran
Given the current state of knowledge, this is the preferred formulation of iron dextran. Unlike the iron salts, FG and IS, this formulation can be administered as an IV bolus or TDI (an approved method of administration in Europe, but not in the United States). Eschbach et al12 were the first to show the benefit of iron dextran in augmenting the response to erythropoietin in patients with the anemia of CKD undergoing dialysis. Subsequently, it has been shown that marked reductions in ESA dosage could be achieved with the addition of iron dextran.39
Following the release of LMW ID in 1991 for dialysis-associated anemia, it became standard for complementing ESAs. The initial review of safety was published in 1996. The authors concluded that AEs with LMW ID were rare, and only a history of allergies predicted otherwise very infrequent AEs.28 Furthermore, TDI is especially useful in the provision of care to patients on home-based maintenance peritoneal or hemodialysis who would otherwise require frequent visits to outpatient facilities for completion of therapy.40 This method of administration to patients on hemodialysis was compared with bolus, in a multicenter, randomized, open-label study and was shown to be of equal safety and efficacy, with considerable cost savings.41
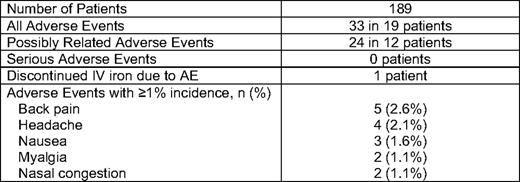
We recently presented data on 226 infusions of 1 gram of LMW ID over 1 hour in 187 consecutive, unselected patients with iron deficiency and no recent IV iron (Table 2). Only two patients received premedication—methylprednisolone—due to an allergic history. No significant toxicities were observed (Table 3). All but one patient, who developed hives, received the complete dose.42
Patient diagnoses in 187 patients receiving 1 gram of IV LMWID over 1 hour span lang=FR
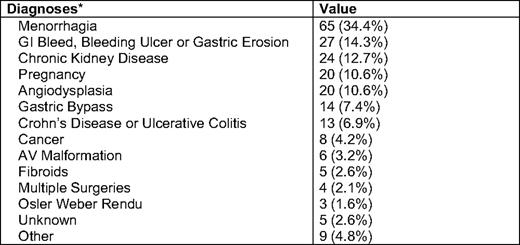
GI indicates gastrointestinal; AV, atrial ventricular.
*Of 189 patients, 154 had a single diagnosis, 34 had two diagnoses, and 1 had three diagnoses.
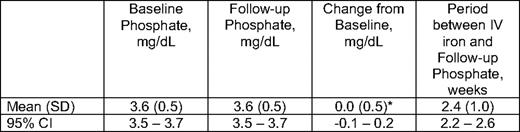
Hypophosphatemia (serum phosphate < 2 mg/dL) has been observed after administration of IS and FC. We examined pre- and postinfusion phosphate levels following LMW ID, and found no change at a median follow-up of 2 weeks (mean change from baseline phosphate level was 0.0 mg/dL (95% CI, -0.1 to 0.2; P = .537) (Table 4).
In patients with cancer or kidney disease requiring weekly or more frequent visits, short infusions of any of the available preparations, with the possible exception of HMW ID, can be given without significant risk. However, if large doses of IV iron are to be given in outpatient settings, the preferred method is a TDI. This method is particularly useful in the following clinical situations: home peritoneal or hemodialysis, iron deficiency due to pregnancy, menometrorrhagia, surgical blood loss, gastric bypass surgery, hereditary hemorrhagic telangiectasias (Osler-Weber-Rendu), angiodysplasia due to other causes, inflammatory bowel disease (IBD), malabsorption syndromes, and in those patients with uncomplicated iron deficiency who are noncompliant with or intolerant of oral iron. This method of administration is especially useful in Jehovah's Witnesses, who refuse all blood products.
Ferric Gluconate
Following its approval for use in hemodialysis patients in 2002, FG (and later IS) rapidly replaced iron dextran as the preferred IV iron preparation. Millions of doses have been given, with AEs being almost exclusively minor infusion reactions. Although AEs have been reported with all IV irons, AEs with this formulation, when high doses are avoided, are rare.15,16 FG administration has been shown to reduce ESA requirements in hemodialysis patients with elevated ferritin levels,43 to improve anemia without ESAs in iron-deficient patients with CKD,44 and to correct anemia in dialysis patients with high serum ferritin and low transferrin saturations.45
Although the maximum recommended dose of FG is 125 mg given as a bolus or short infusion, 250 mg has been reported to be given safely over 1 hour.46 Higher doses are associated with vasoactive reactions consisting of hypotension, acute onset of diarrhea, and swelling of the extremities, and therefore should be avoided. If higher-than-recommended doses are not infused, significant AEs with FG are infrequent.
