Abstract
Peripheral T-cell lymphomas (PTCLs) are a rare and heterogeneous group of disorders that, for the most part, are associated with a very poor prognosis. The standard therapy for PTCLs is CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or a comparable CHOP-like regimen that incorporates anthracyclines. With the exception of anaplastic lymphoma kinase–positive anaplastic large cell lymphoma (ALK+ ALCL), the cure rate for PTCLs with CHOP is low, and limited evidence suggests that anthracyclines do not improve the prognosis. However, there is no compelling evidence that any other regimen or approach is superior. It remains challenging to compare alternative therapies or treatment strategies with CHOP because the majority of data are retrospective and include diverse patient populations. Recently, prospective studies have been initiated exclusively for PTCL, and in some, select histologic subtypes are evaluated in an effort to remove heterogeneity. Encouragingly, there have been several new therapies emerging with activity in PTCLs and exciting novel combinations under consideration that will hopefully move the field forward and improve outcome in this challenging group of diseases.
Introduction
Peripheral TCLs (PTCLs) are derived from post-thymic mature T cells and represent approximately 10%-15% of all non-Hodgkin lymphoma in North America. This broad category encompasses a heterogenous group of diseases. The most commonly encountered subtypes are PTCL not-otherwise-specified (PTCL-NOS), systemic anaplastic large cell lymphoma (ALCL), and angioimmunoblastic TCL (AILT), which collectively represent 66% of all cases of PTCL in North America (Table 1).1
Comparison of the World Health Organization of classifications of mature T-cell and NK-cell neoplasms56
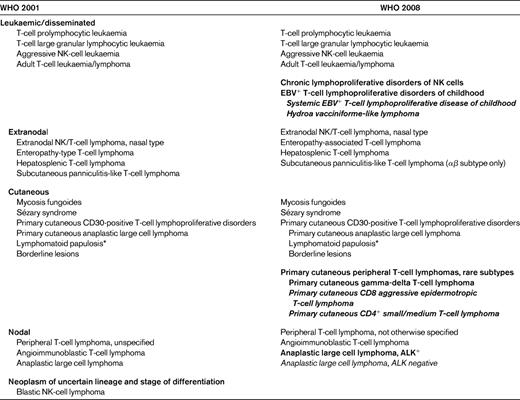
New distinct categories are shown in bold; provisional categories in bold italics.
*Not considered a neoplastic lesion.
The diagnosis of PTCL requires a combination of morphologic, immunophenotypic, genetic, and clinical features; this can be challenging and expert review by an experienced hematopathologist is essential. Immunophenotyping for standard pan-T-cell antigens is typically performed, and the loss of a particular T-cell antigen is considered abnormal. The morphology of PTCL can be quite diverse, particularly for PTCL-NOS and even within the same tumor. It some cases, an inflammatory cell background with sparse malignant cells can be seen, and gene-rearrangement studies are used to document a monoclonal T-cell population. AILT has a characteristic appearance, with increased vascularity and expanded CD21+ follicular dendritic cell networks. Recently CD10, CXCL13, and PDL-1 have been found to be helpful in establishing the diagnosis of AILT, suggesting that the normal cell of origin is a follicular helper T cell. ALCL also has a characteristic appearance, with large, anaplastic cells that are strongly CD30+ and the presence of hallmark cells. ALCL is also tested for anaplastic lymphoma kinase (ALK) expression because it has clinical relevance as outlined below.
It has long been recognized that the majority of PTCLs have an inferior prognosis compared with their B-cell counterparts. Treatment approaches have mirrored diffuse large B-cell lymphoma and, as a result, CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) is considered the “standard therapy” despite consistent results showing that it is largely ineffective. The landmark Southwest Oncology Group (SWOG) trial comparing CHOP with second- and third-generation, dose-intensive regimens in aggressive lymphomas established that CHOP had equivalent efficacy but less toxicity than the more intensive regimens. However, diagnoses in this trial were based on the Working Formulation and immunophenotyping was not yet routine.2 A comprehensive assessment of the outcome of 3287 PTCL patients diagnosed from 1992-1995 and in 13 Surveillance, Epidemiology and End Results (SEER) registries treated with CHOP or CHOP-like regimens reports a 5-year overall survival (OS) of 37.5% for PTCL-NOS.3 This study was limited by the lack of a centralized pathology review; however, it is in keeping with results from other studies, including the large, collaborative International Peripheral T-cell Lymphoma (ITCL) project, which reported a 5-year OS of 32% and a failure-free survival of 22% in this subgroup.4 Further, patients with PTCL-NOS with multiple risk factors according to the International Prognostic Index (IPI) can have a 5-year OS as low as 10%.4 These results are far inferior to aggressive B-cell lymphoma, even in the pre-rituximab era.5 A recent study has also suggested that survival of patients with PTCL-NOS is not affected by the addition of an anthracycline.1 The one exception is ALK positive (ALK+) ALCL, which, in contrast to ALK-negative (ALK−) ALCL has a high cure rate with CHOP (type) chemotherapy, in some cases surpassing diffuse large B-cell lymphoma. This review focuses on recent studies exploring new chemotherapy combinations in PTCLs, discusses the role of transplantation, and highlights exciting novel therapies in the relapsed and refractory setting.
