Abstract
Decisions regarding the optimal treatment of acute myelogenous leukemia in the elderly patient requires the consideration of multiple factors. Population-based studies have demonstrated that, for all age groups, aggressive therapy results in improved survival and quality of life when compared with palliative care. The optimal induction and postremission regimen for older patients has yet to be determined. Furthermore, not all patients are candidates for such therapy. Consideration of patient and disease-related factors can help to determine the appropriateness of intensive therapy in a given patient. For those patients for whom aggressive induction therapy does not seem to be in their best interest, novel agents are being investigated that will hopefully address the issues of induction death and early relapse associated with these patient populations.
How Is Acute Myeloid Leukemia in the Elderly Different?
Acute myeloid leukemia (AML) presents at all ages, but is mainly a disease of the elderly, with a median age of 69 years in the white US population.1 In the Swedish Acute Leukemia Registry, 68% of patients diagnosed with AML since 1973 were over age 60 years; between 1997 and 2005, 75% was aged 60 years or more.2 Prognosis worsens every decade beginning at age 30 to 40.1,3 A report by the German Acute Myeloid Leukemia Cooperative Group looked at patients 16 to 85 years of age enrolled in two consecutive trials in 1992 and 1999 with no upper age limit who had AML.4 In a multivariate analysis of prognostic factors, age ≥ 60 years was a statistically significant poor prognostic factor for complete remission (CR), overall survival (OS), remission duration, and relapse-free survival (RFS). Population-based studies have reported 3- and 5-year survival rates of only 9% to 10% and 3% to 8%, respectively, in patients over age 60, compared with 5-year survival rates of up to 50% for younger patients.2,5,6 Poorer outcome has traditionally been considered to be the result of less intensive therapy in this population, concurrent comorbidities, a higher likelihood of underlying hematopoietic disorders, and biologically poor risk disease. Moreover, because of the perception that older adults are less likely to do well with standard therapy, clinicians are less likely to treat these patients aggressively or refer them to centers that do so. As such, lower levels of aggressive treatment may compound underlying prognostic differences associated with patient factors and disease biology.
In a retrospective analysis of 968 adults with AML in five Southwest Oncology Group (SWOG) trials to understand the nature of how AML changes with age, differences were noted in performance status (PS), presenting white blood cell (WBC) count, percentage marrow blasts, expression of multidrug resistance (MDR), and karyotype.7 (Table 1). When compared with disease in patients younger than age 56, those age ≥ 56 are are more likely to have unfavorable cytogenetics and less likely to have favorable cytogenetics. Differences in disease biology are also apparent. For example, in an analysis of oncogenic pathway dysresgulation in 425 patients with AML, there is a statistically significant difference in the expression of E2F and PI3 (phosphoinositide 3) kinase activation, but a higher probability of RAS, TNF (tumor necrosis factor), Src, and EPI (extrinsic pathway inhibitor) pathway activation.8 In younger patients, a similar profile is of poor prognostic significance and portends for decreased sensitivity to anthracycline.
Patient and disease characteristics at presentation of AML, by age
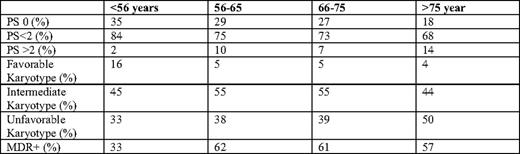
MDR indicates multidrug resistance. Adapted from Appelbaum et al.7
What Is the Standard Therapy for the Older Patient With AML?
The combination of an anthracycline and cytarabine (3+7), with or without other agents, is considered the standard induction regimen for patients under age 60, followed by consolidation therapy that often uses high-dose cytarabine.9–11 As noted previously, the use of the standard 3+7 regimen in elderly patients does not yield results similar to those seen in younger patients. Recent studies have, therefore, focused on dose intensification of anthracyclines and/or postremission therapy (Table 2).
