Abstract
High-dose melphalan with autologous stem cell support has been an integral part of myeloma therapy for more than 25 years, either as salvage therapy or as consolidation of an initial remission. Although multiple phase 3 trials have demonstrated that this therapy results in higher response rates and longer remission times than conventional chemotherapy, the use of thalidomide, lenalidomide, and bortezomib as induction therapy has limited the clinical relevance of these trials. Moreover, ongoing trials have shown that initial induction therapy may affect transplantation outcome, and that long-term disease control can be achieved in a substantial number of patients with a variety of posttransplantation maintenance therapies. This article summarizes the results of ongoing and recently published clinical trials and describes how they have affected current transplantation recommendations.
Introduction and historical perspective
It has now been more than 25 years since McElwain and Powles demonstrated the clinical relevance of melphalan dose and disease response in patients with relapsed and refractory multiple myeloma.1,2 In previously untreated patients, the overall response rate to melphalan at a dose of 140 mg/m2 was 78%, with 27% of patients achieving a complete remission (CR) and 51% a partial remission with a median duration of response of 19 months. However, profound myelosuppression occurred in all patients and there were 10 treatment-related deaths among the first 50 patients reported.2 Barlogie et al demonstrated that the toxicity of high-dose melphalan could be decreased by autologous bone marrow transplantation; however, in patients with refractory disease, response duration was generally short in all patients except those with primary refractory disease.3–5
These results led to the exploration of high-dose therapy with autologous stem cell support as consolidation therapy in patients with newly diagnosed multiple myeloma.6–11 Today, the clinical relevance of these aforementioned trials is limited because many patients receive induction therapy containing an immunomodulatory drug (IMID) such as lenalidomide or thalidomide with or without bortezomib (a proteosome inhibitor) in combination with steroids. For example, in the recently presented CALGB 100104 trial of maintenance lenalidomide after autologous stem cell transplantation (SCT) for myeloma, only 4% of patients received an induction therapy that did not include either an IMID or bortezomib.12 A thorough reevaluation of the role of high-dose therapy with autologous SCT is currently under way. Herein we describe the results of recent trials and how they have affected current practice.
Optimal induction treatment before SCT
Response before SCT has been shown to improve transplantation outcomes, but the optimal type and duration of induction therapy has not been well defined.13 Macro et al reported the results of a randomized trial comparing induction with thalidomide and dexamethasone versus vincristine, Adriamycin, and dexamethasone (VAD). Despite a higher pre-SCT response to thalidomide-dexamethasone (very good partial response [VGPR] rate of 34.7% vs 12.6% for VAD) in this trial, both groups had similar VGPR rates of 42% and 44% after transplantation.14 In contrast to this study, 3 randomized trials have demonstrated that a bortezomib-based induction results in improved outcomes after autologous SCT.15–17 The results of these trials are summarized in Table 1.
Bortezomib-based induction therapy: summary of results of phase 3 randomized trials
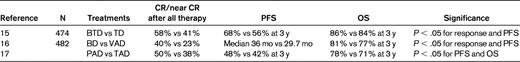
B indicates bortezomib; T, thalidomide; D, dexamethasone; V, vincristine; A, Adriamycin; and P, prednisone.
