Abstract
Monitoring response to therapy for patients with chronic myeloid leukemia using an effective strategy is fundamental for achieving optimal patient outcomes. It will allow the initiation of timely therapeutic intervention for patients with a suboptimal response or kinase inhibitor therapy failure. Evidence is mounting that reaching molecular targets early in therapy is as important as the initial hematologic and cytogenetic response for the identification of patients who may have a poorer outcome. When the molecular target of a major molecular response is achieved at 18 months, patients reach a safe haven where loss of response is rare. However, this benefit is dependent on continuous drug adherence in most patients. As some patients reach their second decade of successful imatinib therapy, how long will frequent response monitoring be necessary? Assuming that very late relapse will be extremely rare for responding patients remaining on kinase inhibitor therapy, there are reasons for maintaining a regular molecular monitoring frequency, including monitoring adherence assessment and confirming sustained undetectable BCR-ABL1 for those considering a discontinuation trial and for late molecular recurrence in patients who maintain response after treatment discontinuation.
Introduction
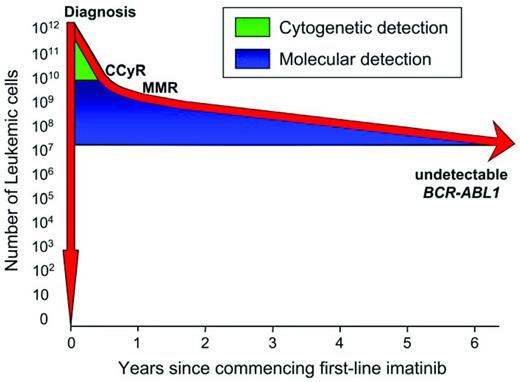
Successful BCR-ABL1 kinase inhibitor therapy is achieved by the majority of patients treated from diagnosis of chronic myeloid leukemia (CML). The Philadelphia chromosome disappears below the level of cytogenetic detection quite rapidly as differentiated leukemic cells are cleared. However, BCR-ABL1 mRNA levels remain detectable for many years and track a slow, biphasic exponential decline in most patients (Figure 1), which represents the turnover rate of leukemic progenitor cells.1–4 Therefore, molecular assessment is important as an essential component of response monitoring along with hematologic and cytogenetic assessment.5 An optimal response to imatinib is considered a stable or improving major molecular response (MMR). This represents an approximately 1000-fold or more clearance of leukemic cells, and the majority of patients with this response are protected from progression to accelerated phase or blast crisis.1 Nevertheless, long-term molecular monitoring remains important. A very small minority of patients will develop resistance and require therapeutic intervention. In these cases, BCR-ABL1 mutation analysis is necessary because mutations are commonly associated with and serve as a marker for resistance. Loss of molecular response in the absence of BCR-ABL1 mutations is problematic, because it may either be related to biologic resistance or due to nonadherence to therapy. The kinetics of a BCR-ABL1 increase could help to distinguish these situations, and BCR-ABL1 levels that fluctuate significantly may be a consequence of intermittent kinase inhibition associated with nonadherence. For patients with long-term optimal response, perhaps the frequency of monitoring can be reduced. However, this may have the potential to be misinterpreted by a responding patient, who may think that drug adherence can also be relaxed. For patients considering a trial of treatment cessation, molecular monitoring is essential to ensure that the qualifying discontinuation criteria are met and for the early detection of molecular recurrence.6,7
Molecular monitoring can detect residual disease during many years of imatinib therapy. There are an estimated 1012 leukemic cells present at diagnosis of CML. The Philadelphia chromosome remains detectable by cytogenetic analysis until the leukemic cells decline by approximately 2 logs, which occurs at an estimated median of 6 months of imatinib. Therefore, the Philadelphia chromosome may never be detectable beyond 6 months in a substantial number of patients. However, we have found that BCR-ABL1 transcripts remain detectable in approximately half of the patients for 6 years and molecular monitoring continues to track the leukemic cell decline. The limit of detection of BCR-ABL1 by RQ-PCR is reached when the leukemic cells decline approximately 4.5-5 logs.
Molecular monitoring can detect residual disease during many years of imatinib therapy. There are an estimated 1012 leukemic cells present at diagnosis of CML. The Philadelphia chromosome remains detectable by cytogenetic analysis until the leukemic cells decline by approximately 2 logs, which occurs at an estimated median of 6 months of imatinib. Therefore, the Philadelphia chromosome may never be detectable beyond 6 months in a substantial number of patients. However, we have found that BCR-ABL1 transcripts remain detectable in approximately half of the patients for 6 years and molecular monitoring continues to track the leukemic cell decline. The limit of detection of BCR-ABL1 by RQ-PCR is reached when the leukemic cells decline approximately 4.5-5 logs.