Intensive regimens for elderly AML

HOVON indicates Stichting Hemato-Oncologie voor Volwassenen Nederland; ED, early death; Ara-C, cytarabine; GO, gemtuzumab ozogamicin; MTZ, mitoxantrone; IL-2, interleukin-2; DAT, daunorubicin, cytarabine, 6-thioguanine; ATRA, tretinoin; ICE, idarubicin, cytarabine, etoposide; IE, idarubicin, etoposide; MICE, mitoxantrone, etoposide, cytarabine.
In a recently published cooperative group study, Lowenberg et al12 reports the results of a comparison of induction therapy with daunorubicin (DNR) at a dose of 45 mg/m2 or 90 mg/m2 in 813 patients over age 60 with previously untreated AML. Although CR rates were improved in the group that received the higher dose of DNR (64% vs 54%, P = .002), there was no significant difference in the two induction groups with respect to OS and event-free survival (EFS). Thirty-day mortality was 11% to 12% in both groups. Two-year probabilities of relapse after CR and death during CR for the standard versus intensified DNR groups were 61% versus 54% for relapse and 10% versus 16% for death. Exploratory post-hoc analysis of patients aged 60 to 65 (n = 299), however, demonstrated a difference in the two dose groups with respect to CR (51% vs 73%), 2-year EFS (14% vs 29%, P = .002), and 2-year OS (23% vs 38%, P < .001). In the subgroup with core binding factor abnormalities (n = 33), escalated dose was associated with increased CR and decreased risk of disease progression or death (CR, 74% vs 93%; disease-free survival [DFS], 31% vs 62%).
Although the study by Lowenberg et al12 would suggest that intensification of DNR leads to superior CR rates with no difference in toxicity, the use of high-dose DNR by the French Cooperative Group in ALFA (Acute Leukemia French Association)-9801 did not demonstrate similar results.13 In their study, DNR at 80 mg/m2/day was compared with standard or high-dose idarubicin (IDA) during induction therapy of patients ages 50 to 70. Neither high-dose DNR nor high-dose IDA demonstrated any clinically relevant superiority over standard dose IDA in this population of patients. Multivariate analysis revealed age > 60 or < 60 to be of prognostic significance for OS, but not for CR or EFS. WBCs and cytogenetics significantly influenced EFS and OS in multivariate analysis. Although the superiority of higher dose anthracycline as induction is not clearly established by these trials, they do demonstrate that there is no increased toxicity with these regimens. In addition, dose-reduced anthracycline led to inferior CR rates in the Lowenberg study, and survival was not as good in certain subgroups of patients. As such, although there is no clear evidence that dose intensification is needed, there does not seem to be any reason to attenuate doses of anthracycline in patients over age 60. Doses of DNR at 60 mg/m2 or IDA at 12 mg/2 for 3 days together with cytarabine would be appropriate to use.
In the National Cancer Research Institute (NCRI) AML 14 trial, randomizations were conducted of anthracycline dose and cytarabine dose in induction, the use of an MDR modulator (PSC 833) in induction, and a number of postremission courses.14 No benefit was seen to any of the interventions tested. The PSC 833 arm was closed prematurely because of an increased early death rate because of infection. A randomized trial by the Eastern Cooperative Oncology Group (ECOG) similarly showed no benefit to the addition of the MDR modulator zosuquidar to induction therapy with DNR 45 mg/m2 and cytarabine.15 The question of whether the short infusion time of zosuquidar in this study inadequately blocked the polyglycoprotein pump has been raised by a subsequent study.16 A review of sequential SWOG studies that differed by the addition of cyclosporine to DNR and cytarabine demonstrated that CR rates were the same with and without cyclosporine (38% vs 44%), with a median OS of 6 months versus 7 months and an RFS of 14 months versus 8 months.17 The lack of benefit of these agents suggests that they are either not effective at inhibiting MDR or that MDR inhibition, even if effective, cannot overcome the drug resistance.
