Abstract
The review summarizes the current understanding of the role of hepcidin and ferroportin in normal iron homeostasis and its disorders. The various approaches to therapeutic targeting of hepcidin and ferroportin in iron-overload disorders (mainly hereditary hemochromatosis and β-thalassemia) and iron-restrictive anemias (anemias associated with infections, inflammatory disorders, and certain malignancies, anemia of chronic kidney diseases, and iron-refractory iron-deficiency anemia) are also discussed.
Introduction: hepcidin, ferroportin, and iron disorders
Systemic iron homeostasis
In healthy humans, the concentration of iron in plasma and extracellular fluid is maintained at a relatively narrow range of 10-30 μM, ensuring that adequate iron is available for essential cellular functions without incurring iron toxicity.1 Plasma iron concentration is controlled by the hepatic peptide hormone hepcidin, which regulates the major iron flows into plasma: dietary iron absorption in the duodenum (1-2 mg/d), iron recycling from senescent erythrocytes (20 mg/d), and the recovery of iron from storage in hepatocytes and macrophages (a few milligrams per day depending on iron needs). Transferrin-bound iron exits the plasma compartment destined predominantly for the BM erythrocyte precursors, where it is incorporated into heme and hemoglobin. Smaller amounts of iron are taken up by other cells, where they are incorporated into myoglobin, redox enzymes, and other iron-containing proteins.
Hepcidin and ferroportin
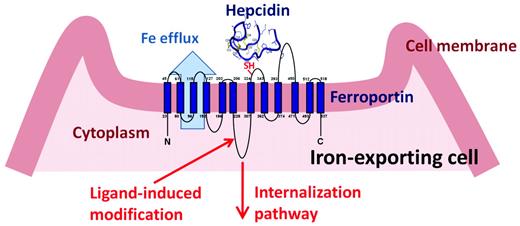
Hepcidin is a 25–amino acid peptide synthesized in hepatocytes as a larger inactive pre-prohepcidin composed of a signal peptide and 60–amino acid prohepcidin. Prohepcidin is then cleaved by the prohormone convertase furin to generate mature hepcidin. The hepcidin structure is a 4-disulfide–cross-linked beta-hairpin with an N-terminal arm that is highly conserved and essential for activity. The sole known molecular target of hepcidin is the protein ferroportin,2 which functions as a transmembrane conduit for the transfer of cellular iron to plasma. Most cells contain very little ferroportin and do not export iron, using it only for their own metabolic needs. The professional iron exporters, including macrophages, duodenal enterocytes, hepatocytes, and placental syncytiotrophoblasts, express ferroportin and provide iron for the entire organism. The binding of hepcidin to ferroportin on the membranes of iron-exporting cells induces the endocytosis and proteolysis of ferroportin and thereby decreases the delivery of iron to plasma2 (Figure 1). The specific pathways required for ferroportin internalization and degradation are an evolving area of investigation, but there is agreement that ferroportin undergoes ligand-induced ubiquitination. The cellular uptake of iron in its various forms (dietary elemental iron and heme for enterocytes, diferric transferrin, heme-hemopexin, hemoglobin-haptoglobin, and senescent erythrocytes for macrophages) is also subject to regulation, but it appears that the regulation of ferroportin expression on the cell membrane is the predominant mode through which iron transport into plasma is controlled.
After binding hepcidin, ferroportin is covalently modified, internalized, and degraded, decreasing cellular iron export.
After binding hepcidin, ferroportin is covalently modified, internalized, and degraded, decreasing cellular iron export.
