Abstract
Patients with chronic myeloid leukemia (CML) who have achieved a complete molecular response (CMR) defined by no detectable BCR-ABL mRNA on imatinib (IM) treatment often ask whether it is necessary for treatment to continue. We now know that approximately 40% of patients with a stable CMR for at least 2 years are able to stop IM treatment and remain in molecular remission for at least 2 years. This exciting observation has raised hopes that many patients can be cured of CML without the need for transplantation and its attendant risks. One might argue that for many patients maintenance therapy with IM or an alternative kinase inhibitor is so well tolerated that there is no imperative to stop treatment; however, chronic medical therapy may be associated with impaired quality of life and reduced compliance. Inferences about the biology of CML in patients responding to kinase inhibitors can be drawn from clinical experience, molecular monitoring data, and experimental observations. We summarize this information herein, and propose 3 possible pathways to “cure” of CML by kinase inhibitors: stem-cell depletion, stem-cell exhaustion, and immunological control.
Introduction
Professor John Goldman suggested years ago that if a treatment is sufficient to prevent the progression of chronic myeloid leukemia (CML) to the advanced phase and to prevent the emergence of resistance, then the treatment can provide an “operational cure.”1 Effectively, CML responsive to imatinib (IM) might become a chronic disease requiring maintenance treatment, just as one might treat hypertension. Our own personal experience of counseling patients about IM cessation in a clinical trial suggests that some patients are comfortable with this view of their disease and feel secure on IM. However, if tyrosine kinase inhibitor (TKI) therapy is required indefinitely to maintain the operational cure of CML, then this ongoing drug exposure raises its own problems: there is a financial cost to the individual and the community, there may be chronic toxicity, and as long as there is residual disease there is at least a theoretical risk of emerging resistance.
With 5 years of follow-up < 10% of patients in the IRIS study stopped IM because of intolerable toxicity.2 Rates of discontinuation for first-line nilotinib and dasatinib are similar at ∼5% in the first year or so.3,4 This is not to say that most patients have no significant toxicity. Chronic grade 1 toxicities such as diarrhea may significantly impair quality of life, and on occasion some patients choose not to take IM to avoid such toxicities.5 Noncompliance has been identified as the single most important risk factor for loss of complete cytogenetic response in long-term IM-treated patients.6 A recent update of progression-free survival in the IRIS study shows that there may still be rare patients who progress to the accelerated phase or to blast crisis after years of IM treatment,7 and long-term compliance could be an important factor in durable disease control.
The potential for late-emerging toxicities remains unknown, given that our experience with TKI therapy spans little more than a decade, and young patients with CML may be facing many decades of exposure to an ABL kinase inhibitor. Potential concerns about cardiotoxicity with IM8 seem not to have been borne out in clinical experience to date, but other side effects may become important in the longer term, including impaired glucose tolerance with nilotinib3 and pleural effusion with dasatinib.4 While such toxicities may be manageable, they impose an additional burden of morbidity.
In recent epidemiological data, the survival of younger patients treated with IM is within 10%-20% of the life expectancy of age-matched peers.9 It is inevitable that patients should pursue other goals, such as parenthood. Patients in their reproductive years may be concerned about impaired fertility and the risk of teratogenesis. Although some women have had normal babies after conceiving on IM, in others, serious birth defects have been reported.10 For some young women with CML the question of whether it is safe to stop IM for the duration of conception and pregnancy is as important as whether the disease is ever cured.
For all of these reasons we feel that an operational cure is only a partial success, and our ultimate aim should be to give the patient long-term disease control without the need for ongoing treatment.
ASCT
There already exists a curative treatment for CML. Allogeneic stem-cell transplantation (ASCT) in carefully selected younger patients may have an overall survival rate similar to that of IM-treated patients.11 However, considering the average age of CML patients at diagnosis, the availability of a well-matched sibling donor, and the variability in outcome according to transplantation center, it is clear that this option applies only to a small minority of CML patients. The potential for chronic GVHD and complications related to myeloablative transplantation conditioning regimens, such as cardiopulmonary disease and an increased risk of secondary malignancy, may reduce quality of life and overall survival in allografted patients. Several lines of evidence indicate that the curative potential of ASCT lies in an immunological GVL effect. If it were possible to harness this benefit by immunological manipulation without the toxicities of allografting this would be a major advance.