Clofarabine, an agent that was initially approved for use with patients with acute lymphoblastic leukemia, has recently been studied in patients with relapsed and refractory AML, as well as untreated elderly patients with AML.18–20 Results of phase II trials are encouraging. The upcoming phase III ECOG trial for elderly patients with AML will randomize patients to induction therapy with DNR and cytarabine versus single-agent clofarabine.
Once CR has been obtained, there is no standard postremission strategy in these patients. No prospective randomized study has ever established the benefit of postremission therapy in older adults; however, all large studies of AML have included postremission therapy, presumably because of the concern that patients are all destined to relapse without postremission therapy. Cycles of high-dose cytarabine, which is felt to be of benefit in younger patients, has not been demonstrated to be of benefit in this age group and is thought to be too toxic.21 Single cycles of high-dose cytarabine have, however, been well tolerated in cooperative group studies.13,22 Recent studies of gemtuzumab, as well as studies of interleukin-2 as postremission therapy, have not demonstrated any benefit.23,24 In the EORTC-GIMEMA (European Organization for Research and Treatment of Cancer-Gruppo Italiano Malattie e Matologiche dell'Adulto) AML 13 trial, in which postremission therapy was randomized between two cycles of intensive intravenous therapy versus oral therapy, no difference in DFS was demonstrable between the two groups.25,26 The median DFS was 9 months for the noninfusional therapy versus 10.4 months for the intravenous regimen and 3-year DFS 13 versus 21% (P = .15). Although no OS benefit was seen, the only patients who enjoyed prolonged DFS had received postremission chemotherapy. This study also offered autologous hematopoetic stem cell transplant (HSCT) as a postremission therapy, and no benefit was demonstrated to HSCT.23 Of the 61 patients with AML in CR in this study who underwent stem cell harvest after first consolidation, 33 of 61 patients had HSCT with a median 3-year OS of 39% and a DFS of 28% for the 33 patients who underwent HSCT and a DFS of 21% for the 61 patients who underwent stem cell harvest.
Although randomized trials have not been done to unequivocally establish the need for consolidation therapy, randomized trials have evaluated duration and types of postremission therapy. The AML 11 trial demonstrated that there was no benefit when four cycles, rather than 1 cycle, of moderate intensity postremission therapy were given.27 In the ALFA 9803 trial comparing a cycle of intensive consolidation versus 6 months of lower dose therapy, prolonged consolidation was associated with improved OS (56% vs 37%) and DFS (28% vs 17%).28 Repeated courses of low-intensity chemotherapy were also associated with shorter hospitalization and fewer transfusions. Age, AML type, and cytogenetics were associated with longer DFS. In the AML HD98 study, in which patients who achieved a CR and after one intensive consolidation were randomized to either a second intensive consolidation with IDA/etoposide or mild oral maintenance with the same drugs, there was a lower relapse rate and superior survival in the group that received the more intensive therapy (median survival 22.3 vs 14.3 months).29 In addition to supporting the use of high-dose consolidation, this study suggests that more than one cycle of consolidation was superior when this regimen was used. Like other studies in the elderly, only 29% of the initial patient cohort was randomized after first consolidation. Moreover, only 2 of 96 randomized patients had high-risk cytogenetics, highlighting the selection bias in many of these trials. One of the other major issues for studies of postconsoldation is that many patients do not receive their assigned therapy, making the trials difficult to interpret. Until there is a randomized trial demonstrating that there is no benefit to postremission therapy, I feel that it should be offered to those patients who achieve a CR and are able to tolerate it.