Building on CHOP in the primary therapy of PTCL
Given disease rarity, there is a paucity of randomized trials comparing new chemotherapy combinations with CHOP, and therefore the majority of information regarding the efficacy of alternative regimens is based on retrospective or phase 2 data with historical comparisons. With encouraging results from a phase 2 study, the Groupe Ouest Est d'Etude des Leucémies et Autres Maladies du Sang (GOELAMS) group compared alternating VIP (etoposide, ifosfamide, cisplatin)/ABVD (Adriamycin, bleomycin, vinblastine, dacarbazine) for a total of 6 cycles against CHOP for 8 cycles in the primary therapy of patients with PTCLs including ALCL and, disappointingly, found that there was no difference in outcome and overlapping survival curves (event-free-survival [EFS] P = .4522).6 With a median follow-up of 110 months, the 2-year EFS for the standard CHOP arm (n = 43, including 3 patients with ALK+ ALCL) was 41% (5-year EFS ∼ 35%) and serves as a benchmark to compare other novel combination therapies in the up-front setting. Not surprisingly, the survival of the non-ALCL PTCL group combining both arms (84% of the trial patients) had an even worse 5-year EFS (∼ 25%-30%), which is not unlike population-based estimates. The German Non-Hodgkin's Lymphoma Group (DSHNHL) retrospectively evaluated the outcome of PTCL patients (n = 331) with a focus on the most common subtypes, PTCL-NOS, ALCL, and AILT (n = 289), who had been included in 7 German high-grade phase 2 or phase 3 aggressive non-Hodgkin lymphoma studies. The main purpose was to determine whether the addition of etoposide or shortening of the interval of chemotherapy from 3 weeks to 2 weeks affected survival.7 In elderly patients, neither the shortening the interval nor the addition of etoposide improved EFS or OS. There were too few younger patients to evaluate the impact of shortening the interval of cycles, but confining the analysis to a very selected population of young patients with a normal lactate dehydrogenase level who were treated on the B1 trial with CHOP-21 or CHOP-14 with or without etoposide demonstrated an improved EFS (P = .003) but not OS (P = .176) in patients who received etoposide (Table 2). Similar results were seen if the same selected patients were combined from the B1 and the Hi-CHOEP phase 2/3 trial (3-year EFS 71% vs 51%, P = .004, Table 2). This benefit was mainly seen in patients with ALK+ ALCL (3-year EFS 91% vs 82%, P = .012) and only a trend toward an improved 3-year EFS (61% vs 48%, P = .057) was observed in the other subtypes; however, patient numbers were small.
Selected studies of novel CHOP-like or other combinations in the primary treatment of PTCLs
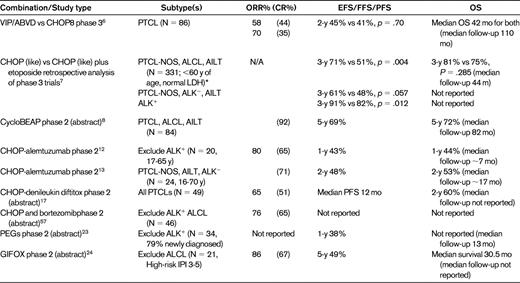
Survival estimates are rounded off.
*Analysis of patients from BH1and CHOEP trials.
