Abstract
Over the past 15 years, more than 1500 patients have received HSCT, mostly autologous, as treatment for a severe autoimmune disease (AD). More than 1000 of these have been registered in the European Group for Bone Marrow Transplantation (EBMT) and European League Against Rheumatism (EULAR) combined database. A recent retrospective analysis of 900 patients showed that the majority had multiple sclerosis (MS; n = 345) followed by systemic sclerosis (SSc; n = 175), systemic lupus erythematosus (SLE; n = 85), rheumatoid arthritis (RA; n = 89), juvenile idiopathic arthritis (JIA; n = 65), and idiopathic cytopenic purpura (ITP; n = 37). An overall 85% 5-year survival and 43% progression-free survival was seen, with 100-day transplantation-related mortality (TRM) ranging between 1% (RA) and 11% (SLE and JIA). Approximately 30% of patients in all disease subgroups had a complete response, often durable despite full immune reconstitution. In many patients, such as in those with SSc, morphological improvement such as reduction of skin collagen and normalization of microvasculature was documented beyond any predicted known effects of intense immunosuppression alone. The high TRM was in part related to conditioning intensity, comorbidity, and age, but until the results of the 3 prospective randomized trials are known, an evidence-based modification of the conditioning regimen will not be possible.1 In recent years, multipotent mesenchymal stromal cells (MSCs) have been tested in various AD, exploiting their immune-modulating properties and apparent low acute toxicity. Despite encouraging small phase 1/2 studies, no positive data from randomized, prospective studies are as yet available in the peer-reviewed literature.
HSCT in autoimmune disease
The European experience of 900 AD patients was recently published.1 The concept of immune ablation, or at least severe reduction, and “resetting” of autoimmunity arose from coincidental case reports (HSCT given for malignancy with coexisting AD improvement)2 and was corroborated by animal model data.3 The first patient published receiving an HSCT for an AD had systemic sclerosis (SSc) with pulmonary artery hypertension,4 responded satisfactorily, and is still stable 15 years later.
From the outset, an international collaboration ensued, which is committed to establishing the place, if any, of HSCT in the treatment of severe therapy-resistant AD in the context of prospective, randomized clinical trials coupled with mechanistic side studies.5 Although many protocols were used, they basically ranged from less aggressive, such as cyclophosphamide 200 mg/kg plus antithymocyte globulin (ATG), to more intensive such as total body irradiation (TBI) plus cyclophosphamide/ATG and CD34 selection.
Successes
Up to one-third of patients in all groups experienced a significant clinical improvement, including full and drug-free sustained remissions.6 In some of those studied (SLE, MS, and SSc), remission was sustained despite full immune reconstitution. In SLE, investigators demonstrated that humoral responses to recall antigens (tetanus, polio, measles, and mumps) were ablated after autologous HSCT, as would be expected, but in addition, eradication of autoantibodies such as anti–double-stranded DNA was also achieved coincident with clinical remission in 5 cases.7 After immune reconstitution, a comparison between the patients and normal subjects TCR Vβ repertoires showed a fully normal pattern. Despite this, no patient had relapsed in the 8-year follow-up reported. Similar findings were described in 7 MS patients who remained in remission up to 3 years after transplantation despite regaining a normal T-cell repertoire.8 These findings are particularly important, because at the onset of the project, many considered autologous HSCT to be doomed to failure, given that the identical “autoaggressive” immune system was being given back to the patient. However, the initial choice of autologous over allogeneic HSCT was mainly based on the lower toxicity of autologous HSCT, mainly due to GVHD. It is now known that in many patients who achieved clinical remission, the autoaggressive immune system was “debulked” rather than fully ablated, allowing reestablishment of normal immune regulation, in part due to increased Treg numbers and activity.9 This was not unexpected, because identical twins have rather low concordance rates for autoimmune disease: ∼ 20% for RA and ∼ 30% for MS. In any case, it would be naive to think that the complexity of autoimmune disease pathophysiology—with its polygenetic, epigenetic, and environmental aspects—could be explained so simplistically.
Several studies have reported reduced collagen deposition in skin10 and normalization of microvasculature in SSc patients11,12 after autologous HSCT. However, these observations are not readily explained by either sustained immunosuppression or direct effects on fibroblasts and endothelial cells, and suggest a more profound modulation of the inflammatory niche by mechanisms yet to be fully elucidated.