Lenalidomide-based induction is being more frequently used in the United States for transplantation-eligible patients.12 Lenalidomide in combination with dexamethasone has been shown to be an effective induction therapy for myeloma, with the advantage of being administered orally and well tolerated.18 A recent retrospective analysis showed that patients treated on E4A03 who underwent an autologous SCT after 4 cycles of lenalidomide/dexamethasone therapy had an overall survival (OS) at 3 years of 94% and a progression-free survival (PFS) of 64%.19
Further intensification of the induction regimen has been shown to improve response rates before SCT, but the impact on OS has not been established. Richardson et al reported on the transplantation outcomes of 28 patients who received induction therapy with lenalidomide, bortezomib, and dexamethasone. This combination is associated with very high rates of VGPR or better (67%). Estimated 18-month PFS and OS for the combination treatment with or without transplantation were 75% and 97%, respectively.20 Finally, induction therapy using 4-drug combinations (alkylator, IMID, bortezomib, and steroids) has also been explored, but is associated with higher toxicity rates and possibly lower survival rates due to this toxicity.22 In summary, for transplantation-eligible patients, a bortezomib-based induction is associated with improved disease control after transplantation and should be considered the standard of care. Lenalidomide-based combinations have as of yet not been compared with bortezomib-based combinations in a randomized trial; however, based on published phase 2 data, the National Comprehensive Cancer Network guidelines consider it an appropriate alternative for induction therapy.19–21
Role of autologous SCT
Before the advent of IMIDs and proteasome inhibitors, the CR rate after induction therapy was less than 10%. Therefore, the rationale to proceed to high-dose therapy with autologous stem cell support was to increase the depth of response and the number of patients who achieve a CR.23 This rationale was supported by multiple retrospective analyses confirming CR as an important surrogate end point for survival and long-term disease control.12,23 Table 2 summarizes the data for 6 of the largest randomized trials comparing single autologous SCT with conventional alkylator-based chemotherapy. CR rates were significantly higher in the autologous SCT arms in 5 of the 6 trials, EFS was superior for autologous SCT in 3 of the trials, and 2 trials showed a survival benefit. A meta-analysis performed on 9 randomized trials confirmed that single autologous SCT was associated with an EFS benefit but not an OS benefit.
Phase 3 trials comparing high-dose therapy (HDT) with conventional chemotherapy (Chemo) as frontline therapy for multiple myeloma
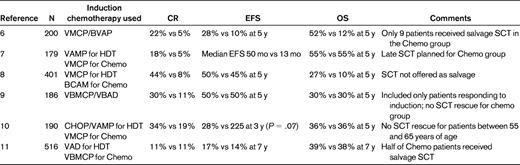
V indicates vincristine; M, melphalan; C, cyclophosphamide; P, prednisone; B, carmustine; and A, Adriamycin.
To increase the dose intensity, Barlogie et al showed the feasibility of tandem autologous peripheral blood stem cell transplantation using melphalan 200 mg/m2 for the first SCT and melphalan 200 mg/m2 or melphalan and total body irradiation for the second SCT. Of 123 patients, 76% completed a second autologous SCT. Tandem SCT was associated with a 40% CR rate and a median EFS of 49 months.24 Two subsequent randomized trials have been completed and published. Attal et al reported on 399 patients < 60 years of age randomized to either single or tandem autologous SCT. Tandem SCT significantly improved 7-year EFS and OS. Cavo et al reported on 321 patients randomly assigned to receive either single or tandem courses of high-dose therapy with stem cell support. Patients in the tandem arm had a significantly increased probability of attaining at least a near CR (33% v 47%) and prolonged EFS (median, 23 vs 35 months); however, no survival benefit was observed. Neither of these studies included induction therapies with either an IMID or a proteasome inhibitor and therefore, as with the initial randomized trials of single SCT versus chemotherapy, are not as relevant today as when they were originally published.25,26
The group from the University of Arkansas Medical Center has over the last 2 decades made important observations regarding the role of tandem transplantation and intensive therapy in myeloma.24 This group demonstrated that long-term disease control was feasible in myeloma and that cytogenetic abnormalities and baseline beta 2 microglobulin levels could predict long-term outcomes.27,28 The recent experience with Total Therapy II and Total Therapy III has underscored the feasibility and efficacy of intensive induction followed by consolidation and maintenance as a strategy of obtaining high rates of CR and durable remissions in patients with standard-risk cytogenetic abnormalities.27,28 Of particular interest has been defining risk groups according to gene-expression profiling that, if validated by others, will allow a more rational use of therapies in myeloma patients to achieve the overarching goal of maximum benefit with minimum burden of therapy.29 However, what remains to be determined is whether such intensive induction therapy and posttransplantation consolidation is warranted for all patients with myeloma, or if it should be relegated to patients with specific risk categories.