Iron Sucrose
By far, the largest experience in the published literature is with this formulation. In 1994, Aronoff et al47 showed the safety of replacement and maintenance regimens of IS in hemodialysis patients. These data were confirmed by Charytan et al,48 who showed the safety of IS in hemodialysis patients, including those intolerant of other parenteral iron products. In a randomized, controlled trial comparing IV IS to oral iron in anemic patients with nondialysis-dependent CKD, Van Wyck et al49 showed that IS, given as 1000 mg in divided doses, was more effective than oral iron. In a study of anemic patients with CKD with absent marrow hemosiderin and high serum ferritins, Gotloib et al50 showed that IV IS administration, with and without ESAs, resulted in increases in hemoglobin levels and achievement of the target hemoglobin of 12 g/dL.
IS has been shown to improve hemoglobin responses in the treatment of anemia in patients with IBD, wherein oral iron is potentially harmful to the intestinal epithelium.51,52 FG, LMW ID, and FC have also been shown to be beneficial in these patients.53 In a review of the efficacy and safety of IV IS as an alternative to blood transfusion in surgery, the authors concluded that IV IS was safe, hastened recovery from anemia, and decreased transfusion requirements.54 IV IS has been shown in observational studies to reduce transfusion requirements in joint replacement, cardiac surgery, and colorectal cancer surgery. A summary of the data on IV iron in surgery is shown in Table 5. These data are reviewed in a recent publication.55
Studies of IV iron in surgery
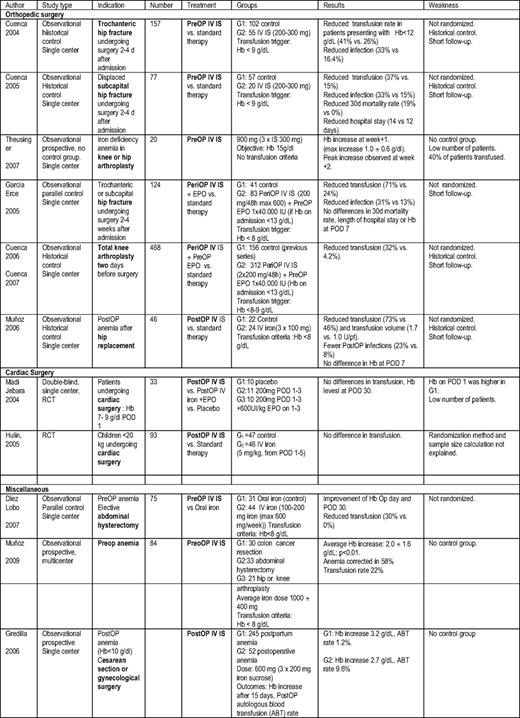
PostOp indicates postoperative; RCT, randomized controlled trial; Hb, hemoglobin; POD, postoperative day; PreOp, preoperative; EPO, erythropoietin; PeriOP, perioperative; G1, group 1; G2, group 2; pt, patient; G3, group 3; ABT, autologous blood transfusion.
Courtesy of Dr. Manuel Munoz, Transfusion Medicine, School of Medicine, University of Malaga, Spain, and Dr. Jose Garcia-Erce, Department of Hematology and Hemotherapy, University Hospital Miguel, Server, Zaragoza, Spain.
IS can be safely administered as a 2-minute bolus or as a short infusion in doses up to 300 mg, but higher doses have been proscribed56 . When higher doses are administered, even with infusions lasting 2 hours, hypotension, nausea, and low back pain are observed..57 If higher-than-recommended doses are not infused, significant AEs are infrequent.
Ferric Carboxymaltose
This formulation of IV iron was the first of the new agents to be approved for more rapid administration of large doses. FC can be administered as an infusion of 500-1500 mg in 15 minutes; however, only doses up to 1000 mg are currently approved. No test dose is required. FC is approved in Europe, Asia, and Australia.
In January 2010, Anker et al58 published their experience with 200 mg boluses of FC in patients with left ventricular ejection fractions of 45% or less. They concluded that treatment with IV FC in patients with chronic heart failure and iron deficiency, with or without anemia, improves symptoms, functional capacity, and quality of life with an acceptable side-effect profile.