Very late relapses (after more than a decade) may occur even in patients allografted in the first chronic phase of CML.12 A stable complete molecular response (CMR) is associated with a much lower risk of relapse after allograft, but even in this population, rare relapses are seen after more than 5 years.13 These observations have implications for how we interpret the outcome of TKI cessation studies, which currently have much shorter follow-up.
Cessation of therapy
Interferon (IFN), with or without cytarabine, was the best drug therapy for CML before the introduction of IM. Approximately 1/4 of patients treated with IFN achieve a complete cytogenetic response, and only a small minority of patients will achieve a CMR.14 Patients who stopped IFN after achieving a deep molecular response have been reported in stable minimal residual disease (MRD) for more than 9 years15,16 and, much more rarely, in a stable CMR.17
The interim results of 2 studies of IM cessation have now been reported. The French STIM trial enrolled 100 patients with a stable CMR for at least 2 years.18 Molecular relapse occurred in ∼ 60% of patients after a median interval of 4 months. Relapses after 6 months were uncommon, but occurred as late as 19 months after stopping IM. The only patient characteristic that proved useful as a predictor of relapse risk was Sokal score: the probability of stable CMR was 54% in the low-risk group versus only 13% in patients with a high-risk score. The majority of relapses occurred only at a molecular level, and resumption of IM treatment rapidly restored CMR. The Australasian Leukaemia & Lymphoma Group (ALLG) CML8 study of imatinib cessation in CMR followed a similar design to the STIM trial, and has shown almost identical results in a smaller number of patients. At last analysis, the relapse rate was 60% (D.M. Ross and T.P. Hughes, personal communication). Given the potential for variability in molecular monitoring methods, it is reassuring to see comparable results from 2 independent investigations. In both studies the resumption of IM treatment resulted in a reduction of MRD, with most patients regaining a stable CMR within 6 months.
There have been no studies so far of therapy withdrawal in patients receiving newer ABL kinase inhibitors. We are, however, aware of a small number of patients who have been treated with either nilotinib or dasatinib after imatinib intolerance19 or failure20 and who stopped the TKI in CMR. Some of these patients remain in a stable remission with follow-up of up to 2 years. The number of such cases is currently too small to know whether the relapse rate after cessation of a second-generation inhibitor is different from that seen after stopping IM. With the increasing up-front use of these more potent ABL kinase inhibitors, it is likely to be only a short time before clinical trials are performed to answer this question.
What determines whether a patient will relapse when therapy is withdrawn? Relapse must arise from a pool of residual leukemic cells that are resistant to therapy. Conversely, drug-free remission might indicate eradication of the leukemic clone. In the ALLG CML8 study we examined this question using a highly sensitive patient-specific PCR for genomic BCR-ABL DNA.21 This method has the advantage that problems of nonspecificity or contamination are eliminated by virtue of the variability in the BCR-ABL fusion sequence.21–23 This study showed that most patients who remained in CMR after stopping IM had circulating cells from the original CML clone. The level of MRD in these patients was ∼ 1-2 log below the limit of detection of conventional real-time quantitative PCR (RQ-PCR). Thirteen of 17 patients had detectable BCR-ABL DNA at study entry, but only 8 of these patients relapsed (62%). Although this is the only study to examine highly sensitive MRD analysis in the context of IM cessation, at least 3 other studies have demonstrated the existence of residual leukemic cells in patients with a stable CMR.22,24,25
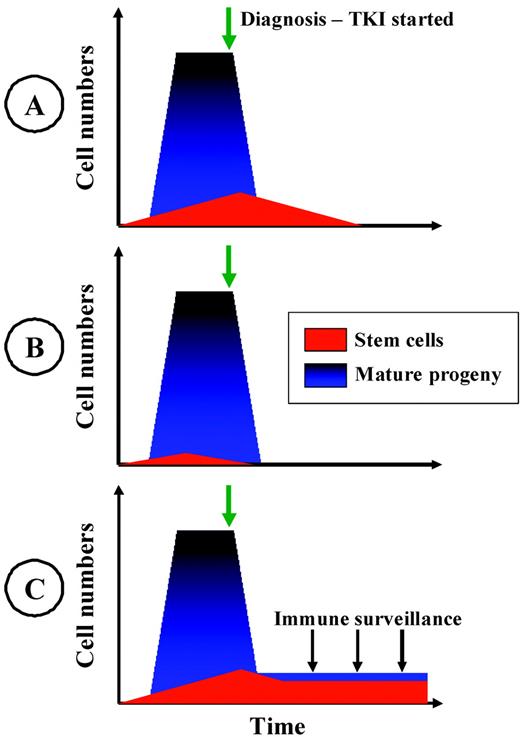
In the remainder of this article, 3 conceptual models for the achievement of a prolonged drug-free remission in CML are presented (Figure 1). The models are not mutually exclusive, and different pathways to drug-free remission might apply in different patients. Clinical implications for each of these models are also discussed.