Initial monitoring requirements for an optimal response
The European LeukemiaNet (ELN) and The National Comprehensive Cancer Network (NCCN) have produced recommendations and guidelines for monitoring and assessing the response to kinase inhibitor therapy, which include hematologic, cytogenetic, and molecular monitoring.5,8 Molecular monitoring involves real-time quantitative PCR (RQ-PCR) assessment of BCR-ABL1 transcript levels and mutation analysis in specific situations. The aim of monitoring is the prevention of disease progression to the accelerated phase or blast crisis by the initiation of timely therapeutic intervention for patients with a suboptimal response or treatment failure. Successful therapeutic intervention is less likely and more complex once the disease progresses beyond the chronic phase.
Lack of achievement of time-dependent treatment milestone responses are trigger points for therapeutic decisions.5,8 In Australia, clinical trials of newly diagnosed CML patients have used a more aggressive intervention approach by shifting therapeutic decision milestones to earlier time points.9,10 Intervention included dose escalation or rapid switching to nilotinib for failure to achieve BCR-ABL1 < 10% on the international reporting scale (IS) by 3 months of imatinib. The aim was to increase the number of patients who achieved an optimal response and to reduce the risk of disease progression. Maintenance of an average imatinib dose intensity of 600 mg/d in the first 6 months of therapy was found to be important for improved responses,9 as was intervention based on the time-dependent molecular targets (BCR-ABL1 10% IS, 1% IS, and 0.1% IS [MMR] at 3, 6, and 12 months, respectively). A BCR-ABL1 value that remains above 10% IS correlates with lack of a major cytogenetic response,11 and failure to reduce BCR-ABL1 below this level by 3 and 6 months is predictive of poorer outcome.1,12,13 High-risk patients were also those in whom BCR-ABL1 remained above 1% IS at 6 months.12 These studies confirm that rapid intervention at early time points is important, and future updates of management recommendations may indeed incorporate these concepts and shift treatment decision milestones to earlier time points.
An important assessment that may be overlooked is molecular analysis using RQ-PCR at diagnosis. The BCR-ABL1 value at diagnosis has no proven prognostic significance. Cytogenetic analysis can validate the diagnosis of CML and is essential in detecting additional abnormalities that may be a marker of a more advanced disease; however, molecular assessment is essential in establishing the BCR-ABL1 transcript type. Rare patients have atypical BCR-ABL1 break points that will not be detectable by standard techniques. Unless the transcript type is determined while the BCR-ABL1 levels remain easily detectable, there is the risk of subsequently reporting a false-negative result. This may be particularly important for patients treated with nilotinib or dasatinib as first-line therapy, because these patients have a more rapid clearance of leukemia.14,15
Continued close monitoring for 3-4 years after commencing therapy is extremely important, because patients are then at their highest risk of loss of response, even those with an apparent optimal response.14 Testing for kinase inhibitor–resistant BCR-ABL1 mutations in the first years of treatment, when appropriate according to recommendations,5,8 uncovers the highest incidence of mutations. The probability of mutations arising depends on the number of cells at risk. Nevertheless, BCR-ABL1 mutations have been detected on occasion in patients who achieved an MMR.4 Our long-term experience in monitoring imatinib-treated patients indicates that the chance of detecting a mutation declines with an increasing duration of therapy. Indeed, mutations were rarely detected in patients remaining on therapy for more than 3 years who met the criteria for mutation screening.4 When considering therapeutic options for a patient who has failed therapy, mutation analysis should be performed to aid in making the appropriate therapy choices.16,17 In addition, it is highly recommended that mutation analysis be performed before a patient ceases inhibitor therapy in cases where a switch of inhibitor is being considered, otherwise mutations may be deselected in the absence of kinase inhibition.18,19 In this case, low-level mutations below the detection limit of conventional sequencing could be selected with subsequent inhibitor resistance.20
An infinite increase of BCR-ABL1 from undetectable to values of around 0.001% IS is meaningless in the absence of loss of an MMR. In other cases, increasing BCR-ABL1 transcripts are recognized as a warning for pending loss of response and a trigger for mutation analysis.5,8 However, the magnitude of the fold increase considered to be of clinical consequence remains controversial. We know that measurement reliability varies between RQ-PCR methods, meaning that some methods generate more variable results than others. This should not be a substantial problem if laboratories are aware of the amount of change in serial BCR-ABL1 measurements that indicates a true biologic change that is readily distinguishable from assay variation. If this is known, clinicians can be advised of the significance of an increase and a repeat analysis suggested. However, determining the measurement reliability of a molecular technique is not a trivial undertaking. Furthermore, the significance of an increase is dependent on the duration of the measurement interval because relapse kinetics vary with the growth rate of the leukemic cells. We found that a rapid BCR-ABL1 increase was associated with blast crisis relapse or loss of kinase inhibition due to imatinib cessation, whereas a slow increase was associated with the detection of BCR-ABL1 mutations and maintenance of chronic phase.21 Overlooking a small increase (eg, 4-fold) that occurs over a short measurement interval of 4 weeks using a method with minimal variability could potentially mean that an early indication of loss of response is missed. In this case, the exponential nature of the increase indicates that BCR-ABL1 is doubling approximately every 15 days.21 In a patient with a BCR-ABL1 value of 1% IS and a 15-day BCR-ABL1 doubling-time, the loss of a major cytogenetic response could occur within 2 months. This suggests the duration of time over which BCR-ABL1 is measured should be considered when assessing the significance of an increase.
