Abstract
Advanced-stage Hodgkin lymphoma (HL) has become a curable disease in the majority of patients. Research during the last decade has challenged chemotherapy with Adriamycin, bleomycin, vinblastine, dacarbazine (ABVD) as the standard of care and debates continue regarding the role of radiation therapy (RT) in this patient population. The incorporation of interim positron emission tomography (PET) imaging and, recently, further characterization of HL on cellular and molecular levels are emerging as tools for treatment stratification and predictors of disease status. Newer targeted therapies have emerged that are very effective in the relapsed setting and are actively being explored as frontline therapy. Lastly, the expanding population of survivors cured of HL outnumbers patients with the disease and needs to be monitored for therapy-related late effects.
Therapeutic advances over the past 3 decades have resulted in the cure of the majority of patients with advanced-stage Hodgkin lymphoma (HL). Several questions emerge when considering what constitutes optimal therapy with a balance between a high cure rate and minimizing short- and long-term toxicity. This review focuses on 3 key elements: (1) what is the optimal chemotherapy?; (2) what is the role of radiation therapy (RT) in advanced HL?; and (3) can we adapt therapy based on clinical biological risk factors?
What is the optimal chemotherapy?
In North America, combination chemotherapy with Adriamycin, bleomycin, vinblastine, dacarbazine (ABVD) is considered the standard of care for advanced HL, providing an excellent balance of efficacy and toxicity.1 Over the past decade, new regimens have been developed for patients with advanced HL based on the premise of either improved efficacy or reduced toxicity (Table 1). The major challenge to the clinician is how to interpret results from various clinical trials to individualize therapy and achieve a high cure rate while at the same time minimizing acute and late toxicity for patients with advanced HL.
Recent randomized clinical trials in advanced HL
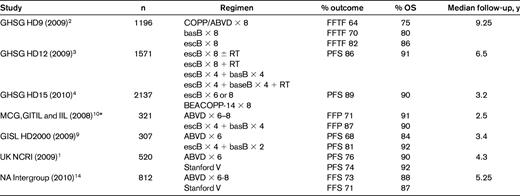
MCG indicates Michelangeo Cooperative Group; GITIL, Italian Group for Innovative Therapies in Lymphoma; IIL, Intergruppo Italiano Linfomi; GISL, Gruppo Italiano per lo Studio dei Linformi; and UK NCRI, United Kingdom National Cancer Research Institute.
*Selection criteria were IPS ≥ 3
Dose-escalated bleomycin, etoposide, doxorubicin (Adriamycin), cyclophosphamide, vincristine (Oncovin), procarbazine, and prednisone (escalated BEACOPP or escB), developed by the German Hodgkin Study Group (GHSG), has emerged as a very effective regimen and yielded significantly better survival compared with conventional-dose regimens in the GHSG HD9 study.2 In a 3-armed trial, patients with stage IIB-IV HL were randomized to 8 cycles of COPP-ABVD, baseline BEACOPP (basB), or escB. Results from the most recent update of the study with 1196 evaluable patients and a median follow-up of 111 months suggest that responses are durable, with the 10-year freedom-from-treatment-failure (FFTF) significantly higher in the escB arm than in the basB and COPP-ABVD arms (82%, 70%, and 64%, respectively, P < .0001). The corresponding 10-year overall survival (OS) rates were 86%, 80%, and 75%, respectively (P = .0005).2 In contrast to the original publication, which indicated an OS benefit across all risk groups, on longer follow-up, the OS advantage of escB was significant only in the patients with intermediate risk according to the International Prognostic Score (IPS 2-3 group), with no difference noted in the patients with the best (IPS 0-1) or worst prognostic group (IPS > 4). In addition, no difference was noted between treatment arms for FFTF and OS in patients older than 60 years. Despite its effectiveness, escB has not been widely adopted in North America because of higher acute hematologic toxicity, significantly higher estimated 10-year cumulative incidence of acute myelogenous leukemia/myelodysplasia (3.2%, 2.2%, and 0.4%, for 3 study arms, respectively, P = .03) and nearly universal infertility.2
To reduce treatment-related toxicity, the GHSG has explored the efficacy of modified versions of the BEACOPP regimen. The GHSG HD12 trial was a 4-arm study for patients with bulky stage IIB and stages III-IV disease comparing 8 cycles of escB with or without RT versus 4 cycles of escB and 4 cycles of basB with or without RT. In a recent update that included 1571 eligible patients with a median follow-up of 78 months, the 5-year FFTF, progression-free survival (PFS), and OS rates of the entire cohort were 85.5%, 86.2%, and 91%, respectively.3 There were no significant differences in these end points between the 2 chemotherapy regimens, but fewer hematologic toxicities were observed in the arm that contained baseline BEACOPP. The GHSG HD15 trial compared 8 cycles versus 6 cycles of escB versus 8 cycles of BEACOPP-14 and evaluated the use of positron emission tomography (PET) response to select patients for consolidative RT, as discussed further below.4 The outcome of patients was similar to results reported in the HD9 and HD12 trials. Therefore, cumulatively, the various versions of escB tested suggest that fewer cycles may suffice and result in a better-tolerated regimen without loss of efficacy.