Three hypothetical models of operational cure of CML. The models are not mutually exclusive. Different models may pertain in different individuals. (A) Stem-cell depletion. The progressive depletion of immature CML cells over years of continued therapy is shown. The risk of relapse on TKI cessation is related to the duration of therapy and the intrinsic sensitivity of CML stem and early precursor cells. (B) Stem-cell exhaustion. The CML stem-cell pool is relatively small and, due to stochastic events that direct self-renewal or proliferation, this pool of cells may become extinct before diagnosis or early in therapy. The risk of relapse upon TKI cessation is related to the depletion of committed CML progenitors and progeny. (C) Immunological control. A reduction in the level of MRD by TKI therapy is sufficient to overcome T-cell anergy and enables the emergence of an autologous immunological response that suppresses, but may not eradicate, the CML clone. The risk of relapse on TKI cessation is dependent on the functional immune response and the intrinsic immunogenicity of the CML cells.
Three hypothetical models of operational cure of CML. The models are not mutually exclusive. Different models may pertain in different individuals. (A) Stem-cell depletion. The progressive depletion of immature CML cells over years of continued therapy is shown. The risk of relapse on TKI cessation is related to the duration of therapy and the intrinsic sensitivity of CML stem and early precursor cells. (B) Stem-cell exhaustion. The CML stem-cell pool is relatively small and, due to stochastic events that direct self-renewal or proliferation, this pool of cells may become extinct before diagnosis or early in therapy. The risk of relapse upon TKI cessation is related to the depletion of committed CML progenitors and progeny. (C) Immunological control. A reduction in the level of MRD by TKI therapy is sufficient to overcome T-cell anergy and enables the emergence of an autologous immunological response that suppresses, but may not eradicate, the CML clone. The risk of relapse on TKI cessation is dependent on the functional immune response and the intrinsic immunogenicity of the CML cells.
Stem-cell depletion
Approximately 60% of patients in CMR are destined to relapse when IM is stopped. In these patients, there must be a population of CML precursors that is resistant to therapy. Analysis of RQ-PCR data from IM-treated CML patients reveals a biphasic response.26 There is a rapid initial drop in the level of BCR-ABL, which is thought to reflect the clearance of mature CML progeny that are sensitive to IM. There follows a second phase with a shallow gradient, which is thought to reflect the gradual depletion of the less-sensitive CML granulocyte-macrophage precursor pool27 which is relatively resistant to IM,28,29 and in which the cellular events leading to blastic transformation are thought to occur.30 The slow depletion of CML progenitors during IM treatment may reflect apoptosis that is dependent on cell cycling. In a kinetic model of cell-cycle–dependent stem-cell depletion, it was predicted that the disease could be eradicated by prolonged IM treatment.27 Beyond the second year of treatment, the average reduction in BCR-ABL mRNA was < 0.5 log per year.27,31 A conservative estimate of the number of residual CML cells in CMR would be ∼ 106 cells, and simple extrapolation would lead us to predict that few patients would eradicate their leukemic clone within 10 years of first achieving CMR.