How long should responding patients be monitored?
When a patient achieves a confirmed complete cytogenetic response (CCyR), the recommendation is that BM cytogenetic assessment be reduced or replaced by molecular monitoring.5 The NCCN guidelines for patients with a CCyR are to monitor BCR-ABL1 transcripts by RQ-PCR every 3 months for 3 years and then every 3-6 months thereafter.8 The International Randomized Study of Interferon and STI571 (IRIS) study, in which molecular monitoring was performed in 4 different laboratories, demonstrated a close concordance between a BCR-ABL1 value of ≤ 1% IS and a CCyR, suggesting that molecular monitoring provides a reasonable surrogate for this cytogenetic response.1 Of course, this is dependent on the use of a molecular method that is adequately standardized to the IS.22,23 Despite many years of working toward international harmonization of BCR-ABL1 assessment, it has proved to be a complex process and is currently unavailable for many laboratories. Nevertheless, for patients with an MMR, there is little value in performing cytogenetic analysis. In a study of more than 300 samples from patients with an MMR, there were no cytogenetic abnormalities detected in the corresponding cytogenetic analyses.11 Indeed, an MMR at 18 months of first-line imatinib in the IRIS study protected patients from disease progression to accelerated phase or blast crisis at 7 years of therapy and the event-free survival was 95%.1 Only 3% of these patients lost a CCyR. With appropriately standardized molecular methods, in the future it may be possible to limit cytogenetic analysis to the diagnosis time point unless loss or lack of response is indicated.
Strict observance of the monitoring recommendations and guidelines will entail life-long molecular analysis for patients treated with kinase inhibitors. As the years pass for long-term responders who remain on kinase inhibitor therapy, it would be very tempting to reduce the frequency of monitoring. However, molecular monitoring will remain important for these patients for several reasons, including an evaluation of drug adherence, to assess eligibility for a discontinuation trial and for the detection of possible late relapse. Molecular monitoring has been ongoing for more than 10 years for imatinib-treated patients and relapse is rare, nevertheless, data are needed for a demonstration of very long-term protection from relapse.
Molecular analysis for an assessment of drug adherence
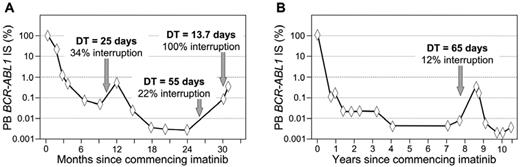
The first patients treated with imatinib as front-line therapy were those enrolled in the IRIS study in 2000 and 2001. After 8 years of follow-up, 55% of these patients remained on imatinib and many had an optimal response.1 Our laboratory has monitored a small proportion of the IRIS study patients since therapy was commenced, and we were surprised to detect loss of molecular response and a greater than 10-fold increase of BCR-ABL1 in a long-term optimal responder after 8 years of therapy.24 This was not a case of resistance; rather, the patient admitted to ceasing therapy for 6 weeks when the molecular relapse was reported (Figure 2B). Studies have demonstrated that poor adherence is the main reason for loss of CCyR for patients on long-term therapy,25 and a BCR-ABL1 increase may be a marker of nonadherence, particularly in the absence of other markers of biologic resistance such as BCR-ABL1 kinase domain mutations.21
Molecular patterns associated with imatinib dose interruption. Consecutive days of zero dose, amounting to at least 10% of days of a BCR-ABL1 measurement interval, is likely to lead to a BCR-ABL1 increase. The dose interruptions in both examples were evident at the molecular level, but without an increase above 1% IS. The molecular equivalent of Philadelphia chromosome detection is above 1% IS. The BCR-ABL1 doubling time (DT) at the increase demonstrates an inverse relationship with the length of the dose interruption. The interruptions were the decision of each patient and were declared to their clinicians. Recommencing imatinib led to rapid reduction of BCR-ABL1.