Another recently reported phase 2 trial evaluated a novel regimen, CycloBEAP, in newly diagnosed PTCL patients with an IPI > 2. This is a regimen with dose intensities equal to or higher than CHOP with the addition of etoposide and bleomycin.8 In total, 84 patients were treated in this study. The complete remission (CR) rate was 92% and the 5-year progression-free survival (PFS) was encouraging (69%), although it was not clear whether ALK+ ALCL patients were excluded (Table 2). Further studies are needed to determine whether the addition of etoposide can improve cure rates in PTCL.
CHOP with alemtuzumab
Alemtuzumab is a humanized mAb that selectively binds to the CD52 antigen, which is expressed on most normal and malignant T and B lymphocytes, making it an attractive target for both T- and B-cell lymphomas. Unfortunately, CD52 is also expressed on virtually all other immune cells and can cause profound immunosuppression and opportunistic infections. Single-agent alemtuzumab has a modest overall response rate (ORR) in relapsed/refractory PTCL (36%),9 but treatment-related mortality was unacceptably high (36%). It also appears that CD52 expression in PTCLs is heterogeneous; in PTCL-NOS, only 35%-40% of cases appear to be CD52+ by immunohistochemistry.10,11
Because the pilot study evaluated heavily pretreated patients, trials were initiated using alemtuzumab combinations in the up-front setting in the hope that reduced toxicity would be observed. In the first published phase 2 study, 20 patients were treated with CHOP and alemtuzumab (30 mg IV) with a lead-in escalating dose and consolidative high-dose chemotherapy and autologous stem cell transplantation (HDC/ASCT) in high-risk patients. The ORR was 80% (65% CR) and the 1-year EFS was 43%, which is not strikingly different from CHOP alone. Furthermore, the toxicity of this combination was high (90% grade 4 neutropenia; 55% febrile neutropenia), including 2 treatment-related deaths, and resulted in early study closure12 (Table 2). To reduce toxicity, the Gruppo Italiano Terapie Innovative nei Linfomi (GITIL) lengthened the cycles of CHOP treatment to every 4 weeks with alemtuzumab (30 mg) given subcutaneously on day −1 of each course, again, after an initial escalation with the first cycle.13 The toxicity was more manageable and there were no treatment-related deaths reported; however, opportunistic infections were still observed, including J-C virus reactivation and aspergillosis. The CR rate was 71% and, with a median follow-up of 16 months, the estimated 2-year failure-free survival was projected to be 48%.
A feasibility phase 2 study was recently reported evaluating alemtuzumab in combination with fludarabine, cyclophosphamide, and doxorubicin (Campath-FCD) in both newly diagnosed and relapsed/refractory PTCL patients. The efficacy was low in the group with relapsed/refractory disease and accrual stopped in this subgroup. Further, the remainder of the trial was stopped prematurely due to 3 treatment-related deaths in 27 newly diagnosed PTCL patients. Overall, there were 6 treatment-related deaths in the trial, including 2 patients who developed an EBV-associated lymphoproliferative disorder due to profound immunosuppression related to the combination of fludarabine and alemtuzumab.14
Currently, there are 2 ongoing phase 3 trials in Europe comparing dose-dense CHOP-14 with CHOP-14 plus subcutaneous alemtuzumab in younger patients (< 60 years, ACT-1) and older patients (> 60 years, ACT-2). Both trials include growth factor support, and ACT-1 also includes HDC/ASCT in responding patients. Hopefully, these landmark studies will clarify whether the addition of alemtuzumab improves outcome in PTCL patients.
CHOP and denileukin diftitox
Denileukin diftitox (DAB389IL-2; ONTAK) is a genetically engineered, recombinant DNA fusion protein that combines the enzymatically active A and B fragments of diphtheria toxin with the sequence of IL-2. The IL-2 domain targets the fusion toxin to tumor cells bearing high-affinity IL-2 receptors (IL-2Rs), resulting in endocytosis of the fusion toxin and rapid cell death.15 A phase 2 trial evaluated denileukin diftitox in 27 patients with relapsed/refractory PTCL,16 and the ORR was 48% (22% CR), with a median PFS of 6 months.
Given that denileukin diftitox is not myelosuppressive and has a non-cross-resistant mechanism of action with cytotoxic chemotherapy, a phase 2 study was initiated combining denileukin diftitox with CHOP for newly diagnosed, untreated PTCL patients.17 With 49 evaluable patients, the response rate was 65% (51% CR, Table 2), and toxicities were mostly infusion related with vascular leak; however, there were 3 deaths after cycle 1 (2 cardiac and 1 rhabdomyolysis).17 Interestingly, the ORR was only 47% in PTCL-NOS patients (n = 19), compared with 80% in AITL (n = 10) and 87% in ALCL (n = 8) patients, suggesting selective sensitivity to this combination. A phase 3 trial is planned comparing this novel combination with CHOP.