The stem cell literature is replete with case reports and small series of dramatic positive outcomes in various diseases, and it is imperative to demonstrate through randomized prospective clinical trials the true impact of HSCT in AD. Currently, only 3 such trials are ongoing: ASTIS (Autologous Stem Cell Transplantation International Scleroderma), SCOT (Scleroderma Cyclophosphamide or Transplant), and ASTIC (Autologous Stem Cell Transplantation International Crohns). Details are available on their respective websites, but Table 1 compares and contrasts the 3 studies. ASTIS and SCOT are similar in patient selection, control arms, and end points, but the transplantation protocols differ. SCOT uses a more intense regimen, including TBI. Each study has experienced its own toxicity issues, none of which was previously unknown in the HSCT field. Only time will tell which approach, if any, imparts a clinically useful and durable outcome. ASTIS has finished recruitment (156 patients) and the first efficacy analysis will be available early in 2012; the last patient reaches the primary 2-year end point (alive with no end-stage organ failure) in October 2011. SCOT will finish recruitment in May 2011 and ASTIC is nearing its target of 40 patients.13
Randomized clinical trials of autologous HSCT in autoimmune disease
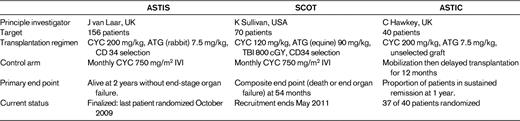
CYC, indicates cyclophosphamide; IVI, intravenous infusion.
Since the initiation of the project, several events have radically changed the environment of AD treatment: the first biologics (anti–TNF-α mAbs) reduced the need for more toxic therapies such as HSCT in RA, and this was closely followed by similar developments in other AD treatments (eg, natalizumab for MS; anakinra, canakinumab, and tocilizumab for JIA; and, recently, belimumab for SLE. However, none of these agents has induced sustained drug-free remission of AD, and for SSc, no such effective disease-modifying agent is available.
Failures
Treatment-related mortality.
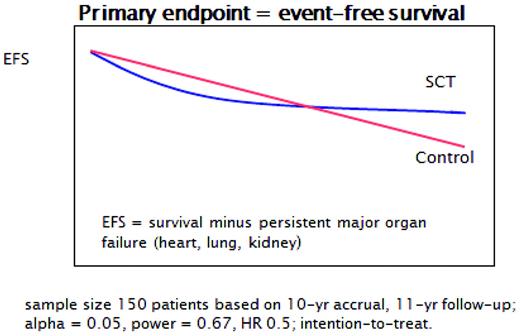
A major issue for physicians dealing with AD was and is that patients rarely die immediately from their disease. However, a growing number of studies suggested that uncontrolled systemic inflammation does lead to premature atherosclerosis and cardiovascular deaths,14 as well as toxicity from chronic immunosuppression, especially glucocorticoids. Despite this, it is still a challenge for a rheumatologist, neurologist, or gastroenterologist to accept an immediate TRM of 5%-10%, especially because long-term benefits have yet to be demonstrated. The hypothesis is that in a randomized prospective trial of HSCT versus conventional treatment, early toxicity from TRM would eventually be surpassed by later deaths and/or organ failure from disease progression in the control arm (Figure 1), but this has yet to be proven.
Early versus late event-free survival power calculation for ASTIS. (Courtesy of J van Laar.)
Early versus late event-free survival power calculation for ASTIS. (Courtesy of J van Laar.)
Apart from the well-known acute toxicity of HSCT (infection and bleeding during the aplastic period and late infection during the T-cell reconstitution phase), several other factors emerged during the program. Some SSc patients experienced serious lung toxicity from TBI, whereas other SSc patients suffered a scleroderma renal crisis during the conditioning phase that was attributed to a combination of rapid fluid and electrolyte shifts and high-dose glucocorticoids given as prophylaxis for ATG-induced cytokine storm. In some JIA children, a fatal macrophage activation syndrome occurred, thought to be infection triggered and due to the profound immunosuppression resulting from TBI and CD34 purging.15 These toxicity problems were mostly eliminated by lung shielding, concurrent ACE inhibition, and reduced intensity of the regimen, respectively. However, an inevitable TRM will always exist and must be weighed against the potential long-term benefit, a calculation that requires efficacy data from randomized trials.