Molecular patterns associated with imatinib dose interruption. Consecutive days of zero dose, amounting to at least 10% of days of a BCR-ABL1 measurement interval, is likely to lead to a BCR-ABL1 increase. The dose interruptions in both examples were evident at the molecular level, but without an increase above 1% IS. The molecular equivalent of Philadelphia chromosome detection is above 1% IS. The BCR-ABL1 doubling time (DT) at the increase demonstrates an inverse relationship with the length of the dose interruption. The interruptions were the decision of each patient and were declared to their clinicians. Recommencing imatinib led to rapid reduction of BCR-ABL1.
Treatment interruption and nonadherence seem to be prevalent.26,27 Adverse effects of imatinib therapy are a driver of nonadherence and include rash, edema, and weight gain.26,28 A higher starting dose of imatinib, a greater number of concomitant medications, and greater disease complexity were also associated with interruptions.26 Interestingly, when nonadherent patients were interviewed, there was a reliance on response monitoring to detect changes in clinical measurements that were adversely associated with their nonadherence.28 If no adverse effect is reported for a nonadherent patient, it may be a signal for continued nonadherence. Therefore, an early marker of nonadherence for a clinician unaware of their patient's compliance issue would be of benefit. We have investigated the molecular changes associated with imatinib interruption or discontinuation. There was a strong association between an interruption of at least 10% of days of a measurement interval and a BCR-ABL1 increase and loss of response.21 Furthermore, complete dose interruption was associated with characteristic kinetics of the BCR-ABL1 increase, as indicated by consistently short BCR-ABL1 doubling times. The kinetics were no different from those observed with molecular recurrence in discontinuation trials.6,7 A short doubling time in a patient in chronic phase may be a marker of nonadherence. The doubling times were more variable for patients with partial dose interruption. A longer interruption during a BCR-ABL1 measurement interval led to a shorter doubling time. More frequent molecular monitoring may be warranted when there is a suspicion of nonadherence. An unexplained transient BCR-ABL1 increase may be an indicator of dose interruption.21,29
Molecular analysis is essential for trials of kinase inhibitor discontinuation
Achieving undetectable BCR-ABL1 at a certain sensitivity level has become an important response. This is not due to strong evidence linking the response to improved event-free or overall survival, which would potentially require molecular monitoring of thousands of patients in a clinical trial. Instead, achieving sustained undetectable BCR-ABL1 using strict sensitivity criteria was used to select patients for participation in trials of kinase inhibitor discontinuation.6,7,30–32 More sensitive methods have been used to detect residual disease in patients with undetectable BCR-ABL1, such as DNA PCR,7,33 nanofluidic digital PCR,34 and replicate RQ-PCR.35 Interestingly, however, the detection of residual disease at a greater sensitivity than conventional RQ-PCR did not predict molecular recurrence after discontinuation.7,35 Therefore, is sustained undetectable BCR-ABL1 before discontinuation essential for continued response after discontinuation? Could an occasional low-positive BCR-ABL1 value be of minimal consequence during the qualifying period for discontinuation or, indeed, after discontinuation? Upcoming discontinuation studies may address these questions.
Monitoring monthly after discontinuation can detect molecular recurrence, which generally occurs within the first 6 months after discontinuation.6,7 However, molecular recurrence has been reported as late as 42 months after imatinib discontinuation.36 This raises the question of how long and how frequently to monitor patients who sustain response after discontinuation. Recent epidemiologic modeling from radiobiologic datasets suggests that there could be highly variable latency times for CML and unpredictable relapses could occur, even after decades.37 Until long-term follow up data of patients who discontinue kinase inhibitor therapy are available, such patients should be monitored regularly using molecular techniques.
How should the molecular response be defined as more patients achieve deep responses?