Other CHOP combinations
Bevacizumab (Avastin) is a humanized mAb against VEGF-A that is strongly expressed in PTCL. The Eastern Cooperative Group (ECOG) initiated a phase 2 study in newly diagnosed PTCL combining CHOP with bevacizumab (15 mg/kg IV) on day 1, followed by maintenance bevacizumab for 3 weeks for 4 cycles. An early report of toxicity was presented at the American Society of Hematology (ASH) meeting in 2009, and one or more cardiac toxicities were identified in 5 of 22 patients, including 4 cases of congestive heart failure, indicating that further development of this combination will be limited.18
Bortezomib is a proteosome inhibitor that down-regulates NF-κB transcriptional activation, potentially sensitizing cells to chemotherapy. A phase 1 study was performed in PTCL patients and established the optimal schedule of bortezomib (1.6 mg/m2 on days 1 and 8).19 The results of a phase 2 study of CHOP and bortezomib including 46 patients with PTCL (including 5 patients with cutaneous TCL) were recently presented. The ORR was 76% (65% CR), but this rate decreased to 30% in patients with extranodal natural killer (NK)/TCL (Table 2). The Groupe d'Etude des Lymphomes de l'Adulte (GELA) group completed a phase 2 study evaluating the CHOP-like regimen ACVBP and bortezomib in newly diagnosed PTCL patients (n = 57), but toxicity was high and the response rate was similar (46% CR) to ACVBP alone.20
Novel chemotherapy and combinations in the primary therapy of PTCLs
Gemcitabine combinations
With some evidence supporting the idea that anthracyclines may not affect survival in PTCL, new chemotherapy agents and combinations are being evaluated. It has been speculated that the chemoresistance may in part be due to overexpression of P-glycoprotein (Pgp), which is known to contribute to anthracycline resistance.
Gemcitabine demonstrated encouraging activity in heavily pretreated, relapsed PTCL patients, with an ORR of 55% (CR 30%). This therapy bypasses the Pgp efflux pump, providing rationale for moving it to the frontline therapy21 (Table 2). A protocol incorporating GEM-P (gemcitabine with cisplatin and methylprednisolone) demonstrated an ORR of 69% (CR 19%) in 16 PTCL patients with mostly relapsed or refractory disease.22 SWOG has completed a phase 2 study evaluating a novel regimen in PTCLs called PEGS (cisplatin, etoposide, gemcitabine, and Solu-Medrol), incorporating drugs that are not substrates for the Pgp. The majority of patients were newly diagnosed (79%), but the 1-year PFS was only 38%23 (Table 2). An Italian study was reported at the 2010 ASH meeting that evaluated a novel regimen in the frontline setting incorporating GIFOX (gemcitabine, ifosfamide, and oxaliplatin, including ASCT in young, chemosensitive patients) for high-risk PTCL patients. The ORR was 86% (67% CR/CR unconfirmed [CRu]) and the 5-year EFS was 49%, but grade 4 thrombocytopenia and anemia were observed in 38% and 24% of patients, respectively, and 33% had a grade 3 infection24 (Table 2).
Dose escalation of chemotherapy in the primary therapy of PTCL
There have been conflicting reports in the literature as to whether dose intensity with or without stem cell support improves outcome in the up-front treatment of PTCL. The M. D. Anderson Cancer Center retrospectively evaluated the outcomes of 135 patients with PTCL treated with CHOP and compared these with a variety of more dose-intensive approaches, including early autologous and allogeneic transplantation, and found no difference in outcome; however, the number of patients receiving each regimen was small.25
Although the German retrospective study suggested that the addition of etoposide may improve cure rates in PTCL in some low-risk patients, a benefit was not observed using simple dose escalation in high-risk patients. The DSHNHL reported the outcome of 33 PTCL patients treated in phase 1/2 trials evaluating Mega-CHOEP with ASCT in patients with an IPI score of 2 or 3 or elevated lactate dehydrogenase and, disappointingly, the 3-year EFS was only 26%26 (Table 3). As a result, the randomized phase 3 trial comparing 4 cycles of mega-CHOEP/ASCT with 8 cycles of CHOEP-14 in high-risk patients was stopped early after only 38 PTCL patients were enrolled; a trend to a worse EFS was actually observed in the dose-intensive group7 (Table 3). These results challenge the use of consolidative HDC/ASCT, which is a commonly used approach in these high-risk, patients.