Late complications include not just the well-known fungal and other opportunistic infections during the T-cell reconstitution phase (which may last up to 2 years or more), but also the emergence of second autoimmunity.16 This second autoimmunity is well known after any major immunoreduction and is thought to be due to emergence of uncontrolled autoreactive clones during the period of homeostatic expansion of self-recognizing residual immune competent cells. It is almost always antigen specific (eg, platelet, erythrocyte, or thyroid) and often, but not always, resolves as the Treg network is reconstituted. However, some patients have died from this second autoimmunity (eg, from acquired hemophilia A antibodies after HSCT for MS17 ).
Nonresponse and relapse.
Two-thirds of patients receiving HSCT either did not respond or responded then relapsed. The factors determining this remain elusive, but some studies in RA suggested that clinical responders (n = 5) had a larger number of cells at baseline expressing CD3, CD4, CD27, CD45RA, CD45RB, and CD45RO in synovium (P < .05), higher activity on human immunoglobulin scans (P = .08), and a trend toward higher concentrations of C-reactive protein in serum than nonresponders (n = 2). Subsequent remissions and relapses in responders paralleled reduction and reexpression, respectively, of T-cell markers. A relatively increased expression of CD45RB and CD45RO on synovial CD3+ T cells was seen after HDC + allogeneic HSCT. No correlations were found between disease activity score and changes in B cells, macrophage infiltration, or synoviocytes.18
Summary
Autologous HSCT for severe AD has demonstrated remarkable clinical, laboratory, and morphological improvement in many patients, but at a high price—including a TRM of up to 10% in some conditions. Retrospective analyses from established databases are inevitably incomplete, especially in those patients “lost to follow-up” and assumed to be still alive. The advent of the biologics has reduced the need for more radical therapies such as HSCT in RA and MS, but for SSc and severe forms of Crohns disease it remains an option. The results of the prospective randomized clinical trials will be critical in deciding the future of this treatment.
Allogeneic HSCT for AD has been investigated, and a summary of 35 patients so treated showed up to 50% remission induction in some AD subgroups.19 However, the lack of a clear advantage over autologous HSCT and the risk of GVHD relegates this to a second-line strategy at the moment.
MSC transplantation for autoimmune disease
The background
MSCs are stromally derived adult progenitor cells, more accurately called multipotent mesenchymal stromal cells, because their true “stemness” has not been established. They may be derived from various tissues, including BM, placenta, umbilical cord, fat, and teeth. Although they are a heterogeneous group of progenitor cells, they have been defined by consensus as being plastic adherent, bearing certain stromal surface markers (CD76, CD90, and CD105) and lacking hematopoietic cell markers such as CD11a, CD14, CD19, CD34, CD45, and MHC class II. In addition, MSCs have adipogenic, osteogeneic, and chondrogenic differentiation potential.20
First applied in humans for HSC graft enhancement more than 15 years ago,21 there has since developed a major interest in their potential for immune modulating and anti-inflammatory and tissue-protective properties, including autoimmune disease.22 Originally considered as “regenerative” due their ability to transdifferentiate into other tissues, it is now appreciated that the positive effects seen in vitro in animal models and in some clinical studies is most likely due to their capacity to initiate various paracrine events, resulting in tissue protection. Some of these effects are via soluble factors such as TGF-β, 2,3-indoleamine dioxygenase, soluble HLA-G, and others via cell-cell contact and “reprogramming” of target cells.23 In addition, MSCs display certain special properties such as immune privilege (survival in allogeneic environments) and active homing to distressed tissues via surface molecules such as CXCR4. They also preferentially home to tumor stroma,24 potentially inhibiting tumor immune surveillance.
The role of the MSCs in normal tissue homeostasis and repair is not fully clear, but it seems likely that they participate in the inflammatory niche, possibly as part of the resolution phase of injury. Their origin in adult animals is also not defined fully, but potential sources are pericytes released from blood vessels during injury,25 epidermal-to-mesenchymal transition (especially in lung and kidney26 ), and direct release from the BM. In any case, the use of supraphysiological numbers of exogenous MSCs in vivo may evoke different biological pathways from those used during homeostasis.