As the importance of deep molecular response takes center stage in many clinical situations, it may be time to reconsider the definitions of response.38 It has long been recognized that a sample returning an undetectable BCR-ABL1 value assessed using molecular techniques does not equate to an absence of leukemia, because several million leukemic cells could potentially be present (Figure 1). Furthermore, the ability to detect low BCR-ABL1 levels is dependent on several technical factors associated with the molecular method, as well as the degradation status of the RNA sample. The rapid degradation of BCR-ABL1 mRNA means that a delay in processing or stabilizing a sample by 24-36 hours could lead to a loss of signal below the level of detection in a patient with a deep molecular response.39 This will essentially lead to a false-negative result, meaning that BCR-ABL1 could have been detectable if degradation had not occurred. Nevertheless, the term “complete molecular response,” often abbreviated to CMR, has been a convenient pseudonym. Defining the sensitivity level achieved with individual samples aids in the interpretation of undetectable BCR-ABL1. A limit of sensitivity of more than 4.5 logs below the standardized baseline was used to define undetectable BCR-ABL1 in the IRIS study and to select patients for the Australian imatinib discontinuation trial.7 It is considered a deep molecular response and has been adopted as an end point in subsequent clinical trials of more potent kinase inhibitors.14,15,40 The molecular response level now equates to a value of ≤ 0.0032% IS. The term CMR4.5 was used to define the response in the trial of nilotinib in newly diagnosed patients.14 However, in this case, patients with detectable BCR-ABL1 of ≤ 0.0032% IS were also counted as having achieved CMR4.5. This highlights the unsuitability of the term and may contribute to confusion in interpretation.
To overcome the issue of how to define deep responses, Cross et al have suggested standardized definitions of molecular response.38 CMR will be replaced with MR (molecular response) at a certain sensitivity level. MR4.0 equates to BCR-ABL1 ≤ 0.01% IS, MR4.5 to BCR-ABL1 ≤ 0.0032% IS and MR5.0 to BCR-ABL1 ≤ 0.001% IS. These definitions will encompass samples that achieve this level of sensitivity irrespective of whether BCR-ABL1 is detectable. When BCR-ABL1 is undetectable, certain control gene transcript levels must be attained for each response level. However, further method standardization may be required to adopt specific transcript values according to the control gene used. It is suggested that the term MMR (BCR-ABL1 ≤ 0.01% IS, MR3.0) be retained because it is an established term. These are rational recommendations and will doubtless be widely adopted.
Is molecular monitoring widely accepted?
Because a molecular end point is considered by a panel of experts to be an optimal response to kinase inhibitor therapy, it would be intuitive to assume that standardized molecular techniques are widely available and adopted. This is the case in Australia and Europe, but less so in the United States, although an increasing number of laboratories have converted their data to the IS.41 A survey conducted between November 2005 and January 2006 found a discrepancy between the monitoring practices of European and US clinicians.42 At that stage, the follow-up of the IRIS study was only 5 years. In the United States, commercial laboratories were used by 50% of respondents, which was far in excess of Europeans. There were several possible explanations for the discrepancy, including affiliations between health insurers and commercial laboratories. European clinicians were more familiar with tests for BCR-ABL1 kinase domain mutations, whereas some US clinicians were neither familiar with mutation testing or RQ-PCR analysis to monitor CML patients. In 2012, it is anticipated that both European and US clinicians are now more familiar with—and more frequently use—molecular tests to monitor their patients. For US clinicians to embrace molecular techniques, consistency of reporting is essential, but may not be readily available yet and further work is required.
Summary
As the prevalence of CML increases due to the outstanding success of kinase inhibitor therapy for prolonging survival, the requirement for good-quality, sensitive, and standardized molecular monitoring of residual disease will expand. Long-term monitoring of patients with an optimal response will continue to be necessary for many patients. The exciting potential for safe treatment discontinuation will be reliant on close molecular monitoring to aid the appropriate selection of patients for a cessation trial and for prompt restart of therapy for molecular recurrence. Nonadherence may become more frequent as some patients face life-long therapy with bothersome side effects that affect their quality of life. In some circumstances, molecular monitoring can aid in the assessment of adherence because a BCR-ABL1 increase may be a marker of the degree of kinase inhibition and adherence to therapy during the prior measurement interval. The refinement of the terms to describe molecular responses should aid in the interpretation of results.
Disclosures
Conflict-of-interest disclosure: The author is on the board of directors or an advisory committee for Novartis and Ariad; has received research funding from Novartis, Bristol-Myers Squibb, and Ariad; has consulted for Cepheid; and has received honoraria from Novartis, Bristol-Myers Squibb, and Ariad. Off-label drug use: None disclosed.
Correspondence
Susan Branford, Department of Genetics and Molecular Pathology, SA Pathology, PO Box 14 Rundle Mall, Adelaide, South Australia, 5000, Australia; Phone: 61-08-8222-3899; Fax: 61-08-8222-3146; e-mail: susan.branford@health.sa.gov.au.