Prospective studies of consolidative high dose chemotherapy and autologous stem cell transplant in the primary treatment of PTCL
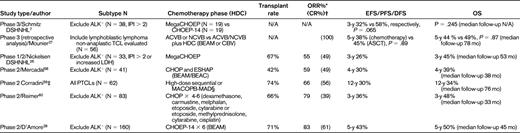
Survival estimates are rounded off.
*ORR after the chemotherapy phase.
†Includes CRu.
‡Combined results of 2 phase 2 studies.
§High-dose sequential was APO × 2 (doxorubicin, vincristine, prednisone) and DHAP × 2, MACOPB × 8 weeks, then a 3-day course of MAD (mitoxantrone, AraC, dexamethasone).
Multiple other retrospective studies have also been published evaluating the impact of up-front transplantation in the treatment of PTCLs. Trial interpretation and comparisons are difficult due to several factors: heterogeneous patient populations, inclusion of relapsed/refractory patients, inclusion of the favorable ALK+ ALCL patients, and selection bias because intention-to-treat results are not described.
There are no phase 3 randomized controlled studies evaluating up-front consolidative transplantation in PTCL patients; however, similar to the DSHNHL study initiatives, the GELA group has included PTCLs in their aggressive lymphoma phase 3 studies, thus enabling subgroup analyses. In one analysis, patients with high-risk TCL (including lymphoblastic lymphoma) who achieved a CR after frontline therapy were pooled from the randomized phase 3 trial LNH-87 comparing ACVB (or NCVB with mitoxantrone substitution) with or without HDC/ASCT and LNH-93, which initially compared ACVB with shortened induction chemotherapy with HDC/ASCT. However, the shortened induction chemotherapy arm was closed due to inferior results and the trial continued with the same transplantation arm as in LNH-87 and the trials were pooled for this analysis.27 Patients in a CR/CRu after ACVB/NCVB were matched (1:1) by several clinical factors to patients who underwent subsequent HDC/ASCT after CR/CRu. There was no difference in DFS (the 5-year DFS was 38% with chemotherapy alone versus 45% with ASCT, P = .89) or OS (P = .87), but the treatment groups were small (Table 3).
Several phase 2 prospective studies of up-front transplantation have been published and represent more homogenously populations of treated patients; with rare exceptions, patients with ALK+ ALCL were excluded (Table 4). The transplantation rate varied from approximately 40%-70%, and in the intention-to-treat analysis, the EFS ranged from 30%-50% (Table 4). The Nordic group completed the largest prospective phase 2 trial of up-front transplantation (NLG-T-01) in 160 patients with PTCL excluding ALK+ ALCL (Table 3). The planned treatment scheduled was CHOEP-14 for 6 cycles, followed by BEAM/ASCT in responding patients.28 The transplantation rate was 70% and, with a median follow-up of almost 4 years, the 5-year PFS was 48% and the 5-year OS was 50%. Interestingly, patients with ALK− ALCL (n = 31) appeared to have a superior 5-year PFS (64%) compared with PTCL-NOS (42%) and AILT (54%) patients and with historical comparisons, suggesting that this group may have a greater benefit from this approach.29
Selected study of novel drugs currently under investigation in the treatment of relapsed/refractory PTCLs
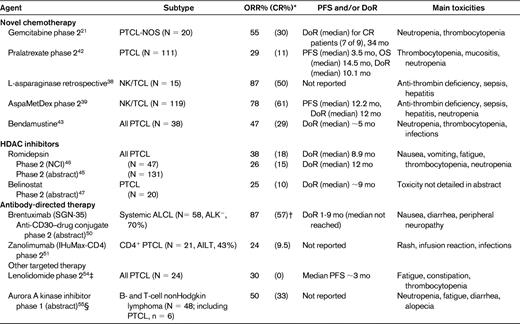
*Response by central review.
†Interim analysis reported in abstract based on 30 patients; responses reported in abstract by investigator assessment.
‡Interim analysis.
§Responses described in table are for PTCL patients only.