To evaluate and incorporate these results into clinical practice, the outcomes reported with the various versions of escB need to be compared with recent results with ABVD chemotherapy (Tables 1 and 2) rather than older studies in which the dose intensity varied.6 It is important to note that in the HD9 study, comparisons were made with COPP/ABVD (rather than ABVD) that was delivered over a median of 46.3 days rather than the planned 30 days, whereas escB, due to mandated growth factor use, was delivered in 24.7 days. Compared with the older ABVD series, in which doses were delayed until adequate count recovery, current retrospective data suggest that 99% dose intensity can be maintained (median of 28.2 days) without growth factors with an excellent 5-year freedom from progression (FFP) of 87%.7,8
Outcome of patients treated with ABVD in randomized trials
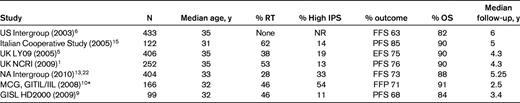
NR indicates not reported.
*Selection criteria were IPS ≥ 3.
Recently, 2 Italian studies compared ABVD with variations of the BEACOPP regimen. The Gruppo Italiano per lo Studio dei Linformi trial (HD2000) reported that 4 cycles of escB and 2 cycles of basB resulted in superior PFS compared with 6 cycles of ABVD (5-year rates, 81% and 68%, respectively, P = .038).9 The magnitude of the advantage of BEACOPP over ABVD was more evident in patients with IPS ≥ 3. However, this did not translate into a significant improvement in OS (92% vs 84%, P = .89). Despite the use of only 4 cycles of escB, higher rates of acute grade 3-4 neutropenia and severe infections were reported compared with ABVD. The second trial by the Michelangelo, Italian Group for Innovative Therapies in Lymphoma and the Intergruppo Italiano Linfomi cooperative groups compared 6-8 cycles of ABVD versus 4 cycles of escB followed by 4 cycles of basB in patients with stage IIB-IV and/or IPS ≥ 3 as frontline therapy, with preplanned high-dose therapy as salvage for patients with a partial response (PR) < 80%.10 Again, despite an unconventional definition of response in terms of requiring salvage therapy, results were similar to the previous Italian trial with a significantly higher 3-year FFP rate (87% vs 71%, P = .01), more infections, and no differences in OS (90% vs 91%) in the BEACOPP arm compared with the ABVD arm, respectively.10
These studies suggest that some version of escB results in better PFS, albeit with increased toxicity, than ABVD. To date, studies have not shown an OS advantage with escB over ABVD, because patients who relapse after ABVD can be salvaged with second-line treatment. These findings are supported by the recent Cochrane review, a meta-analysis of the major trials comparing various versions of escB with ABVD-like regimens, and confirmed the superiority of escB over ABVD for PFS; however, this result for OS was not established.11 Results of the European Organization for Research and Treatment of Cancer (EORTC) protocol 20012 comparing 4 cycles of escB followed by 4 cycles of basB with 8 cycles of ABVD in stage III-IV patients with an IPS ≥ 3 are awaited.