Posttransplantation therapies
Despite intensive induction and tandem SCT consolidation, myeloma recurrence occurs almost universally in patients not receiving some form of posttransplantation therapy. IFN was the first agent extensively studied in the context of posttransplantation maintenance, with conflicting results, and is now only rarely used after transplantation due to toxic side effects and poor tolerance.30 Maintenance therapy after SCT was reviewed in 2009 at the American Society of Hematology meeting by Drs Ghobrial and Stewart. At that time, only thalidomide maintenance had been conclusively shown to increase PFS and to potentially affect survival after SCT.31 Since that time, thalidomide has been studied in several randomized trials that are summarized in Table 3. Two of these studies have shown an OS benefit for patients receiving thalidomide; however, the side-effect profile has made it difficult for patients and physicians to adopt.32–37
Phase 3 trials of thalidomide maintenance therapy after autologous SCT for myeloma
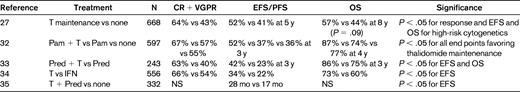
T indicates thalidomide; Pam, pamidronate; Pred, prednisone; and NS, not significant.
Lenalidomide, with its better side-effect profile, was a logical candidate drug to study as posttransplantation maintenance therapy. Two randomized trials of lenalidomide have been performed and recently reported. The IFM 2005-02 trial reported on 614 patients randomized to receive either lenalidomide or placebo posttransplantation after 2 cycles of lenalidomide intensification. With a median follow-up of 34 months, the median PFS was 42 months for the lenalidomide arm versus 24 months for the placebo arm (P < 10-8).37 At the time of the last report, no significant difference in OS was reported, but a significantly increased risk of secondary malignancies was seen in the lenalidomide arm (primarily hematologic malignancies). The CALGB 100104 trial was also updated at the International Myeloma Workshop meeting in Paris. As with the IFM 2005-02 study, there was a significant improvement in time to progression for patients receiving lenalidomide maintenance (median 43.6 months vs 21.5 months for placebo). With a median follow-up of 18 months, there were significantly fewer deaths in the lenalidomide arm versus placebo (21 vs 37, P < .019) despite the crossover design. Post hoc analysis suggests that the benefit of lenalidomide maintenance is seen regardless of response to SCT and type of induction therapy.38
Bortezomib maintenance has also been demonstrated to reduce the risk of relapse after SCT. Sonneveld et al reported on the outcomes of 626 patients randomized to receive bortezomib during induction in combination with doxorubicin and dexamethasone, followed by posttransplantation bortezomib every other week for 2 years or thalidomide during induction and posttransplantation. Bortezomib therapy was associated with a higher response rate (60% VGPR or greater vs 40% for thalidomide). On multivariate analysis, bortezomib therapy was also associated with improvements in PFS and OS.17
In summary, both IMIDs and bortezomib have been shown to enhance disease control when given as post-SCT therapy in patients with multiple myeloma. The increased risk of secondary malignancies seen after lenalidomide require further study, careful discussion, and risk assessment before this can be considered standard of care. Although the data to date suggest that most patients may benefit from the institution of post-SCT therapies, particularly if they still have evidence of residual disease, the optimal agent, timing, and duration of such therapy remains to be defined. Therefore, continued design, planning, and implementation of clinical trials addressing these issues will be essential.
Role of allogeneic SCT in myeloma
The role of allogeneic SCT has been recently reviewed by the International Myeloma Working Group (IMWG).39 Numerous studies comparing autologous SCT with allogeneic SCT after reduced intensity conditioning were summarized in that review. The results of the largest study performed through the Blood and Marrow Clinical Trials Network had not been reported at the time of the IMWG review. In that study, 625 patients with standard-risk myeloma were biologically assigned to receive either a tandem autologous SCT with melphalan 200 mg/m2 (n = 436) or autologous SCT with melphalan 200 mg/m2 followed by an allogeneic SCT conditioned with fludarabine and 200 cGy of total body irradiation (n = 189). The 3-year PFS was 46% for the tandem autologous arm versus 43% for the autologous-allogeneic arm (P = .67). OS at 3 years was also not significantly different between the groups: 80% for the tandem autografts versus 77% for the autologous-allogeneic arm (P = .19). Exploratory multivariate analysis between treatment arms revealed that assignment to the autologous-allogeneic arm was associated with worsened survival in patients with stage I and II disease, but not in those with stage III disease.40
Allogeneic SCT as part of “frontline therapy” should continue to be explored in the context of clinical trials. Young patients (< 55 years of age) with high-risk disease (eg, del17p, plasma cell leukemia, chromosome 1 abnormalities, or suboptimal response to induction therapy) should be considered for this approach if an optimal donor is identified. In patients with low-risk disease and low tumor bulk, allografting is probably better used as salvage therapy.