Role of HDC/ASCT in relapsed/refractory PTCL
In eligible patients, HDC/ASCT is the standard of care for relapsed and refractory PTCL. In the original PARMA phase 3 randomized controlled study, HDC/ASCT emerged as superior to second-line chemotherapy for relapsed aggressive non-Hodgkin lymphoma with diagnoses based on the Working Formulation classification. There has been no similar study in PTCLs; however, many centers have done retrospective studies that are all very heterogeneous with respect to the patients included and some did not separate patients who were receiving transplantation as part of the frontline therapy. A detailed discussion is beyond the scope of this chapter; however, the cumulative evidence has been recently summarized in a comprehensive review30 and supports that patients with PTCL can be cured with HDC/ASCT if chemosensitivity to a second-line regimen is demonstrated, with results comparable to that seen in diffuse large B-cell lymphoma.
Is there evidence of a GVL effect in PTCLs? The role of allogeneic SCT
The use of allogeneic SCT, either full myeloablative or reduced-intensity conditioning could add an additional GVL effect in PTCL. Data are limited, particularly in the frontline setting, but there are several studies supporting the existence of a GVL effect in PTCL. The largest study published to date evaluated 77 previously treated patients with mainly myeloablative conditioning (74%); the 5-year PFS was 53%, but the treatment-related mortality was 34% at 5 years.31 A phase 2 trial evaluating reduced-intensity conditioning and allogeneic SCT in 17 patients, including 8 who had failed frontline HDC/ASCT, demonstrated a 3-year PFS of 64% with a treatment-related mortality of 6%.32 In both of these studies, responses were seen with donor lymphocyte infusions, supporting the existence of a GVL effect. Allogeneic SCT is promising in the treatment of PTCL, but is limited by the availability of stem cell donors and toxicity related to GVHD.
Should treatment be tailored for specific PTCL subtype?
Treatment approaches to date have been similar among the PTCL subtypes and, because of disease rarity, it is common to include all subtypes other than cutaneous PTCLs in clinical trials. Unfortunately, this approach obscures differences in treatment sensitivity and can make trial comparisons difficult. There is also some evidence to support that for at least some subtypes, treatment should be tailored.
Conventional CHOP-based combined modality therapy in localized NK/TCL has disappointing cure rates, likely due to an inherent resistance of these tumors to anthracyclines due to frequent Pgp expression. It is appears that radiotherapy is the key treatment modality, with more favorable outcomes observed using high doses of radiotherapy (50-60 Gy) early in the frontline setting.33 Recently, the use of a platinum as radiosensitizer has been explored and may allow for the use of lower, less-toxic doses of radiation.34 Further, because systemic relapse can occur with single-modality radiotherapy, other novel combinations are being tested. Kim et al evaluated concurrent radiation (40 Gy) with cisplatin (30 mg/m2 weekly), followed by 3 cycles of VIPD (etoposide, ifosfamide, cisplatin) in stage nasal IE/IIE NK/TCL. Although this was a highly selected population, the outcome was very encouraging, with a CR rate of 83% and an estimated 3-year PFS of 85%. These results compared favorably to a previous cohort treated with dose-intensified CHOP followed by radiotherapy (P = .044 for PFS). Similarly, concurrent radiotherapy (50 Gy) and DeVIC (dexamethasone, etoposide, ifosfamide, carboplatin) was evaluated in a phase 1/2 trial in localized nasal NK/TCL, with good results (CR, 77%; 2-year PFS, 67%).35 In the absence of a randomized trial, the most recent National Comprehensive Cancer Network (NCCN) guidelines suggest either high-dose radiotherapy alone (>50 Gy for stage 1 with no risk factors) or concurrent chemoradiotherapy (stage 1 or 2) using either of the above regimens.36
The outcome of patients with advanced-stage nasal NK/TCL is poor and cures are uncommon using anthracycline-based chemotherapy,37 prompting the evaluation of novel chemotherapy approaches. L-asparaginase has emerged as an extremely active agent in NK/TCL. In vitro evidence supports that L-asparagine depletion results in NK-cell apoptosis. A retrospective study of 15 patients with relapsed/refractory NK/TCL treated with asparaginase, methotrexate (3 g/m2), and dexamethasone demonstrated an ORR of 87% (CR 50%), but toxicity can be problematic and antithrombin levels need close monitoring38 (Table 4). A phase 2 study evaluating L-asparaginase in combination with methotrexate and dexamethasone (AspaMetDex) was recently reported. This combination appears to be extremely active in this relapsed/refractory population, with an ORR of 78% (CR 61%) and a median duration of response (DoR) of 12 months.39 Additional studies incorporating L-asparaginase in the frontline treatment of both localized and advanced-stage NK/TCL are ongoing.