The available evidence from studies of primary CML stem cells in vitro strongly suggests that the most primitive compartment of quiescent leukemic stem cells is resistant not only to IM, but also to the more potent second-generation ABL kinase inhibitors,32 and this should mean that ongoing therapy is necessary to suppress the CML clone. This pessimistic prediction is at odds with the observed relapse rate when IM is stopped after as little as 2 years in CMR. The absence of early relapse does not indicate that these patients are cured, but it is inconsistent with unchecked proliferation of CML from a level that is only 1-2 log below the limit of detection of RQ-PCR. Furthermore, the duration of CMR before IM cessation was not significant as a predictor of relapse risk in the French study, although total duration of TKI therapy and Sokal risk score were.18 A plausible explanation for the correlation between Sokal score and relapse risk is that the clinical risk score is a surrogate for the sensitivity of CML progenitors to IM. If this is the case, then stable CMR after IM withdrawal might be explained by more rapid depletion of CML stem cells in a subset of patients.
While we have so far considered the level of MRD in CMR based on extrapolation from the kinetics of response, it is also possible to extrapolate from the kinetics of relapse. Modeling of relapsing CML using RQ-PCR data predicts a rapid increase in BCR-ABL with a doubling time of about 2 weeks, which is consistent with relapse from a pool of committed myeloid precursors rather than from slow-cycling stem cells.26,27 This model fits with the clinical observation that patients who relapse usually do so within several months of stopping IM, and indicates that, for these patients, IM treatment has not effectively targeted the stem-cell compartment.
In both the French STIM study and the Australian CML8 study, occasional patients have been observed with late relapses more than a year after stopping imatinib. One hypothesis to explain this observation is that there is a change in the proliferation rate of the residual CML cells so that their doubling time is significantly prolonged. This hypothesis is not supported by experimental data showing that CML cells that survive TKI therapy can proliferate efficiently when the TKI is withdrawn.33 However, it remains possible that prolonged TKI exposure in vivo might modify the interaction between the CML cells and the stroma or microenvironment to impair the proliferative potential of residual CML cells.
What of those patients who have evidence of MRD based on the detection of genomic BCR-ABL DNA while in CMR without therapy? These data would seem to indicate that stable CMR is, in most cases, not dependent on complete eradication of the leukemic clone. This is an important objection to the stem-cell depletion model of cure. One possible explanation is that the residual leukemic cells measured are mature progeny with no self-renewal potential—that is, that the cells in the peripheral blood arise from a population that is doomed to proliferate for a short time and then become extinct. A similar model has been proposed as an explanation for the detection of BCR-ABL in normal individuals.34 Whether this hypothesis explains our observations in CML patients after IM withdrawal will be answerable with longer follow-up.
If the principal determinant of relapse risk is the depletion of CML stem cells by treatment, then there is a need for strategies to target these cells in at least 60% of patients. Because there is a range of biological pathways that typify CML stem cells, there is a similarly wide range of agents to target this population. CML stem cells share many properties with normal stem cells,35 so it may be challenging to develop a therapy that is selective for leukemic stem cells with acceptable toxicity. Studies on primitive CML cells in vitro have shown multiple mechanisms of resistance to ABL kinase inhibitors,28 including decreased intracellular uptake and retention of cytotoxic drugs and TKIs,28 decreased expression of HLA costimulatory molecules and targets of adaptive immunity (eg, myeloid granule proteins), and resistance to apoptosis.33,36 Treatment with ABL kinase inhibitors in vitro results in an increase in the fraction of quiescent cells that is highly resistant to TKI therapy.32,37 IFN may induce the proliferation of hematopoietic stem cells,38 and if this is also true of BCR-ABL+ stem cells, then it could mean that the combination of IFN with a TKI might then be more effective against quiescent CML stem cells. The combination of IM and IFN has been used successfully, with promising early molecular responses in 2 studies,39,40 a result that has not been observed in 2 other trials41,42 possibly due to high rates of intolerance or differences between IFN preparations. The combination of IFN and IM did not improve cytogenetic responses or progression-free survival in any of the 4 studies reported. The long-term effect on the rate of CMR and the potential for drug-free remission is unclear.