Recent analyses have further tried to elucidate the prognostic importance of cytogenetics and molecular prognostic factors in elderly patients. As in younger patients, core binding factor abnormalities have a more favorable prognosis. In a retrospective French study of 147 patients who received at least one dose of induction chemotherapy, 60 had t(8;21) and 87 had inversion (16). There was an 88% CR rate and a 10% 8-week early death rate.30 With a median follow-up of 48 months, the 5-year probability of OS and leukemia-free survival (LFS) was 31% and 27%, respectively. On multivariate analysis, high WBC count, poor PS, and del(9q) were of negative impact, and trisomy 22 and intensive consolidation—when compared with maintenance therapy—were of positive impact. Despite a CR rate of 88%, median LFS was only 18 months. In the t(8;21) group, the difference in survival following intensive consolidation was statistically significant when compared with maintenance (not yet reached vs 10 months). In a recent analysis of the impact of NPM1 (nucleophosmin) mutation in elderly patients in 148 patients ≥ 60 years of age, with de novo cytogenetically normal AML enrolled onto CALGB (Cancer and Leukemia Group B) 9720 and 10201, those patients with NPM1 mutations have a higher CR (84 vs 48) and longer 3-year DFS (DFS 23 vs 10) and OS (35 vs 8). In multivariate analysis, this was particularly true in patients ≥ 70 years of age (CR 87% vs 39%, DFS 30% vs 7%, and OS 49% vs 5%). Several investigators have suggested that, in determining therapy for older patients with AML, it would be appropriate to wait for results of cytogenetic and perhaps molecular studies before discussing therapeutic options.31
Overall, these studies demonstrate that results of intensive therapy in elderly patients remain poor. Although CR rates of 40% to 80% can be achieved, in highly selected populations, long-term survival is poor. Even in patients with favorable cytogenetics who have 80% CR rates, relapse rates are high. As such, novel strategies are needed. With the use of reduced-intensity conditioning regimens, allogeneic transplantation has become a valid option. Recent reports have suggested that age, at least up to 70, does not impact outcome of reduced-intensity transplant and that reduced intensity transplantation may yield better outcomes than chemotherapy in patients aged 60 to 70. Retrospective registry studies have demonstrated that age does not appear to be a factor in transplant outcome. In a retrospective analysis of 629 reduced-intensity allogeneic transplants reported to the French transplant registry, outcomes (OS, transplant-related mortality, and acute graft-versus-host disease [GVHD]) in patients over age 65 were comparable with those for patients who were between ages 60 and 65.32 Two-year OS was 48% vs 47% for the two age groups. Similarly, data from the Center for International Blood and Marrow Transplant Research (CIBMTR) on 1080 patients undergoing reduced-intensity conditioning transplant for AML or myelodysplastic syndrome (MDS) demonstrated no difference in nonrelapse mortality, grade 2 to 4 GVHD, chronic GVHD, or relapse, when patients ages 60 to 64 or over age 65 were compared with patients aged 40 to 59.33 Although 2-year survival rates were lower in patients over age 60, 2-year survival rates were greater than 30% in all age groups, and multivariate analysis revealed no significant impact of age on DFS or OS. In a prospective study of the feasibility of transplant in patients over age 50 at M.D. Anderson who entered CR, outcome for those patients who underwent an allogeneic transplant with reduced-intensity conditioning had a superior RFS and OS when compared with those who received postremission chemotherapy.34 A retrospective analysis of over 1,000 elderly patients with AML in first CR who underwent HSCT or chemotherapy demonstrated improved OS and DFS in the HSCT group.35 Further prospective studies are needed.
Who Should Not Receive Intensive Therapy?