Another regimen tested as an alternative to ABVD is Stanford V, a combined modality therapy (CMT) approach. The premise of this protocol was to substantially lower the cumulative doses of agents known to contribute to late effects and modify RT for the same reason. In this CMT, chemotherapy is administered weekly for 12 weeks, followed by 36 Gy to sites of initial tumor burden > 5 cm and/or macroscopic splenic disease.12 Compared with ABVD × 6, the cumulative doses of Adriamycin and bleomycin are substantially lower (150 mg/m2 vs 300 mg/m2 and 30 units/m2 vs 120 units/m2, respectively). Excellent phase II results were reported, with FFP and OS > 80%, along with preservation of fertility, low risk of pulmonary toxicity, and no leukemia or myelodysplasia.12,13 In the Stanford experience, patients with a high IPS score (> 4) had an inferior outcome compared with those with IPS scores of 0-3. At a median follow-up of 10 years, the FFP and OS for IPS 0-3 versus 4-7 were 92% versus 68% and 98% versus 81%, respectively.
An assessment of these results for practical clinical use requires a direct comparison of Stanford V with recent studies on ABVD (Tables 1 and 2). Recently, 2 large randomized clinical trials for patients with advanced HL comparing Stanford V with ABVD were reported. Both study designs included patients with bulky stage I-II mediastinal disease.1,14 The United Kingdom National Cancer Research Institute reported identical 5-year PFS (75%; hazard ratio [HR] = 1.12, 95% confidence interval [95% CI] = 0.78,1.61, P = .55) and OS (90%; HR = 0.75, 95% CI = 0.41,1.38, P = 0.35) for ABVD compared with Stanford V.1 Initially, RT was used in both arms of the trial in accordance with the Stanford V regimen, but was subsequently restricted in the ABVD arm to only those patients with bulky mediastinal disease. This resulted in 53% of patients receiving RT on the ABVD arm compared with 73% on Stanford V. The hematologic toxicity was similar in both arms. Compared with ABVD, significantly fewer pulmonary events and slightly more neuropathy were reported with Stanford V.1
The North American (NA) Intergroup has recently reported results of a randomized clinical trial for patients with advanced HL.14 Compared with the UK study, RT in the ABVD arm was delivered only to patients with bulky mediastinal disease. At a median follow-up of 5 years, no differences were reported between ABVD and Stanford V for failure-free survival (FFS) and OS (73% vs 71%, P = .29, and 88% vs 87%, P = .87, respectively). Patients with high IPS scores (≥ 3) in the NA Intergroup trial had an inferior FFP but no difference in OS with Stanford V compared with ABVD. The 5-year FFS and OS for the latter subset were 58% versus 68%, P = .013, and 75% versus 77%, P = nonsignificant, respectively, a similar result to the outcome for high-risk patients reported by the Stanford group.
A single Italian study has reported an inferior PFS with a modified version of Stanford V compared with ABVD with no differences in OS.15 In this trial, the study design deviated considerably from the published Stanford V regimen, because RT was limited to patients with initial bulky disease defined as size > 6 cm with 2 sites of disease and/or with PR to chemotherapy. In addition, because response assessments were performed at different time points on study arms, results are difficult to interpret.15
In general, randomized clinical trials have shown that the Stanford V regimen is not significantly superior compared with ABVD.1,14 It is important to note that in both of these trials results with ABVD were better than those reported in older studies.6 Longer term follow-up of both the United Kingdom National Cancer Research Institute and NA Intergroup trials will be required to assess the impact of the modified RT and reduced cumulative doses of Adriamycin and bleomycin in the Stanford V arm on the long-term outcome of these patients.
The above data suggest that no regimen provides a significant OS advantage over ABVD. Therefore, in North America, ABVD remains the standard of care for patients with advanced HL and provides the appropriate balance between efficacy and toxicity. For selected patients, the Stanford V regimen remains a valid option as frontline therapy because of the brief duration of treatment and lower cumulative doses of Adriamycin and bleomycin, although long-term follow-up is required to accurately assess the impact of the modified RT on outcome. It has yet to be determined where escB will fit into the management algorithm of advanced HL. PET-adapted stratification studies are ongoing to identify patients with high risk of failure who might benefit from intensified regimens.
What is the role of radiation therapy?