Hepatosplenic TCL is a rare PTCL subtype that mainly affects young men who may have a history of immunosuppression. Typically, patients present with constitutional symptoms and hepatosplenomegaly. The outcome is extremely poor with CHOP-based treatment, with only occasional survivors, and therefore many investigators have advocated transplantation in the primary setting after chemotherapy with a platinum-based regimen. Particularly encouraging results have been seen with allogeneic SCT. A recent review of the literature of 17 hepatosplenic TCL patients who had undergone allogeneic SCT demonstrated 7 (41%) patients alive and in remission40
Novel therapies in relapsed disease
Chemotherapy
Pralatrexate.
Interest in pralatrexate, a novel folate analog, for the treatment of PTCLs stemmed from a phase 1/2 study in patients with multiply relapsed and refractory hematologic malignancies suggesting that pralatrexate had a greater selectivity for TCLs.41 The phase 2 PROPEL study recently evaluated pralatrexate in combination with vitamin B12 and folate in the treatment of relapsed/refractory PTCL. By central review, the ORR was 29% (n = 111) with a median PFS of 3.5 months and the median DoR was 10.5 months42 (Table 4). The main toxicities were mucositis, thrombocytopenia, and neutropenia. These results led to US Food and Drug Administration approval of pralatrexate in September 2009 for the treatment of relapsed/refractory PTCL, the first agent to be approved exclusively in this disease (Table 4). Studies are ongoing combining pralatrexate with other agents.
Bendamustine.
Bendamustine is a cytotoxic agent with alkylating and antimetabolite therapy but no cross-resistance. It is extremely active in indolent lymphomas and has recently been tested in a phase 2 study in relapsed/refractory PTCLs. Of 38 evaluable patients, 23 (47%) had a response, including 29% CRs with a median DoR of approximately 5 months. Neutropenia (74%) and thrombocytopenia (47%) were frequent complications.43
HDAC inhibitors.
Histone deacetylases (HDACs) and histone acetyltransferases regulate chromatin structure and function through the removal and addition, respectively, of the acetyl group from the lysine residues of core nucleosomal histones, regulating gene expression. HDAC inhibitors are epigenetic therapies that increase the acetylation of histones, as well as other nuclear factors, thus modulating the expression of genes as well as other cellular effects ultimately resulting in cell cycle arrest and apoptosis. Multiple HDAC inhibitors are currently under investigation in clinical trials, particularly in cutaneous TCL and PTCL due to an apparent class effect.
Romidepsin (depsipeptide or FK228), a member of this novel class of antineoplastic agents, was one of the first HDAC inhibitors studied in PTCL. With encouraging results observed in a phase 1 study,44 two phase 2 studies have now been completed in relapsed/refractory PTCLs. In the first, conducted by the National Cancer Institute (NCI), 47 patients were treated with romidepsin as a 4-hour infusion on days 1, 8, and 15 for 28 days. In 45 evaluable patients, the ORR was 38% (CR 18%). The median DoR was 9 months and patients in CR had a median DoR of 30 months (Table 4). A second, industry-sponsored phase 2B study was recently reported at the 2010 ASH meeting. In total, 130 patients with histologically confirmed PTCL with relapsed/refractory disease were treated. The ORR was 26% (CR 15%) with a DoR of 12 months45 (Table 4). Romidepsin was generally well tolerated, and the most common side effects encountered in these studies were nausea, fatigue, thrombocytopenia, and neutropenia.46
Other HDACIs are also in clinical trial in PTCLs, including belinostat, a pan-HDAC inhibitor (1000 mg/m2 IV D1-5 for 3 weeks), which demonstrated an ORR of 20% (CR 10%) in 20 patients with relapsed/refractory PTCL in a recent phase 2 trial.47 With in vitro evidence of synergy or an additive effect, several trials are evaluating combinations of HDACI with other targeted therapies and chemotherapy.