Selection criteria commonly used for inclusion into clinical studies have a major impact on reported outcome. Furthermore, the interpretation of clinical trials must be done with caution, in that not only are eligibility criteria different, but also investigator choice and patient preference clearly influence patient inclusion. In an analysis by the Swedish of 9729 patients diagnosed with AML in Sweden since 1973, the proportion of older patients who were considered eligible for intensive remission induction therapy decreased from 92% in those aged 60 to 64 years to 80%, 67%, 45%, 23%, and 4% in those aged 65 to 69, 70 to 74, 75 to 79, 80 to 84, and 85+ years, respectively.2 Importantly, this report found that early death rates were improved with intensive treatment, compared with palliation alone. This was seen in all age groups and all PS levels. For example, within patients age 76 to 89, with a PS of > 2, those who received intensive treatment had a 36% early death rate, compared with 52% for those who received palliation alone. The assessment of eligibility for intensive therapy is, however, quite subjective, and the reason that certain patients within the cohort received intensive therapy whereas others did not is not available in this report. An analysis was done within the six Swedish health care regions among patients age 70 to 79 years.2,36 Percentage of patients who were deemed candidates for intensive therapy differed by region, probably because of physician and patient preferences, because baseline characteristics of the patients seem no different. In those regions in which only 40% of patients were felt to be eligible for intensive therapy, there was a 16% early death rate with intensive treatment, compared with 27% with palliation only. The early death rate was similar in those regions wherein 75% of patients were felt to be eligible for intensive treatment. When comparing regions where only 40% versus 75% were treated intensively, CR rates with intensive treatment were 41% and 49%, respectively, and CR rates for the entire group in the region was 19% versus 37%. Given the lower proportion of patients who received intensive therapy in those regions, the 2-year OS was decreased at 24% versus 50% and 5-year OS was 1% versus 13%. Furthermore, although PS is more predictive for early death rate than age, long-term survival may be achieved in patients with initial poor PS. Based on their report, they state that standard intensive treatment decreased early death rates in most patients up to age 80.
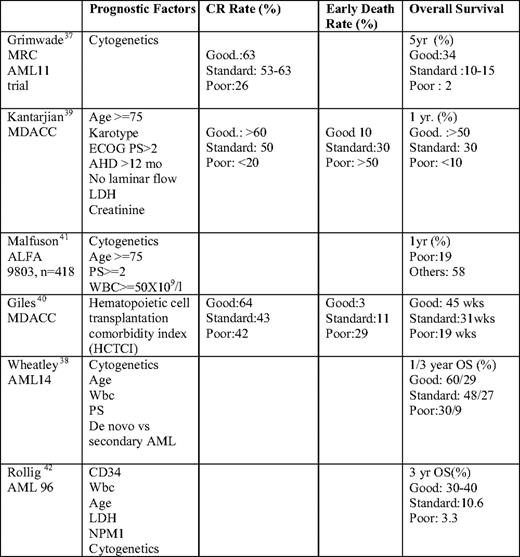
The MRC (Medical Research Council) AML 11 trial allowed for the use of cytogenetic data to identify prognostic groups in older adults who were treated with intensive therapy on study.37 More recently, investigators have become interested in developing tools to allow us to discriminate between patients who are likely to benefit from intensive chemotherapy versus those who should be candidates for less aggressive therapy (Table 3).
Patients are now being considered “unfit for intensive therapy” and more appropriate for alternative or investigational therapy if there is either a high risk of early mortality or a poor chance of long-term DFS. In a retrospective analysis by Wheatley of the patients in the AML 11 trial, five risk factors were identified in AML patients that were weighted according to the values of their multivariate coefficients and used to define three subgroup risk classifications that were then able to be validated in the more recent MRC AML 14 trial.38 Cytogenetic group, age, WBC count, PS, and de novo vs secondary AML were all found to be highly significant. Good, standard, and poor risk groups had a 1-year survival of 53%, 43%, and 16%, respectively (P < .0001). In a retrospective analysis by Kantarjian et al39 of 998 patients aged 60 or older who received intensive therapy at M.D. Anderson Cancer Center, a multivariate analysis was performed to calculate a prognostic score. They identified prognostic risk factors: age (≥ 75 years), unfavorable cytogenetics, poor PS (> 2), ≥ 12-month history of antecedent hematologic disorder (AHD), lactate dehydrogenase (LDH) > 600, elevated creatinine, and treatment outside a laminar flow room. Three risk subgroups were defined based on the number of poor prognostic factors. Those with none of the adverse risk factors have an expected CR rate of > 60%, induction mortality of 10%, and 1-year survival over 50%. A poor risk group with more than or equal to three poor prognostic risk factors had CR, induction mortality, and 1-year survival rates of < 20%, > 50%, and < 10%, whereas those in an intermediate risk group had rates of 50%, 30%, and 30%, respectively. Several reports have focused on the impact of comorbidities on decision making in AML. A refined Hematopoetic Cell Transplantation Comorbidity Index (HCTCI) has been found to be useful in identifying patients who are not candidates for intensive therapy.40 Malfuson et al41 analyzed 416 patients treated on the ALFA 9803 multicenter trial to identify independent prognostic factors that would impact on OS. Based on their analysis, they propose that, in those patients with unfavorable cytogenetics and the presence of two poor prognostic factors (age ≥ 75 years, PS ≥ 2, and WBC count ≥ 50 × 109/L), the OS is only 19% at 12 months, and these patients should, therefore, not be considered for standard chemotherapy.