Because combination chemotherapy can be expected to cure a high proportion of patients with advanced disease, the incremental benefit of consolidative RT is controversial. Most of the long-term data suggest that RT results in improved tumor control rates but no OS advantage after 10 years due to excess mortality related to treatment complications, mainly cardiac and secondary malignancy.16 It is important to consider that these risks are based on older chemotherapy regimens such as MOPP and on relatively large RT fields and doses than currently used. Current RT uses involved-field RT (IFRT) in advanced HL, and results of trials are summarized in Table 3. A landmark randomized clinical trial, the EORTC 20884 study, evaluated the role of RT in patients with advanced HL.17 Patients with a complete response (CR) defined by computed tomography (CT) criteria after MOPP-ABV chemotherapy were randomized to either 24 Gy IFRT or no further treatment, with no differences in FFTF or OS reported. Whereas this is an extremely important report and often cited as the basis for challenging the role of RT, it is imperative to note that the chemotherapy used, a MOPP-ABV hybrid, is an obsolete regimen and response criteria used to define a CR were based on CT results, which now have been replaced by PET assessments.18 More importantly, in a follow-up analysis, patients who achieved a PR by CT criteria underwent IFRT 30 Gy to nodal areas and 18-24 Gy to extranodal sites, with 8-year event free survival and OS of 76% and 84%, respectively. These results are not statistically different from those in patients who achieved CR to chemotherapy, and suggest a beneficial role for consolidative RT in patients who achieve a PR after 6 cycles of chemotherapy.19 Although the EORTC 20884 trial failed to support the routine use of IFRT after patients achieved a CR to a full course of conventional chemotherapy, for approaches with attenuated chemotherapy, such as Stanford V, RT is an integral part of treatment and variations lead to inferior results.15
Randomized trials of involved field radiation therapy in advanced HL
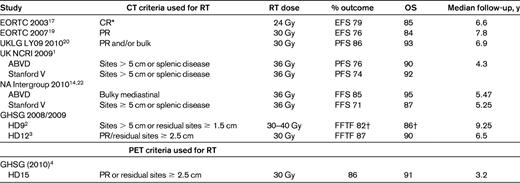
EFS indicates event-free survival
*CR randomized to RT.
†escB arm only.
The United Kingdom Lymphoma Group analyzed the outcomes of nonrandomized consolidative IFRT after chemotherapy with ABVD or multidrug regimens in the LYO9 trial. At least 30 Gy was delivered to residual masses or sites of original bulky disease at presentation (MMR>1/3 or 10 cm on CT).20 Postchemotherapy RT for consolidation was reported in 300 (43%) patients. With a median follow-up of 6.9 years, the 5-year outcomes were superior for patients who received RT (PFS = 86% vs 71%, P < .001, HR = 0.43; 95% CI, 0.30-0.60, and OS 93% vs 87%, P = .014, HR = 0.47; 95% CI, 0.29-0.77).20 The investigators concluded that RT contributed significantly to the cure rate for advanced HL, although a better definition of patient selection for CMT is necessary for prospective trials.
A major issue today is how to extrapolate results that were largely based on CT criteria to the current PET-based criteria to define a CR. This has been systematically studied only in the context of escB chemotherapy, as reported in the HD15 trial in which RT was omitted in patients with PET-negative residual masses at completion of chemotherapy with excellent outcomes. Patients with PET-positive residual sites underwent RT to 30 Gy, with a 3-year PFS of 86%. These results, although inferior to the outcome of patients who achieved a PET-CR in the study, are far more favorable than those reported in other series in which treatment decisions were not based on PET findings.21
Patients with stage I-II bulky mediastinal disease, another group in which RT is routinely used, is a subset included in trials of advanced HL in North America. For this group, a subset analysis of the recently reported NA Intergroup trial showed equal efficacy for both CMT approaches tested, ABVD followed by IFRT 36 Gy and Stanford V, without differences in FFS (5-year FFS = 85% vs 77%, P = .13, HR = 1.56; 95% CI 0.87-2.88, respectively) or OS (5-year OS = 95% vs 92%, P = .31, HR = 1.69; 95% CI 0.60-4.75, respectively).22 In Europe, patients with stage I/II bulky mediastinal disease are treated differently based on the presence of additional risk factors.23 Therefore, management of this group varies considerably between North America and Europe. Within the GHSG, patients with B symptoms or extranodal sites are treated on protocols for advanced HD, such as the HD9, HD12, or HD15 trials, and outcomes for this subgroup have not yet been reported on separately. In contrast, patients without B symptoms or extranodal disease are treated on protocols for unfavorable early-stage HL. In the HD14 trial, patients were treated with ABVD × 4 + 30 Gy IFRT or escB × 2 and ABVD × 2 + 30 Gy IFRT.24 A statistically significant 6% improvement in FFTF was reported for the 2 × 2 arm, but at the cost of more acute toxicity with no OS advantage.24 In contrast to the GHSG, in the EORTC trials, all patients with stage I/II bulky mediastinal disease were treated on protocols for early-stage unfavorable disease. The EORTC/GELA H9-U trial reported no significant differences between 4 or 6 cycles of ABVD followed by IFRT.25 It is again important to note that this subgroup of patients with bulky mediastinal disease has not been reported on separately by either the GHSG or the EORTC, making outcome comparisons of this group difficult because of the variable therapy delivered.