In a prospective study of 200 patients with IBD who received FC as a 1000-mg infusion in 15 minutes, Kulnigg et al59 concluded that FC was safe and noninferior to oral ferrous sulfate in treating patients with iron deficiency, and resulted in a rapid hemoglobin increase and repletion of iron stores. In a recently published clinical trial, Covic et al60 studied 163 hemodialysis patients with IDA, who received FC boluses of 100 to 200 mg on the day of dialysis, and concluded that the drug was well tolerated and effective in the correction of hemoglobin levels and iron stores.
In a study of 477 women with IDA randomized to receive ≤1000 mg of FC over 15 minutes or oral ferrous sulfate, Van Wyck et al61 showed improvement in hemoglobin responses, physical function, and fatigue, compared with oral iron. No significant toxicity was observed.
However, in February 2008, the FDA failed to approve FC for distribution in the United States due to unexplained hypophosphatemia following infusion to patients with CKD, an increased number of adverse cardiac events, and an imbalance in death rates in the treatment arm, compared with the control arm. The results of ongoing, adequately powered safety trials with this agent are awaited.
Ferumoxytol
This formulation was approved by the FDA in June 2009 for iron replacement in patients with IDA and CKD. No test dose is required. This product is unique in that it can be administered as a relatively large dose (510 mg, exclusive vial size) in < 20 seconds. There is no data on higher doses. The published safety profile is consistent with that of LMW ID, FG, and IS.
In a study of 230 patients, in whom 116 received ferumoxytol and 114 oral iron, Provenzano et al concluded that two rapid (17 seconds) injections of 510 mg of ferumoxytol, administered within 1 week, led to a greater hemoglobin increase, compared with oral iron with comparable tolerability. No significant AEs were observed.62 In a crossover study enrolling 750 patients with IDA and CKD not on dialysis, Singh et al63 administered 510 mg of ferumoxytol or placebo in 17 seconds twice in 1 week to 711 patients or placebo to 713. One significant AE (anaphylaxis) was observed in the treatment arm in a patient with a history of multiple drug allergies. The patient recovered. The authors concluded that ferumoxytol is well tolerated and has a safety profile similar to placebo in anemic patients with CKD.
Ferumoxytol has the potential to facilitate iron replacement if the safety profile remains unchanged in other patient populations. It is not currently approved in Europe. We look forward to more postmarket safety and dosing data with this new agent in patients with the anemia of CKD as well as other clinical settings.
Iron Isomaltoside
This formulation is the newest of the three agents able to provide large doses of IV iron more rapidly than iron dextran. II is approved for administration in doses up to 20 mg/kg. It was approved in Europe in November 2009.
Wikstrom et al (World Nephrology Congress, Milan, Italy, 2009) reported on a cohort of 182 patients with anemia of CKD who received 584 injections given as a 100- to 200-mg bolus or TDI in doses ranging from 462 to 1800 mg in 30 to 60 minutes. No serious AEs were observed. Efficacy was consistent with established guidelines for CKD.64 As with FC, no test dose is required. II contains strongly bound iron that enables a controlled and slow release of bioavailable iron to iron-binding proteins with minimal free iron release. Clinical trials in nephrology, oncology, gynecology, and gastroenterology evaluating more rapid administration of both boluses, and TDIs are ongoing.
Long-term Toxicity of IV Iron
There are no prospective data on long-term outcomes with the use of IV iron. The largest experience with IV iron's use is in the nephrology community, with the majority of the published data coming from studies in dialysis patients. The most comprehensive work on this subject was done by Kalantar-Zadeh et al,65 who examined time-dependent associations between IV iron administered and both all-cause and cardiovascular mortality in maintenance hemodialysis patients. Prospectively collected data (2001–2003) from an historical cohort of more than 58,000 patients, representing virtually all DaVita dialysis clinics in the United States, were analyzed. The investigators reported that, after extensive time-dependent and multivariate adjustment for case mix, administered IV iron and erythropoietin doses, and surrogates of nutritional status and inflammation, the lowest all-cause and cardiovascular death risk was associated with the following iron parameters: serum ferritin levels between 200 and 1200 ng/mL, transferrin saturations between 30% and 50%, and serum iron levels between 60 and 120 μg/mL. They also found that, compared with those who did not receive IV iron, maintenance doses up to 400 mg per month were associated with improved survival.65
In a retrospective analysis of all-cause mortality in a cohort of 32,566 hemodialysis patients from 1996 to mid-1998, Feldman et al66 found no adverse effect on 2-year survival, with doses up to 1000 mg given over a 6-month period, but increased mortality for doses ranging from 1000 to 1800 mg. However, using multivariable models to account for time-varying measures of morbidity and iron administration, the authors found no statistically significant association between any level of iron administration and mortality. As a result of this observation, they concluded that previously observed associations between iron administration and higher mortality may be confounded and provided cautious support for the safety of the judicious administration of cumulative iron doses > 1000 mg over a 6-month period if needed to maintain target hemoglobins in patients on hemodialysis