In a recent analysis of the AML 96 trial of 909 patients with a median age of 67, univariate and multivariate analyses identified karyotype, NPM1 mutation status, WBC count, and LDH of independent prognostic significance for OS, DFS, and relapse.42 Although favorable and poor cytogenetic risk groups had markedly different OS, those patients with intermediate risk karyotype could be subdivided based on CD34 expression, along with the abovementioned factors identified on multivariate analysis, allowing for the designation of four groups: (1) favorable cytogenetics, (2) intermediate cytogenetic with favorable features, (3) intermediate cytogenetics with adverse risk features, and (4) poor risk cytogenetics, with 3-year OS of 39.5%, 30%, 10.6%, and 3.3%, respectively. These scoring systems can be used to identify patients who are likely to respond to standard therapy, as well as those who are not likely to benefit and should therefore be considered for alternate or investigational therapy.
What Treatment Options Are Available for Patients Who Are Not Candidates for Intensive Induction Therapy?
For those patients who are not considered to be candidates for intensive induction therapy, one would hope to identify agents and regimens that are more effective and less toxic to address the concerns regarding early induction death, inadequate response rate, and high risk of relapse. The NCRI AML 14 study was designed to allow for randomization of patients between intensive and nonintensive therapy, but only eight patients agreed to randomization.14,43 As such, data available on novel agents comes from a variety of pilot and phase II studies with differing eligibility criteria. When evaluating the outcomes, it is important to also look at the characteristics of patients who were ultimately enrolled. Table 4 reviews available data from some of these studies.
Novel therapeutic regimens
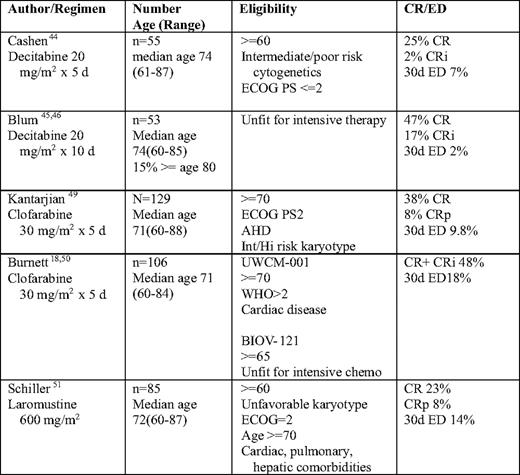
Int indicates intermediate; Hi, high; UWCM, University of Wales College of Medicine; WHO, World Health Organization; chemo, chemotherapy; CRi, complete remission with incomplete blood count recovery; ED, early death; CRp, complete remission with incomplete platelet recovery.
As part of the NCRI AML 14 study, 212 patients who were deemed unfit for intensive treatment options by the local investigator were randomized to receive supportive care alone with hydroxyurea or cytarabine 20 mg twice daily by subcutaneous injection for 10 days every 4 to 6 weeks.43 Outcome was improved for the low-dose (LD) cytarabine arm when compared with supportive care with hydroxyurea alone. CR was 18% versus 1%, and median survival was 575 days for those who achieved CR, compared with 66 days in nonresponders. DFS for responders was 8 months. Survival benefit was seen in all age groups, even those over age 75. As none of the patients with adverse cytogenetics achieved a CR, no survival benefit was, however, seen in that group. The early death rate was 39% at 8 weeks. Although no criteria were used to define unfit patients, 78% were over age 70, 27% had secondary AML, 30% had PS ≥ 2, 27% had heart disease, 49% had other comorbidities, and 59% had a poor risk score by the Wheatley Risk Index.38 Based on this study, LD cytarabine became the standard of care for the treatment of patients felt to be unfit for intensive chemotherapy, although one could argue that it should not be given to those with poor risk cytogenetics.
