Abstract
An 80-year-old man has newly diagnosed chronic myeloid leukemia. His BM and blood examination at diagnosis confirms chronic-phase disease, with the Philadelphia chromosome as the sole cytogenetic abnormality. He has intermediate Sokal and Hasford risk,1 and is started on imatinib 600 mg once daily. He lives 5 hours away from the nearest specialist hematology service and prefers followup with his local physician, who cannot perform BM examinations. In patients such as this, is it acceptable to monitor his therapeutic response solely with molecular studies of his peripheral blood?
Introduction
Tyrosine kinase inhibitors (TKIs) have markedly improved outcomes for patients with chronic-phase chronic myeloid leukemia (CML), beginning with the widespread use of imatinib2,3 and now with the broad availability of nilotinib4 and dasatinib.5 These agents allow the majority of CML patients to achieve an ever greater reduction in disease burden and prolong survival compared with the previous gold standard of interferon.6 However, suboptimal treatment response and treatment failure still occur. Ideally, an optimal monitoring strategy would provide prognostic information and separate good-risk from bad-risk patients at an early stage.
As demonstrated by the International Randomized Study of Interferon and STI571 (IRIS) study, cytogenetic analysis of BM metaphases by G-banding is well established as a monitoring technique in imatinib-treated CML patients and is well correlated with clinical outcome.3 However, its lack of sensitivity in patients with low disease burden led to the development of quantitative PCR (RQ-PCR) as a monitoring tool, which can at its best detect the presence of disease at 0.001% when optimized.7 RQ-PCR is also more convenient from a patient perspective because it only involves peripheral blood sampling and has been adopted as the end points of phase 3 TKI studies.5,8
Methods
We sought to review response monitoring and long-term survival in chronic-phase CML patients treated with TKIs in the frontline setting. A PubMed literature search was performed using the MeSH terms “Fusion Proteins, bcr-abl” OR “Leukemia, Myelogenous, Chronic-Phase” AND “Piperazines” (the MeSH term that encompasses TKIs), and excluding the terms “resistance,” “resistant,” “failure,” “acute lymphoblastic leukemia,” “accelerated phase,” and “blast crisis.” Publications were restricted to phase 2 and phase 3 clinical trials and randomized controlled trials. The search resulted in 45 citations and 24 papers on 15 suitable trials were retrieved.9–32 The remaining citations were excluded because they described auxiliary outcomes of the associated trials (eg, pharmacogenomic and pharmacokinetic or quality-of-life data),33–40 described diseases other than newly diagnosed chronic-phase CML,41–48 were not in English,49 or did not include TKIs as the main therapy.50–53 Three other studies were accessed from references cited by other papers: the DASISION,54 French SPIRIT,55 and PETHEMA studies.56 Relevant results of these studies from conference proceedings57–59 and follow-up publications were included.60–63
Any prognostic information relating cytogenetic or molecular response to disease outcome, such as landmark analyses of progression-free and overall survival were collected from the selected trials. The correlation between survival and the achievement of complete cytogenetic response (CCyR) or different levels of molecular response on the International Scale (IS)7 are collated in Table 1.
Association of overall survival (OS) and achievement of time-dependent molecular and cytogenetic milestone responses
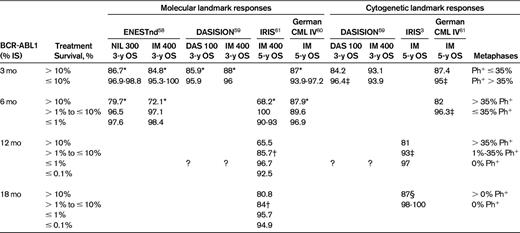
Deeper molecular responses were significantly associated with better OS, similar to the differential rates of OS observed when patients were segregated according to cytogenetic responses achieved on therapy. OS and progression-free survival (PFS) comparison between CCyR vs no CCyR in both the dasatinib and imatinib arms were significant, as was major molecular response (MMR) vs no MMR. Ph+ indicates the number of Philadelphia-positive metaphases in a BM cytogenetic preparation by G-banding; DAS 100, dasatinib 100 mg once daily; IM 400, imatinib 400 mg once daily; NIL 300, nilotinib 300 mg BID; and ?, values not published.
*Statistically significant comparison between BCR-ABL > 10% and ≤ 10% at P < .05.
†Statistically significant comparison between BCR-ABL > 1% and ≤ 1% at P < .05.
‡Statistically significant comparison between Ph+ > 35% and ≤ 35% at P < .05.
§Statistically significant comparison between CCyR vs no CCyR at P < .05.
Results
No published data allow for the conversion of cytogenetic responses into an equivalent molecular response expressed as BCR-ABL1%, although there is excellent correlation between patients achieving CCyR and having BCR-ABL1 < 1% (IS).57,61 Landmark analyses available from DASISION,59 ENESTnd,58 IRIS,61 and German CML IV60 studies covering all 3 currently available TKIs did report survival and its correlation to both cytogenetic and molecular response in the same cohort and these results are summarized in Table 1. There was good discriminatory value in the published data between differing levels of cytogenetic response and long-term outcome in patients treated with TKIs. The correlation between survival and molecular response was also significant: patients with worse outcomes may be identified as early as 3 months and at subsequent time points; BCR-ABL1 of > 10% at 3 months appears to identify a particularly poor-risk group.58,59 It is interesting that the outcomes stratified by treatment response were independent of TKI chosen for therapy, although patients treated with more potent TKIs were more likely to achieve early therapeutic responses58,59 (data not shown).
Discussion
For the majority of patients with chronic-phase CML with the Philadelphia chromosome as the sole cytogenetic abnormality at diagnosis, it is adequate to rely primarily on molecular studies for disease monitoring if there is access to a laboratory with good quality assurance and the ability to report as IS (grade 2A).64 This will identify a subset of poor-risk patients who fail to achieve an optimal level of molecular response (eg, patients with BCR-ABL1 > 10% at 3 months) and need cytogenetic analyses to assess for the possibility of clonal evolution or disease progression associated with treatment failure. Cytogenetics have very little to add for patients who meet all their treatment targets.
The 5%-10% of patients who have additional cytogenetic abnormalities (ACAs) at diagnosis should have both cytogenetic and molecular tests, at least until achievement of CCyR or major molecular response (BCR-ABL1 ≤ 0.1% IS), and disappearance of ACAs (grade 1C). Data from the German CML IV study suggest that certain abnormalities (second Philadelphia chromosome, trisomy 8 or 19, and isochromosome 17q) had significantly worse 5-year progression-free and overall survival.63 These patients took longer to reach CCyR and major molecular response compared with patients without ACAs; this was confirmed by an Italian study.62 There is, however, no evidence to suggest that once these patients reach a milestone, it is any less durable.63
Acknowledgments
The authors thank Professor Timothy Hughes for his valuable input and critical review of the manuscript.
Disclosures
Conflict-of-interest disclosure: D.T.Y. has received research funding and honoraria from Novartis Pharmaceuticals and Bristol-Myers Squibb. S.B. is on the board of directors or an advisory committee for Novartis and Ariad; has consulted for Cepheid; and has research funding and honoraria from Novartis, Bristol-Myers Squibb, and Ariad. Off-label drug use: None disclosed.
Correspondence
Dr David Yeung, Department of Haematology, SA Pathology, IMVS, PO Box 14, Rundle Mall, Adelaide, SA 5000, Australia; Phone: 61-8-8222-3942; Fax: 61-8-8222-3037; e-mail: david.yeung@adelaide.edu.au.