The phosphorylation status of BCR-ABL substrates depends on the balance between the activity of tyrosine kinases and protein phosphatases. Many substrates of BCR-ABL are dephosphorylated by protein phosphatase 2A (PP2A), and the activity of PP2A is decreased in CML and in various other hematologic malignancies.43 The small molecule FTY72044 and the plant derivative forskolin43 both increase PP2A activity, providing a rationale for the combination of one of these agents with an ABL kinase inhibitor. There is preliminary evidence that self-renewal of CML stem cells is not dependent on BCR-ABL kinase activity. FTY720 treatment of quiescent BCR-ABL+ stem cells degraded β-catenin and induced apoptosis, with no effect on normal stem cells.45,46 One of the most important pathways for self-renewal is the Wnt-β-catenin pathway. Inhibitors of Smo, a molecule in the Hedgehog pathway, have stem-cell–targeting activity, and the combination of LDE 225 and nilotinib in vitro resulted in a decrease in CML stem cells in a transgenic mouse model.47 Another Smo inhibitor, GDC449, has been used in patients with solid tumors with some response and acceptable toxicity.48
Another possible mechanism by which CML stem cells survive ABL kinase inhibition is autocrine or paracrine signaling through cytokine receptors. The addition of hematopoietic growth factors to the culture medium of primary CML cells exposed to dasatinib in vitro increases the survival of these cells.49 Because many cytokines signal through the JAK-STAT pathway, the combination of a TKI with a JAK2 inhibitor might be beneficial to target MRD. Stromal interactions have also been proposed to protect stem cells in vivo, providing a rationale for disrupting marrow homing through the CXCR4 with the mobilizing agent plerixafor.50 Other agents that have promise include farnesyl transferase inhibitors,51 histone deacetylase inhibitors,52 and proteasome inhibitors.53 Choosing the best combination of stem-cell–targeting agent to move into clinical trials will depend on the balance of in vitro efficacy and clinical safety data.
Stem-cell exhaustion
An alternative hypothesis to explain eradication of the CML clone without the need for activity of ABL kinase inhibitors against CML stem cells is stem-cell exhaustion. It is believed that the numbers of stem cells or early progenitor cells are regulated by a balance between symmetric and asymmetric cell division. Symmetric division, in which the daughter cells are committed either to differentiation or to self-renewal must occur in order for the stem-cell pool to expand and markedly increase the output of mature progeny. In a large population of stem cells, regulators of hematopoiesis such as cytokines and stromal interactions will ensure that the proportion of cells that commits to self-renewal is sufficient to maintain long-term hematopoiesis. However, for an individual cell, it is possible that both progeny should commit to differentiation and that the “clone” would become extinct once its progeny complete differentiation and senesce. This may be one of the reasons for graft failure after autologous stem-cell transplantation with a marginal cell dose.54 Using X chromosome inactivation patterns in women of various ages, it is possible to estimate the number of hematopoietic stem cells contributing to hematopoiesis throughout the human lifespan. Combining these observations with data from animal experiments, the number of true hematopoietic stem cells in a young adult was estimated at ∼ 10 000, and the critical number needed to ensure stable hematopoiesis after autologous stem-cell transplantation was ∼ 100.54
In a mathematical model of CML incorporating a stochastic process in which stem cells could undergo symmetric division and commit to differentiation, the CML stem-cell pool was already exhausted before most virtual patients reached diagnosis.55 After commencement of IM treatment, the proportion of “patients” in whom the CML stem-cell pool became extinct increased progressively over time. Similar conclusions were reached by a second group using a stochastic model of myeloproliferative neoplasms in small animals in which hematopoiesis has been well-characterized.56 The introduction of a single neoplastic stem cell in the model led to clonal predominance in only ∼ 15%-20% of simulations. Assumptions about normal hematopoietic stem cells may not always be applicable to CML stem cells. The presence of BCR-ABL in stem cells may slightly shift the balance between self-renewal, apoptosis, and proliferation to reduce the self-renewal potential of the CML stem cells relative to normal stem cells.35 Such a shift would increase the probability of spontaneous exhaustion of the CML stem-cell pool when small numbers are present.
If this hypothesis were correct, what implications would it have for our approach to the treatment of CML? It would mean that all patients could potentially be cured by prolonged suppression of the more mature CML cells. It is thought that the acquisition of new mutations in the granulocyte-macrophage precursor compartment is responsible for the acquisition of ectopic self-renewal capacity and transformation to blast crisis.30 Therefore, perhaps these more mature progenitor cells should be our main targets for eradication. Numerous assumptions go into a stochastic model, and the possibility of spontaneous exhaustion of the CML clone remains for now a tantalizing hypothesis. Nevertheless, it provides us with a different perspective on CML stem cells and their significance as the ultimate targets of therapy.