Beguin et al67 published the only oncology study to examine long-term outcomes after IV iron administration. The authors studied 127 anemic autologous stem cell transplant patients with lymphoproliferative malignancies, who received either darbepoietin with or without IV iron. At > 1500 days, similar outcomes were observed for both groups.67
Potential Negative Effects of IV Iron
Iron is a pro-oxidant, an important nutrient for many bacteria, and in laboratory animals has been shown to exacerbate sepsis. Whereas human studies have shown transient increases in markers of oxidative stress with all forms of IV iron, no clinically negative outcomes associated with the increase in these markers has ever been reported.68
Iron repletion with oral iron has been associated with adverse outcomes in certain infections. In a study of 137 iron-deficient Somali nomads, 67 of whom were treated with placebo and 71 with oral iron, Murray et al69 noted seven episodes of infection in the placebo group and 36 in the group who received iron. Although IV iron has been theoretically implicated as a cause of increased infections.70,71 Hoen et al72 prospectively examined 985 dialysis patients receiving IV iron as part of the regimen for dialysis-associated anemia and found that, while central venous catheters, history of bacteremia, arteriovenous grafts, and immunosuppression were associated with increased risk of infections, ferritin levels and total dose of IV iron administration were not. Furthermore, in the Anker study, patients with heart failure (a relatively high-risk group)—including approximately 40% with CKD in a 6-month placebo-controlled trial—had improvements in quality of life and functional status without an increase in infections.58 The current state of the literature relating to iron administration and infection risk does not allow for firm conclusions. The risk, if any, of IV iron causing infection and related morbidity and mortality is probably very small. Clinicians should weigh this information with the well-established benefits of effective iron management and anemia correction, including decreased morbidity and improved quality of life.
Summary
Given the demonstrated safety and efficacy of IV iron in a broad spectrum of diseases associated with IDA, the current paradigm that oral iron is first-line therapy should be reconsidered. Furthermore, IV iron is the preferred method of iron replacement as an adjunct to ESAs in patients with both dialysis and nondialysis-associated CKD, in patients with IBD and in oncology patients receiving ESAs for chemotherapy-induced anemia. The IV iron can be given as multiple boluses of IS, FG, FC (Europe only), II (Europe only), or LMW ID until the desired dose is achieved: two rapid injections of ferumoxytol (United States only) 2 to 3 days apart or a TDI of LMW ID (usually 1 gram) in approximately 1 hour (approved only in Europe). In Europe, TDIs of FC (1000 mg) and II (20 mg/kg) are approved for administration in 15 minutes. Premedication should be avoided unless there is a history of hypersensitivity. In our practice, we routinely administer 1000 mg of LMW ID in 250 mL of normal saline in 1 hour without premedication. A test dose of 10 to 25 mg is infused over 3 to 5 minutes. If no acute reaction is observed, the remaining solution is infused over the balance of 1 hour. For those with a history of drug allergies, an allergic diathesis or a history of inflammatory arthritis, wherein both parenteral and oral iron have been shown to exacerbate symptoms, 125 mg of methylprednisolone is infused prior to the test dose.
Although the safety of IV iron has been demonstrated in studies comprising thousands of patients with numerous clinical entities associated with iron deficiency, safety concerns still abound. All iron products can cause hypersensitivity or other reactions, some of which will be severe. Outside of retrospective reviews of spontaneously reported events, the comparative frequencies of reactions remain unknown. However, until the real incidence of AEs is known more precisely, one might consider avoiding HMW ID based on the preponderance of published evidence. A commonly accepted AE definition should be promoted and adopted by the FDA so that types of events can be calculated. Reporting to a central agency should be mandatory and the data reviewed regularly. Until reliable comparative data becomes available, one product cannot, and should not, be considered superior in terms of safety profile. As hematologists, we should acquaint ourselves with the incidence, clinical nature, and significance of reactions to the existing preparations. As experts on diseases of blood, it is incumbent on us to gain increased familiarity with this important therapy for the most common anemia we encounter.
Disclosures
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Off-label drug use: Total dose infusion of iron dextran. This method of iron replacement allows for full replacement of iron deficits in a single setting.
Correspondence
Michael Auerbach, MD, Clinical Professor of Medicine, Georgetown University School of Medicine, Private Practice, 9110 Philadelphia Rd., #314, Baltimore, MD 21237; Phone: (410) 780-4050; e-mail: mauerbachmd@aol.com