These studies support a role for RT in patients who have achieved a PR after ABVD chemotherapy. This is of paramount importance in patients with bulky mediastinal disease, who often have residual masses on postchemotherapy CT scans. Studies omitting RT for this patient population based on PET responses are being evaluated, and until results are available, CMT remains the standard of care for this subgroup of patients.
Can we adapt therapy based on clinical or biological risk factors?
The challenge in deciding the best treatment for patients with advanced HL is largely due to our inability to reliably identify subgroups of patients who will respond differently to primary chemotherapy.
The IPS reported in 1998 by Hasenclever identified 7 risk factors (3 clinical and 4 laboratory based) that adversely affected the FFP and OS in advanced HL patients treated with chemotherapy with or without RT.26 Patients without risk factors have a 5-year FFP of 80%, whereas the higher-risk group (IPS score ≥ 5), as represented by < 20% of patients, had an FFP of 42%. Whereas the IPS has allowed for retrospective comparisons of therapies, it was developed in an era (before 1992) when therapy and supportive care varied considerably from the current decade and needs to be used with caution to interpret therapeutic advances. The British Columbia Cancer Agency has reported markedly improved outcomes in the current era (1990-2008). Most notable are improvements in patients with the highest scores according to IPS, and whereas distribution of patients across scores has not changed, the 5-year FFP and OS in this subgroup have improved from 42% to 71% and 56% to 73%, respectively.27
The IPS score is also useful for comparisons across prospective trials. Irrespective of the chemotherapy used, ∼80% of patients with an IPS of 0-3 had excellent outcomes in all of the recent randomized clinical trials reported.2,14 Therefore, the question that arises is can the IPS be used to choose optimal therapy up front to offer high-risk patients more intense approaches? In terms of PFS, the advantage of escB is seen among all IPS subgroups and is not just restricted to the high-risk group; accordingly, selection of high-risk patients by IPS is not a reliable strategy to discern the subset of patients who may benefit from dose intensification.
Autologous stem cell transplantation has also been evaluated as a consolidative strategy for patients with high-risk disease. Although the definition of “high-risk” has varied and included only elements of the IPS, randomized clinical trials have concluded no benefit for early intensification with autologous stem cell transplantation in patients with unfavorable disease responding to anthracycline-based therapy.28–30
The pertinent question becomes how to identify up front the ∼ 20% or so of cases that may not do well with standard approaches. Emerging data support that a negative PET may be predictive after 2 cycles of chemotherapy.21 The application of the prognostic significance of a negative PET after 2 cycles of ABVD chemotherapy has led to several reports of therapy escalation or de-escalation with encouraging results.31,32 The temptation to adapt these results into clinical use outside of a clinical trial should be resisted, because most data come from small sample sizes with inconsistency on the definition of a negative PET scan. Whether PET-based risk-adapted approaches can be used to select patients at high risk of failing ABVD form the basis of most of the ongoing trials both in North America (US Intergroup trial: S0816) and Europe (UK RATHL, GHSG HD18 trial), and it is imperative that we endorse and actively enroll patients in these clinical trials.