The DNA methyltransferase inhibitors have been the subject of several recent studies. In a multicenter phase II study of 55 patients over age 60 with untreated AML, decitabine was administered for 5 days monthly until disease progression.44 With a median of three cycles, the overall response rate was 24%, median survival was 7.7 months, and 30-day mortality was 7%. Responses were seen in all cytogenetic risk groups, as well as in those patients with prior MDS. An alternate schedule of decitabine was reported by Blum et al.45,46 Patients received an initial one to two courses of 10 days of decitabine, followed by a course over 3 to 5 days every 4 weeks for 1 year. Of the 53 patients with a median age of 74, 36% had secondary AML, and 34% had a complex karyotype. Eighteen patients had a HCTCI score of ≥ 3. There was a 64% response rate after a median of three cycles of therapy. CR occurred in all subsets, regardless of age, karyotype, presenting WBC, and prior AHD. One-year survival of poor risk patients was 30% (compared with 10% in patients with a similar Wheatley risk score in the AML 11 trial38 ).
In a study of azacitidine in AML with 20% to 30% blasts, patients who were deemed unfit for standard induction chemotherapy were randomized against either supportive care or LD cytarabine.47 OS survival was superior in the azacitidine arm. There was a statistically significant difference seen in OS for patients with poor risk cytogenetics in favor of azacitidine, compared with conventional care regimens (12.3 vs 5.3 months, respectively, with 2-year OS of 38% vs 0%).
Gemtuzumab ozogamicin (GO) has been the subject of a recent study by the EORTC and GIMEMA leukemia groups (AML 19).48 In this randomized multicenter study, 84 patients were randomized to receive one of two schedules of GO at attenuated doses or best supportive care. The proportion of patients either achieving a response or maintaining stable disease was greater in patients who receive GO at a dose of 6 mg/m2 on day 1 and 3 mg/m2 on day 8, when compared with a schedule of GO 3 mg/m2 on days 1, 3, and 5 (63% vs 38%, respectively). Results of the comparison with patients who were randomized to standard care are not yet available, and a phase III trial is ongoing.
Clofarabine has been studied as an agent in elderly patients with AML. In a phase II study of the agent in 112 patients over age 60 with untreated AML with at least one unfavorable baseline prognostic factor, there was a 46% response rate.49 The median age of the patients was 71. Twenty-two percent of patients had a baseline PS of 2, 47% had a prior hematologic disorder (AHD) or secondary AML, 55% had an unfavorable karyotype, and 62% were ≥ age 70. Overall response rate (ORR) was 395 for patients ≥ 70, 32% for PS 2, 51% for patients with AHD, 54% for intermediate karyotype and 42% for unfavorable karyotype, and 38% for patients with three risk factors. Median DFS was 37 weeks, and median OS was 41 weeks for all patients, 59 weeks for patients with CR/complete remission with incomplete platelet recovery (CRp), and 72 weeks for patients with CR. Early death rate (within 60 days) was 16%.
In two consecutive European studies of 106 untreated older patients with AML who were considered unfit for chemotherapy, participants were given four to six 5-day courses of clofarabine.18,50 In the UWCM (University of Wales College of Medicine)-001 study, patients who were either over age 70 (68%) or over age 60, with a PS of 2 or cardiac comorbidity, were treated with clofarabine for 5 days every 28 days for 2 to 4 courses. In the BIOV-121 study, patients were treated for 5 days every 4 to 6 weeks for up to six courses. All patients were age ≥ 65 and deemed unfit for chemotherapy. Overall, 36% of patients had a PS ≥ 2, 30% had adverse risk cytogenetics, 46% had Wheatley poor risk disease, and 65% were age ≥ 70. The ORR was 48%, and the median OS was 19 weeks for all and 45 weeks for those who attained a CR/complete remission with incomplete blood count recovery (CRi). Responses were seen in patients with adverse cytogenetics (44% ORR), patients with secondary AML (31%), and patients age ≥ 70 (49%). The death rate within 30 days was 18%.