Immunological control
In the ALLG CML8 study of IM withdrawal in CMR we showed that a stable level of MRD could be measured in patients who did not relapse.21 Because cessation of TKI therapy commonly results in relapse within 6 months, it is possible that in those patients who do not relapse, there is ongoing suppression of the CML clone, potentially mediated by an autologous T-cell response. In contrast to stem-cell–targeted therapies, in which the target effect is almost impossible to measure, an immunological approach has the advantage that functional immune responses can be measured in the peripheral blood or BM, and might prove useful as surrogate markers of response.
The strongest evidence for immunological control of CML comes from ASCT, with responses to donor lymphocyte infusion highlighting the importance of T cells. In IFN-treated patients, there is an association between clinical response to IFN and the emergence of CTLs specific for myeloid-associated antigens.57 Higher expression of the myeloid-associated antigen proteinase 3 was associated with more indolent disease,58 suggesting that a more immunogenic CML clone is more effectively suppressed by the host immune system. If immunological control of MRD is important in determining relapse risk, what are the therapeutic implications? There are currently no data on CTL responses as a predictor of relapse risk after TKI withdrawal. Clonal T-cell proliferations occur in some dasatinib-treated patients and have been associated with a good response to therapy.59 Similar CTL responses may occur during IM treatment,60 and rare CTLs against CML-associated antigens can be found even in the blood of normal individuals.61 Studies of CTL responses in CMR patients should help to answer this question, and might help to improve the selection of candidates for a trial of TKI withdrawal.
CTL responses against leukemia-associated antigens may emerge in response to a reduction in disease burden.57,60 In CML, the reduction of the antigenic burden by TKI therapy may enable the recovery of a functional CTL response that had been rendered anergic. The potential importance of anergic CTL responses was shown in a mouse model of CML in which up-regulation of the receptor for a T-cell–costimulatory molecule, Programmed Cell Death-1, was associated with immune exhaustion and disease progression.62 Pharmacological manipulation of costimulatory molecules involved in the CTL response could provide a novel therapeutic approach in CML.
One of several mechanisms by which IFN might contribute to control of CML is by increasing the expression of leukemia-associated antigens and augmenting the CTL response.57,63 Burchert et al devised a rational approach to combination therapy with IFN and IM in which both drugs were given together to reduce disease burden, and then IFN monotherapy was used as maintenance treatment.64 Leukemia-specific CTLs were observed more frequently after IM was stopped, and there was a suggestion that the loss of response was associated with failure to develop an immunological CTL response. An alternative approach that has been used to augment leukemia-specific CTLs is vaccination.65,66
Summary
Various strategies might improve the odds of drug-free remission of CML, and some of these have already been used in clinical trials. Given the excellent safety and tolerability of TKI therapy, the safety of any novel treatment for this patient group is of paramount importance. Any augmentation of TKI therapy must carry a risk of new, additional, or unexpected toxicity, and this risk must be balanced against the potential benefit. The same consideration of patient safety applies to a therapeutic test of TKI withdrawal. It is essential that careful monitoring is undertaken to detect relapse as early as possible so that effective TKI therapy may be reinstituted. It is therefore recommended that, at present, such approaches are only considered in the context of carefully designed, controlled clinical trials.
Sustained CMR after withdrawal of TKIs is a therapeutic success that many clinicians and scientists would not have anticipated when IM treatment was first introduced. By analogy with the treatment of HIV with antiretroviral drugs, it seemed likely that even a highly effective targeted treatment would need to be continued indefinitely to suppress the CML clone. Perhaps contrary to expectation, this is not always the case in chronic-phase CML. Early and deep response translates to long-term disease control with the potential for safe withdrawal of treatment in carefully selected patients. The challenges now are to identify those patients in whom treatment can safely be withdrawn, and to increase the number of patients eligible for withdrawal of therapy.
Disclosures
Conflict-of-interest disclosure: J.V.M. declares no competing financial interests. D.M.R. has received research funding and honoraria from Novartis Pharmaceuticals. Off-label drug use: None disclosed.
Correspondence
Professor Junia V. Melo, Department of Haematology, Centre for Cancer Biology, SA Pathology, Frome Road, Adelaide, SA 5000, Australia; Phone: 61-8-8222-3441; Fax: 61-8-8222-3139; e-mail: junia.melo@health.sa.gov.au.