Hepcidin regulation by iron
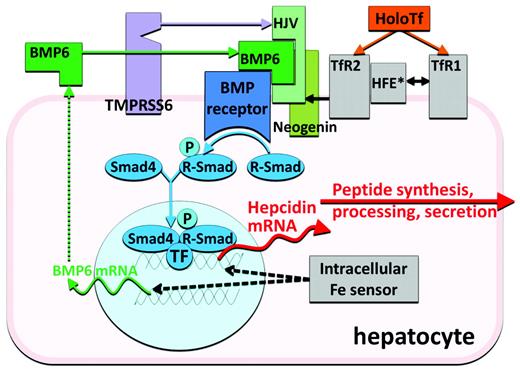
As would be expected of an iron-regulatory hormone, the production of hepcidin is homeostatically regulated by plasma iron concentrations and iron stores,3 predominantly through a transcriptional mechanism. Increased hepcidin release in response to increased iron concentrations generates a negative feedback loop that limits iron absorption and retains iron in stores. The regulatory mechanism centers on a bone morphogenetic protein receptor (BMPR) and its SMAD signaling pathway that regulates hepcidin transcription4 (Figure 2). The canonical pathway, which has other important roles in development and tissue remodeling, is adapted for iron regulation by its interaction with proteins specialized in iron sensing or iron-related signaling. BMP6 is an iron-regulated ligand with no other known function except the regulation of hepcidin expression5,6 Similarly, GPI-linked hemojuvelin (HJV)4,7 is the BMPR coreceptor involved solely in hepcidin regulation. HJV membrane expression is modulated by 2 other proteins. Matriptase 2 (also called TMPRSS6) is a transmembrane serine protease that degrades HJV, possibly in an iron-regulated manner,8 and thus is a negative regulator of the BMP pathway. Neogenin, a receptor for netrins, was also found to interact with HJV and BMPRs, although the specific link to iron sensing is still unknown. Finally, BMP pathway signaling is also adjusted by 2 potential sensors of holo-transferrin concentrations, transferrin receptors 1 and 2, and their interacting partner, transmembrane protein HFE.9 Increasing concentrations of holo-transferrin shift the interaction of HFE from TfR1 to TfR2, promote stabilization of the TfR2 protein, and enhance SMAD signaling. Although the important role of each of these proteins in hepcidin regulation is supported by the known effects of human and murine mutations on hepcidin regulation, the biochemistry of their interactions is only beginning to be uncovered.
Hepcidin regulation by erythroid factors
Low hepcidin concentrations are observed in iron-deficiency anemia, in hereditary anemias with ineffective erythropoiesis, and in mouse models of anemia due to bleeding or hemolysis. Substantial evidence points to the existence of a hepcidin-regulating signal originating in erythroid precursors in the BM.1 This “erythroid” factor, the biochemical nature of which is not yet known, physiologically suppresses hepcidin in proportion to the erythropoietic activity of the BM. Suppression of hepcidin in turn allows for greater availability of iron for erythropoiesis.
Hepcidin regulation by inflammation
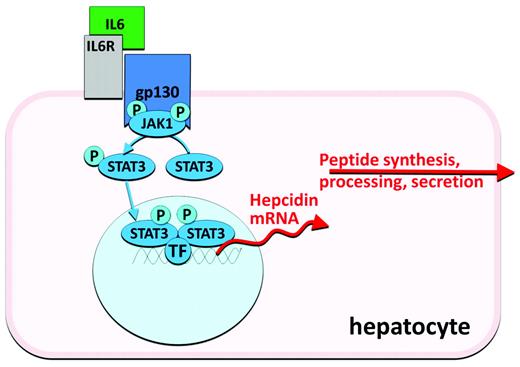
Infection and inflammation generate signals that dramatically increase hepcidin synthesis and release, resulting in the characteristic hypoferremia, restriction of iron flow to erythropoiesis, and anemia of inflammation (anemia of chronic diseases). Hepcidin transcription is particularly responsive to stimulation by IL-6, an inflammatory cytokine that activates the JAK-STAT pathway (Figure 3). After phosphorylation by JAK2, the STAT3 transcription factor binds to cognate motifs in the hepcidin promoter and increases hepcidin transcription.10 Other inflammatory cytokines and microbial products may also activate hepcidin transcription.