Antibody-directed therapy
Given that CD30 is expressed in all ALCLs and is highly restricted, it was an obvious target to use to develop an antitumor antibody. SGN-30, a chimeric anti-CD30 mAb, was studied in a phase 2 study of relapsed/refractory ALCL, and the ORR was only 17% (CR 5%).48 Another anti-CD30 mAb, MDX-060, has also been studied in ALCL and, although it was well tolerated, objective responses were uncommon.49
To enhance antitumor activity, an antibody-drug conjugate, brentuximab, was designed (SGN-35) by conjugating monomethyl auristatin E, an antitubulin agent, to the CD30-specific mAb cAC10 by an enzyme-cleavable dipeptide linker. Monomethyl auristatin E is released into the cell after endocytosis and interferes with mitosis, causing cell arrest and apoptosis. A phase 1 trial was performed in patients with CD30+ hematologic malignancies, the majority of whom had Hodgkin lymphoma, however, the 2 ALCL patients responded, including 1 CR. Results of a phase 2 study of 58 patients with relapsed/refractory systemic ALCL were recently updated.50 Most patients had ALK− ALCL (72%), and the ORR of this single-agent therapy was 86% with CRs in 53% of patients. The median DoR has not yet been reached (range, ∼.08-11 months). Toxicities were manageable, and nausea, diarrhea, and peripheral neuropathy were the most commonly encountered (Table 3). A study combining CHOP and brentuximab in the primary treatment of systemic ALCL is currently under way.
The majority of PTCLs, including PTCL-NOS, AITL, and ALCL, have a T-helper cell phenotype expressing CD4 on the cell surface. Zanolimumab (HuMax-CD4) is a human mAb directed against CD4 that abrogates signaling by the TCR and also induces killing of CD4+ cells by antibody-dependent cellular cytotoxicity. A phase 2 trial evaluating this agent in 21 patients with relapsed/refractory PTCL was recently reported, and the ORR was 24% (Cru, 10%) (Table 3).51 The most frequently encountered toxicities were rash, pyrexia, and infusion reactions, and 29% of patients had infectious episodes.
Other targeted therapy
Bortezomib
Bortezomib has a non-cross-resistant mechanism of action with a variety of agents. A phase 1 study of gemcitabine (800 mg/m2) and bortezomib (1.6 mg/m2 on days 1 and 15 for 28 days) was evaluated in a variety of hematologic malignancies, with early responses seen in PTCL.52 Phase 2 studies are also ongoing evaluating bortezomib in combination with pralatrexate given evidence of synergy in preclinical models.53
Lenalidomide
Lenalidomide (Revlimid) is an immunomodulatory agent with several proposed mechanisms of action, including direct cytotoxicity to the tumor cell and alteration of the microenvironment. It is active in a variety of hematologic malignancies, most notably multiple myeloma. The interim results of a phase 2 study evaluating 24 patients treated with lenalidomide in relapsed/refractory PTCL have recently been published, and the ORR was 30% with no CRs. The most common side effects were fatigue, constipation, and thrombocytopenia (Table 4).54
Aurora A kinase
Aurora A kinase is a serine-threonine kinase that regulates mitosis in addition to multiple cell signaling pathways has recently been explored as a target in hematologic malignancies. A phase 2 trial evaluating the efficacy of an oral aurora A kinase inhibitor, MLN8237, has been initiated in relapsed/refractory aggressive nonHodgkin lymphomas, and the early results suggest some selectivity for PTCLs. Of 6 treated PTCL patients, 3 responded, including 2 CRs.55 The most commonly encountered toxicities were neutropenia, fatigue, diarrhea, and anemia (Table 4).
Conclusion
The development of optimal treatments to improve outcome for PTCLs remains a challenge given their rarity and biological heterogeneity. Nevertheless, in the last few years, there have been several trials initiated and quickly completed in PTCL, demonstrating that it is possible to conduct prospective trials in this rare group of diseases. The goal of future studies should be to focus on new combinations and the most active agents in PTCL, with the ultimate goal of improving upon CHOP alone in the primary treatment setting.
Disclosures
Conflict-of-interest disclosure: The author has served as a consultant for and received honoraria from Celgene and Seattle Genetics and served as a consultant for Allos Therapeutics. Off-label drug use: Use of the following drugs for the treatment of PTCLs: gemcitabine, alemtuzumab, etoposide, L-asp, bortezomib, brentuximab, denileukin diftitox, zanolimumab, lenalidomide, and belinostat.
Correspondence
Kerry J. Savage, BC Cancer Agency, 600 West 10th Ave, Vancouver, BC V5Z 4E6; Phone: (604) 877-6000; Fax: (604) 877-0585; e-mail: ksavage@bccancer.bc.ca.