Reduced intensity conditioning regimens have now become the standard for allografting, although an increased risk of relapse was seen compared with myeloablative therapies in a recent registry analysis.41 This suggests that dose intensity is important for long-term disease control in myeloma, and that intensification of the conditioning regimen should be an important component of the future research agenda for allografting in this context.39
What is the “standard of care” for transplantation-eligible patients in 2011?
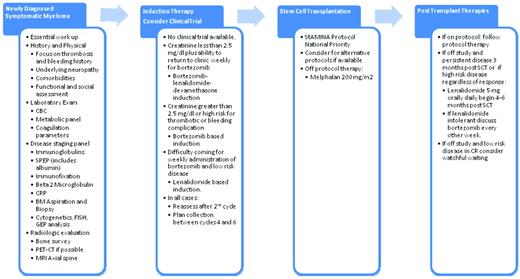
With the primary objective of achieving the longest possible remission with the “least burden of therapy,” most experts would recommend that all transplantation-eligible patients be offered participation in a prospective clinical trial. Outside of the context of a clinical trial, optimal induction therapy would be either a bortezomib-based or a lenalidomide-based combination. In patients with high-risk disease, induction therapy with bortezomib, an IMID (lenalidomide or thalidomide), and dexamethasone should be considered the treatment of choice there is no specific contraindication to these agents. The optimal duration of induction therapy has not been established, but most experts recommend between 4 and 6 cycles of induction before proceeding to stem cell collection and consolidation with high-dose therapy.42 My current algorithm for patients with newly diagnosed myeloma is summarized in Figure 1.
The author's recommended approach to the treatment of patients with symptomatic multiple myeloma who are eligible for SCT.
The author's recommended approach to the treatment of patients with symptomatic multiple myeloma who are eligible for SCT.
Posttransplantation therapies should now be considered routinely for all patients failing to achieve a CR to initial induction and consolidation. Patients who have achieved a CR should be advised that there is evidence to suggest that continued therapy with either an IMID or a proteosome inhibitor is associated with prolongation of the remission duration, and may be associated with improved survival. However, whether patients treated preemptively at the sign of first relapse could have the same degree of benefit has not been determined. In the meantime, it is reasonable to observe patients with low-risk disease who have achieved a CR or a VGPR after induction and consolidation, and then intervene at the time of first disease progression.
Addressing the questions of tomorrow
A significant amount of effort is being spent in defining the role of early versus late consolidation with autologous SCT, as well as defining the optimal role of posttransplantation consolidation. However, most patients are being treated the same regardless of their risk category. Identification of high-risk myeloma as defined by cytogenetics and gene-expression profiling has allowed investigators to develop “risk-stratified” strategies. With the exception of Total Therapy 4 and 5, no other trial is addressing issues according to risk stratification. As important as it is to develop specific strategies for high-risk myeloma, clinical trials aimed at reducing the burden of therapy for patients with lower-risk disease (as defined by International Staging System staging and cytogenetics) also need to be explored. Improving transplantation outcomes over the next 5 years will require the exploration of novel strategies aimed at addressing the following issues:
Reducing the morbidity of high-dose therapy
Reduction of symptom burden through anticytokine approaches (anti-IL6)
Enhancing immune recovery (mega doses of stem cells)
Identification of patients at high risk for gastrointestinal toxicity and dose adjusting melphalan
Improving the efficacy of the conditioning regimen
New melphalan combinations
Novel posttransplantation therapies
Combination therapies for patients with high-risk disease
Immunotherapies for relapse prevention (vaccines)
Improved minimal residual disease detection
Continued support and participation in well-conducted clinical trials, as well as continued global collaborative efforts, will make long-term disease control for most myeloma patients an achievable goal within the next 5-10 years.
Disclosures
Conflict-of-interest disclosure: The author has consulted for and received honoraria from Millennium, Novartis, Genzyme, Amgen, Onyx, and Celgene, and has been affiliated with the speakers' bureaus for Millennium, Genzyme, and Celgene. Off-label drug use: Melphalan for transplantation and lenalidomide for maintenance.