Hepcidin clearance
As a small peptide that does not appear to be strongly bound to plasma proteins, hepcidin is cleared from plasma by the kidneys and by ferroportin-mediated endocytosis and proteolysis. Accordingly, plasma hepcidin concentrations can also be increased by disease processes that diminish the clearance of hepcidin by the kidneys.11
Hepcidin deficiency in iron-overload disorders
Dysregulation of the hepcidin-ferroportin axis gives rise to 2 phenotypically opposite groups of disorders. In iron-overload disorders, ferroportin is hyperactive, stimulating intestinal iron absorption and the release of iron from macrophages, and causing an increase in plasma iron concentrations, transferrin saturation, and iron deposition in the liver and other organs. In hereditary hemochromatosis, increased ferroportin activity is caused by deficient hepcidin production due to genetic lesions that dysregulate the signals controlling hepcidin transcription, disrupt the hepcidin gene itself, or, in rare cases, make ferroportin resistant to internalization by hepcidin. In iron-loading anemias associated with ineffective erythropoiesis (eg, β-thalassemias and congenital dyserythropoietic anemias), hepcidin production is suppressed12 by a pathological signal13 from an expanded population of erythrocyte precursors that fail to mature to fully differentiated erythrocytes.
Hepcidin excess in iron-restrictive disorders
In iron-restrictive disorders, plasma hepcidin concentrations are excessive because of increased production of hepcidin driven by systemic inflammation,14,15 autonomous production by tumors,16 or genetic lesions in TMPRSS6/MT-2,17 the negative regulator of hepcidin transcription. Alternatively, hepcidin concentrations may increase because of decreased hepcidin clearance by diseased kidneys.11,18 Regardless of the specific cause, increased hepcidin concentrations inhibit iron absorption in the duodenum and restrict iron release from macrophages, limiting the flow of iron to hemoglobin synthesis and resulting in iron-restricted anemias. Other mechanisms, including shortened erythrocyte lifespan, direct suppression of erythropoiesis by cytokines, and diminished erythropoietin production may also contribute to anemia in these settings depending on the underlying disease.
Hepcidin agonists
Rationale for hepcidin agonists
In hereditary hemochromatosis, phlebotomy is an inexpensive and effective treatment for iron overload that is acceptable to most but not all patients. Conversely, iron-loading anemias cannot be treated in this manner and require iron chelation therapy, which is not well tolerated by many patients both because of toxic side effects (all current chelators) and the burden of parenteral administration (deferoxamine). Alternatives to existing treatments are needed. In both of these disorders, hepcidin agonists, agents that replace hepcidin activity or stimulate its endogenous production, would be expected to prevent iron accumulation. Although this strategy would not reverse established iron overload, it could diminish iron-mediated tissue injury by redistributing iron from parenchymal tissues to macrophages,19 where iron is less toxic. Recent studies in a mouse model of β-thalassemia suggest that hepcidin agonists could not only prevent iron overload but could also improve erythropoiesis,20 perhaps by decreasing excessive α-globin synthesis or diminishing oxidative stress in erythrocyte precursors.