After many positive animal models of inflammation, organ transplantation, autoimmunity, critical ischemia, radiation damage, and tissue scarring, MSCs entered clinical trials for inflammatory disorders, first in GVHD and later in MS, Crohn disease (including fistula closure), SLE, and SSc.22 In addition, many trials relating to ischemia in myocardium, CNS, kidney, and limbs have been performed. There are more than 160 trials registered on www.clinicaltrials.gov, more than 50 patents pending, and more than 900 reviews on the subject have been published.
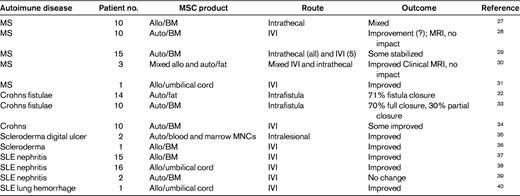
Despite this activity, only 14 phase 1/2 clinical trial in autoimmune disease have been published (Table 2), all of which involve < 15 patients as of the time of writing this (May 2011). Two large randomized prospective trials on Crohns disease and GVHD were reported as failing to reach their primary end points, but as yet have not been published in the peer-reviewed literature.
The issues
There is a lack of standardization of cell product regarding heterogeneity, potency, impact of expansion media on phenotype, and suitability of source. Many expansion media use growth factors such as FGFβ, which has been shown to induce proliferation-dependent MHC class II expression41 and, in one study, suspected karyotypic changes.42 The clinical trials so far have used MSCs derived from various sources including fat, BM, placenta, and umbilical cord, the former 2 being either autologous or allogeneic. MSCs from the BM of autoimmune disease patients have been shown to be defective regarding certain functions such as differentiation potential and hematopoietic support, but seem as equally potent as healthy allogeneic MSCs in terms of in vitro antiproliferative potential.43
The efficacy data so far available are difficult to interpret due to variable pre-MSC transplantation treatment regimens, nonstandardized outcome measures, and lack of long term follow-up. So far, no acute toxicity signals have emerged from the experiences of ∼ 1000 patients,44 although longer-term data are important regarding tumor surveillance.
The future
Clearly, additional small phase ½ trials will not shed further light on the long-term benefit of MSC transplantation, and we now need larger, randomized, double-blind clinical trials, including mechanistic side studies. There are major gaps in our knowledge, such as duration of engraftment, impact on normal tissues and organs, and phenotypic changes occurring in MSCs exposed to inflammatory/ischemic target tissue. INF-γ and FGF-β are both known to cause expression of MSC class II on MSCs, and in the latter case, this molecule is able to present antigen.41 It would seem that costimulating molecules are never expressed by MSC, thus rendering the expression of MHC class II molecules potentially “tolerogenic.”
Several groups of investigators are planning such studies. The EULAR Stromal Cell Group is finalizing a prospective, double-blind, comparative, multicenter trial of renal lupus using allogeneic MSCs, and an MS consortium is planning a prospective comparative trial using autologous MSCs.45
It will require a determined effort from investigators, regulators, and industry to determine through adequately powered, prospective clinical trials the potential benefit of MSC transplantation in autoimmune and other human disorders. Because many of these are investigator-initiated, strategy-based studies, the bureaucratic burden may hamper development.46 In the meantime, it is important to recall the advice of a leader in the field, Irving Weissman, on his election to president of the International Society of Stem Cell Research47 :
Every week, I am asked whether a family should take their loved one with an incurable disease to clinics that promise cures with supposed stem cell therapies. Usually, the costs for such therapies are in the tens of thousands of dollars. When I go to the websites of the providers, usually very slick, the therapies are often transplantation of tissue or umbilical cord stem cells for a variety of nonhematopoietic or nonmesenchymal disorders (claiming transdifferentiation), or even claim to be embryonic stem cell (ESC) therapies. I tell those that ask that there is no scientific basis for such transdifferentiation to date and no approved therapies using ESCs.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: Use of adult stem cells in experimental clinical trials.
Correspondence
Alan Tyndall, Professor and Head, Department of Rheumatology, University of Basel, Switzerland, Felix Platter Spital, Burgfelderstrasse 101, Basel, 4012, Switzerland; Phone: 41-61-326-4003; Fax: 41-61-326-4010; e-mail: alan.tyndall@fps-basel.ch.