The identification at diagnosis of biomarkers associated with poor response or outcome is another strategy that may help in the development of a rational, risk-adapted treatment approach based on a molecular risk algorithm. In HL, neoplastic Reed Sternberg (RS) cells are a minority and are surrounded by a heterogeneous background population of nonneoplastic cells, mostly B and T cells, but also macrophages (eosinophils, basophils, and monocytes). Recently, there has been increasing interest in the role of these bystander cells in the pathogenesis of HL. Expression of a variety of cytokines and chemokines by the RS cells is believed to be the driving force for an abnormal immune response, and additional factors secreted by reactive cells in the microenvironment help to sustain the inflammatory background that allows for immune evasion.33 Tumor-associated macrophages evaluated by CD68 IHC have been reported to correlate with clinical outcome independently of the stage of disease.34,35 Preliminary results of IHC-based studies combining 2 markers, CD68 and FoxP3 (a marker for regulatory T cells), suggest an improvement over predictive value of individual markers alone.36 There is also renewed interest in the serological marker CC thymus and activation-related chemokine (TARC/CCL17), a protein that is highly expressed by malignant RS cells and can be detected in human serum in the majority of HL patients.37 It has been determined that this chemokine contributes to the microenvironment that supports the survival of RS cells, and may therefore be useful as a prognostic marker to identify those patients at the time of diagnosis who are at a higher risk for relapse or to predict a patient's response to chemotherapy regimens.38
The Spanish HL study group has recently reported a molecular risk score based on 4 functional pathways in advanced HL.39 Using formalin-fixed paraffin-embedded samples and using RT-PCR, the best prediction genes were integrated into an 11-gene model, including 4 functional pathways (cell cycle, apoptosis, macrophage activation, and interferon regulatory factor 4) that identified low- and high-risk patients with a 5-year FFS of 67.5% versus 46.3%, respectively (P = .022). When this model was combined with stage IV, a group of patients with a particularly poor outcome (FFS of only 25.5%) was identified.39
It is important that these emerging biomarkers be built into future prospective studies for evaluation and comparison with other disease markers such as IPS and PET imaging both during and after treatment. The next decade will provide exciting new data on whether these biomarkers can be integrated into routine clinical practice to select patients for either escalation or de-escalation of primary therapy. In addition, novel therapeutic agents targeting the reactive cells in the microenvironment are in clinical development.33
In conclusion, ABVD remains the standard of care in North America for most patients with advanced HL. When deciding what the best primary therapy option is for patients with advanced HL, it is important to take into account that patients who relapse can be cured by subsequent high-dose therapy and stem cell support (hematopoietic stem cell transplantation).40–42 Therapeutic advances over the last few decades have resulted in a large group of patients cured of HL. The Surveillance Epidemiology and End Results database has reported significantly improved outcomes for HL patients treated in 2000-2004 compared with 1980-1984.43 In the current era, further refinement of therapy and improvement in supportive care will likely result in further gains in outcome, resulting in a growing population of cured HL patients. Recent data of excellent durable remissions in a high proportion of patients with targeted therapies in relapsed/refractory HL with brentuximab, vedotin, and panobinostat also provide an excellent opportunity to move these agents to the frontline setting, and such trials are ongoing.44–46 However, success comes with a price and requires a commitment by the medical community to meet the needs of this expanding population outnumbering patients with active disease. In 2006, the Institute of Medicine published recommendations on optimizing survivorship.47 One of the main components of the guideline was that at the end of therapy, patients receive a personalized treatment summary outlining therapy delivered and possible related late effects to enable appropriate surveillance. Depending on the “era” in which patients were treated, different surveillance strategies apply. It is important to recognize that the increased risk of solid tumor and cardiovascular risks after RT in HL are from an era when larger treatment fields and higher RT doses were used.16 Results of trials evaluating PET-adapted strategies and biomarkers should be available in several years, and these will help to refine the management of patients with advanced HL. As long as an OS advantage is not demonstrated, choices need to be driven by comparison of both acute and long-term toxicity.
Disclosures
Conflict-of-interest disclosure: The author is on an advisory board committee for and has received research funding from Seattle Genetics. Off-label drug use: SGN35 for refractory or relapsed HL.
Correspondence
Ranjana Advani, Stanford University Medical Center, 875 Blake Wilbur Dr, CC-2338, Stanford, CA 94305; Phone: (650) 725-6456; Fax: (650) 725-8222; e-mail: radvani@stanford.edu.