Small peptides that mimic the action of hepcidin
The extracellular loop of ferroportin surrounding the thiol cysteine C326 appears to be essential for hepcidin binding (Figure 1), as indicated by the lack of hepcidin binding to otherwise fully functional ferroportin containing the isosteric mutation C326S.21 Structural and mutagenesis analysis of the interface between hepcidin and ferroportin showed that the segment composed of a 9 N-terminal amino acid segment of hepcidin is sufficient for binding and internalization of ferroportin.22 Further refinement by the introduction of unnatural amino acids and lipid/bile acid modification of this mini-hepcidin scaffold has generated peptides with molar activity that exceeds that of hepcidin. These small modified mini-hepcidin peptides show bioactivity in vivo, as determined by their ability to induce hypoferremia in mice and to prevent iron accumulation in hepcidin-deficient mice. When engineered for resistance to proteolysis and uptake by intestinal transporters, some of these peptides retain activity even when administered by gavage.22
Stimulants of hepcidin synthesis
In murine models, proof of principle has been demonstrated for 2 approaches to stimulating hepcidin production in patients with hepcidin deficiency. In the first, pharmacologic doses of BMP6, the natural ligand of the BMP receptor involved in hepcidin regulation, were shown to activate the pathway that regulates hepcidin transcription23 (Figure 2) and to correct the high iron saturation and iron maldistribution in the HFE model of hereditary hemochromatosis.23 Relatively high doses of BMP6 were required for this effect, and chronic administration caused peritoneal calcifications.23 In the second approach, transgenic inactivation of the membrane protease TMPRSS6 in HFE mice increased hepcidin expression and reversed their iron-overload phenotype,24 suggesting that the administration of a specific inhibitor of the enzymatic activity of TMPRSS6 could be used to treat hereditary hemochromatosis. Titration of the inhibition may be necessary because the HFE mice developed iron-restricted anemia when TMPRSS6 was completely inactivated.
Hepcidin antagonists
Rationale for hepcidin antagonists
Iron-restrictive anemias caused or exacerbated by hepcidin excess due to inflammation, renal failure, or genetic lesions are currently treated by a combination of erythropoiesis-stimulating agents (ESAs) and parenteral iron.25 Although these modalities are often effective, concerns about their side effects have spurred the search for alternatives. Hepcidin antagonists, agents that decrease hepcidin production or interfere with its effect on ferroportin, would be expected to relieve hepcidin-mediated iron restriction and to release more iron for erythropoiesis. Because iron-restrictive anemias are not life-threatening, the safety profile of effective hepcidin antagonists will be the key determinant of their therapeutic use. Mouse models have provided a proof of concept for the potential benefit of hepcidin antagonists, and several have reached the preclinical stage, with a few reaching human trials for iron-restrictive anemia indications. In principle, hepcidin antagonists can neutralize hepcidin-stimulating cytokines (eg, BMPs and IL-6), target the cytokine-signaling pathways (eg, STAT3 and BMPR-SMAD), stimulate erythropoiesis, bind and neutralize the hepcidin peptide (eg, antibodies and other binding molecules), prevent hepcidin binding to ferroportin, or interfere with ferroportin-internalization pathways.
Antagonists of BMPs or their pathways
The BMP pathway is at the core of transcriptional regulation of hepcidin synthesis by iron. Various sulfated proteoglycans are known to bind BMPs, and the glycosaminoglycan heparin was recently shown to decrease hepcidin concentrations in mice and in humans with treated for venous thrombosis.26 Other natural and modified natural BMP antagonists, including the BMP family protein noggin,27 the soluble form of the BMP coreceptor hemojuvelin,28,29 and the soluble ALK3 component of the type I BMP receptor,30 manifested hepcidin-lowering activity in vitro and/or in mouse models. Dorsomorphin and its congeners, synthetic small-molecule antagonists of the kinase activity of the BMP receptor, also lowered hepcidin in mouse models.30,31 BMPs and their pathways are involved in bone formation, wound healing, hematopoiesis, morphogenesis, and many other essential processes. It remains to be seen whether further development of anti-BMP agents can achieve sufficient activity and specificity for iron-related signaling to make them safe and useful for the treatment of anemia.
Antagonists of the IL-6 pathway
Reflecting the important role of IL-6 in inflammatory hepcidin regulation, anti-IL-6 strategies originally developed for other indications appear to have robust hepcidin-lowering effects.32,33 Although published reports used the anti–IL-6-receptor antibody, approaches targeting IL-6 itself34 or its signaling pathway35 would also be expected to suppress hepcidin when it is increased due to infection or inflammation. The beneficial effects of these agents on other manifestations of inflammatory diseases will facilitate studies of their effects on anemia in these settings. As these agents are introduced for human use in various inflammatory disorders, we will learn more about their safety profile and effects on host defense, and whether they are suitable as first-line agents for the treatment of anemia of inflammation.