A novel agent, laromustine (VNP40101M), a sulfonylhydrazine alkylating agent, has been studied in 85 patients with poor risk AML age ≥ 60 years.51 Patients received one to two cycles of laromustine at a dose of 600 mg/m2, followed by one cycle of cytarabine. Seventy-eight percent of patients were age ≥ 70, 47% had an adverse karytype, 41% had a PS of 2, 77% had pulmonary disease, 73% had cardiac disease, and 3% had hepatic disease. All patients with unfavorable karyotype or ECOG PS had at least one other risk factor at the time of enrollment. Seventy-five percent of patients had ≥ 3 risk factors. The ORR was 32% and was similar in patients over age 70 (32%), with a PS of 2 (32%), with baseline pulmonary or cardiac dysfunction (27%–34%). There was a 14% 30-day mortality. OS was 3.2 months (12.4 months for those with CR/CRp), and 1-year survival was 21% (52% for those with CR/CRp).
These phase II studies are encouraging, in that responses are seen in all poor risk categories, and early death rates are acceptable. Randomized trials are needed. Although randomized trials of intensive versus nonintensive therapy have not been successful, the ongoing AML 16 trial was designed to randomize patients who are considered not fit for intensive treatment to LD cytarabine versus LD cytarabine with GO, LD cytarabine with arsenic trioxide or tipifarnib, or LD clofarabine.52 The arsenic arm has been closed because of ineffectiveness with CR/CRi of 29%, compared with 24% and a 12-month OS of 27%, compared with 41%. The other arms continue to accrue patients.
Approach to the Elderly Patient With AML
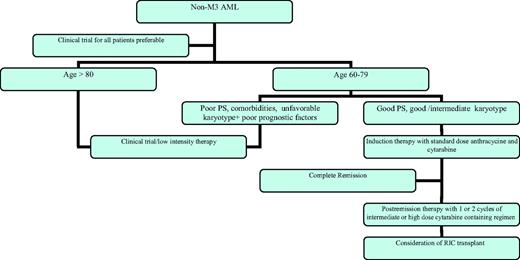
AML is a disease of the elderly, with the majority of patients over age 60. As our population ages, that percentage will only increase. Unfortunately, the standard regimens that are successful in treating younger patients will AML are not as beneficial in the majority of older patients with the disease. Figure 1 outlines my approach to the elderly patient with AML. Understanding of the disease biology, as well as the prognostic factors associated with the host, allows us to better determine which patients are likely to benefit from standard therapy and which require alternative approaches. Objective scoring systems are being developed that allow us to define patients unfit for intensive chemotherapy on the basis of increased risk of induction death, low response rate, and/or low long-term DFS. Optimal induction and postremission therapy for patients appropriate for intensive therapy have yet to be defined, again, because results are not satisfactory with our current regimens, even in those patients who do not have definable poor prognostic factors. When compared with young patients with similar disease-related features, outcomes are inferior. For patients who are not candidates for intensive therapy because of comorbid conditions, low-intensity therapies appear to be superior to palliative care alone. Whenever possible, patients should be enrolled in clinical trials that will allow us to address these issues.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests.
Off-label drug use: Use of gemtuzumab ozogamicin, clofarabine, and decitabine.
Correspondence
Selina M. Luger, MD, Professor of Medicine, and Director, Leukemia Program, Hospital of the University of Pennsylvania, Perelman Center for Advanced Medicine, 3400 Civic Center Blvd., Philadelphia, PA 19104; Phone: (215) 614-1847; Fax: (215) 615-5888; e-mail: selina.luger@uphs.upenn.edu