Nucleic acid-based inhibitors of hepcidin synthesis
Antisense oligonucleotides and siRNAs directed against hepcidin or its regulators (Figures 2 and 3) may be well suited as inhibitors of hepcidin synthesis because of their preferential effect on hepatocytes. Other components of the hepcidin-regulatory network in hepatocytes could also be targeted by these approaches. Development programs in this area have been announced by Alnylam and the Isis/Xenon consortium.
ESAs
Erythropoietin and ESAs have been reported to suppress hepcidin production in animal models and in humans.36 Generation of the putative hepcidin-suppressing “erythroid factor” by developing erythrocytes is a likely mechanism mediating this effect. In addition, agents such as prolyl hydroxylase inhibitors37 may also reduce hepcidin expression by increasing the activity of hypoxia-inducible factor (HIF), a pathway proposed to be directly involved in the regulation of hepcidin transcription.38
Agents that bind and neutralize hepcidin
Human or humanized mAbs against hepcidin have been developed by scientists at Lilly (US Patent 7820163) and Amgen (US Patent Application 12/022515). Amgen's anti-hepcidin mAb reversed iron restriction and erythropoietin resistance in a humanized mouse model of anemia of inflammation,39 in which mouse hepcidin genes were replaced by a human hepcidin gene. Lilly's mAb (LY2787106) is already undergoing phase 1 human trials in cancer-related anemia. In addition to mAbs, other protein and nonprotein scaffolds could be used to developed hepcidin-trapping agents. Considering the high rate of hepcidin synthesis during inflammation, the main hurdle is whether it is technically and economically feasible to deliver effective amounts of these neutralizing drugs into the bloodstream. This concern could be addressed by developing agents that recycle after delivering their hepcidin cargo for degradation by macrophages.
Antagonists of hepcidin effect on ferroportin
Agents that block the hepcidin-binding site on ferroportin without inducing its internalization are also under development. Researchers at Lilly developed a mAb that targets the extracellular ferroportin loop adjacent to the hepcidin-binding site (WIPO Patent Application WO/2010/065496 and Figure 1). As LY2928057, the anti-ferroportin mAb is undergoing studies in healthy volunteers. Our group developed a high-content screen using cells expressing the ferroportin-GFP fusion protein to identify small molecules acting as hepcidin antagonists.40 We identified compounds that allow continuous iron export from cells in the presence of hepcidin. The small-molecule hepcidin antagonists include thiol modifiers that inhibit hepcidin binding to ferroportin. It remains to be seen whether any of these activities have sufficient selectivity to form a basis for drug development.
Conclusions
Considering the central role of the hepcidin-ferroportin axis in iron regulation and in the pathogenesis of common iron disorders, it is not surprising that this system has been targeted for drug development. In the coming years, these efforts are likely to yield new medications for the treatment of anemia and iron-overload disorders.
Disclosures
Conflict-of-interest disclosure: T.G. has been employed by, is on the board of directors or an advisory committee for, and has equity ownership in Intrinsic LifeSciences; has received research funding from Amgen and Xenon Pharmaceuticals; and has consulted for Alnylam, Pieris, Xenon Pharmaceuticals, and J&J/Ortho. E.N. has been employed by, is on the board of directors or an advisory committee for, and has equity ownership in Intrinsic LifeSciences and has consulted for Pieris and Centocor. Off-label drug use: None disclosed.
Correspondence
Tomas Ganz, Department of Pulmonary & Critical Care Medicine, University of California-Los Angeles, Box 951690, 37-055 CHS, Los Angeles, CA 90095-1690; Phone: (310) 825-6112; Fax: (310)-206-8766; e-mail: tganz@mednet.ucla.edu.