Abstract
The use of umbilical cord blood (CB) as a source of hematopoietic progenitor cells for patients with high-risk hematologic disorders receiving allogeneic hematopoietic cell transplantations (HCTs) has increased significantly. Single-institution and registry studies have shown a decreased relapse rate and an increased transplantation-related mortality rate with similar overall survival rates after allogeneic HCT with CB compared with other donor sources. The transplantation of double CB units has overcome the dose limitation inherent in a single CB unit and thus has markedly extended the use of CB to larger children and adults. Similarly, the use of reduced intensity conditioning in the CB transplantation setting has allowed the treatment of older patients who would be unable to tolerate the myeloablative regimens used in the original CB transplantation protocols.
Introduction
In recent years, there has been exciting progress in the use of umbilical cord blood (CB)–derived immune cells in the hematopoietic cell transplantation (HCT) setting. There are now several strategies designed to reduce graft-versus-host disease (GVHD) and to improve CB homing, engraftment, and immune reconstitution. These novel approaches will likely change the current application of CB transplantation (CBT) with the goal of producing even better clinical outcomes.
Only 30% of patients who need allogeneic HCT have a matched sibling donor. The National Marrow Donor Program (NMDP), which was established in 1986, and its cooperative international registries have 16 million volunteer donors.1 It is estimated that 60% of white patients, but only 20%-45% of African-American and other minority patients, will be able to find a suitably matched unrelated donor (MUD) and proceed to transplantation. Therefore, there are an estimated 5000 patients per year who are candidates for alternative donor HCT. Their options include mismatched related (often haploidentical), CB, or mismatched unrelated donors (MMUD).
Since the first CBT was performed in 19892 to treat a child with Fanconi anemia, CB has emerged as an alternative source of hematopoietic stem cells.3 Expanding on the success in pediatric CBT pioneered by Drs Kurtzberg, Gluckman, Wagner, Broxmeyer, and others,4,5 the field grew rapidly. As of 2011, more than 25 000 CBTs have been performed worldwide and more than 500 000 CB units have been donated for public use. CB is readily available and donors can be found for diverse patient populations.6
The use of CB for HCT provides some potential advantages compared with the use of bone marrow (BM) or mobilized peripheral blood progenitor cells (PBPCs). Advantages include ease of collection, with little to no risk to the mother or newborn; prompt availability, with patients receiving CBT a median of 25-36 days earlier than those receiving unrelated BM7 ; low risk of infection transmission; decreased stringency of human leukocyte antigen (HLA)–matching requirements; and the relatively lower risk of GVHD with preserved graft-versus-malignancy effects. In contrast to BM or PBPCs, which generally require a high degree of HLA match between donor and patient,8 this reduced incidence of GVHD with partially HLA-mismatched CB is likely due to the lower numbers of T cells and the relatively immunologically naive status of the lymphocytes in CB.9
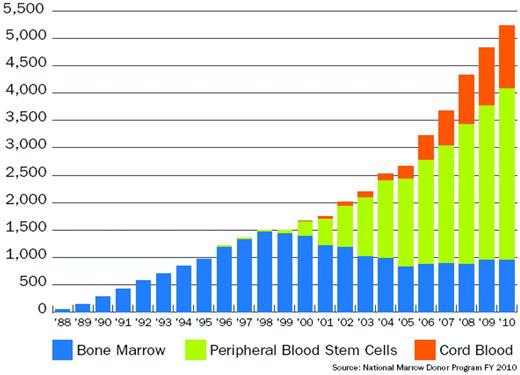
CB is now an accepted source of allogeneic HCT. Figure 1 presents the growth in the number of BM transplantations, PBPC transplantations, and CBTs facilitated by the NMDP worldwide in pediatric and adult patients over time.
NMDP transplantations by hematopoietic cell source. In 2010, more than 1150 CBTs were facilitated by the NMDP, which represents 22% of the total number of NMDP transplantations in 2010.
NMDP transplantations by hematopoietic cell source. In 2010, more than 1150 CBTs were facilitated by the NMDP, which represents 22% of the total number of NMDP transplantations in 2010.
CBT in pediatric patients
Although several studies have demonstrated a benefit of CBT in children with hematological malignancies,10–15 the first prospective, multicenter trial of CBT in 191 pediatric patients with hematologic malignancies was reported by Kurtzberg et al on behalf of the Cord Blood Transplantation Study (COBLT).16 The overall survival (OS) of the study population, 77% of whom had high-risk disease, was 57.3% at 1 year. Those results were compared favorably with those published from registry and single-center data showing disease-free survival (DFS) of 50%-60% in those with early-stage disease and 10%-30% in those with more advanced and active disease. A landmark study was reported by Eapen et al on behalf of The Center for International Blood and Marrow Transplant Research (CIMBTR).17 That study compared the outcomes of 503 children under the age of 16 years with acute leukemia who had received transplantation with HLA-matched (4-6/6) CB with outcomes of 282 HLA-matched (7-8/8) unrelated donor BM recipients. CBT compared favorably to the gold standard of 8/8 allele-matched unrelated BM transplantation, supporting the use of HLA-matched or HLA-mismatched CBT in children with high-risk acute leukemia without a matched related donor (MRD). The recipients of 1-antigen–mismatched CB units with a lower cell dose had engraftment similar to 2-antigen–mismatched units, whereas 1-antigen–mismatched CB units with a higher cell dose had superior engraftment, indicating that cell dose partially compensated for the degree of HLA mismatch.
There has been an increasing interest on the use of CBT for the treatment of metabolic diseases, hemoglobinopathies, and immune deficiencies in children.18–22 Kurtzberg et al has pioneered the use of CBT with very encouraging preliminary results in children with inherited metabolic disorders,18,23 including Krabbe disease and Hurler syndrome. Registry studies also showed acceptable outcomes after CBT in patients with severe combined immune deficiency compared with other donor sources.24
CBT in adults
The first large series of adults receiving CBT was reported by Laughlin et al in 2001.25 This analysis of 68 heavily pretreated patients with advanced hematologic malignancies demonstrated the feasibility of performing CBTs after myeloablative conditioning (MAC) in adult patients, with an event-free survival (EFS) of 26%. Similar to what had been described in pediatric series, a higher cryopreserved nucleated cell dose (≥ 2.4 × 107/kg) was associated with faster and a higher probability of neutrophil recovery and a higher cryopreserved CD34+ cell dose (≥ 1.2 × 105/kg) was associated with a better EFS rate in this groundbreaking study. Neither patient age nor HLA matching appeared to influence EFS. Cornetta et al subsequently reported a 30% 6-month survival rate for the COBLT prospective study of 34 adult patients (median age, 34 years) who received MAC for advanced malignancies.26 Over time, results of CBTs have been improving. Two large registry studies compared disease outcomes for adults after CB or unrelated BM transplantation with MAC. Rocha et al observed similar leukemia free survival (LFS), transplantation related mortality (TRM) and relapse incidence despite delay of engraftment in CB recipients with acute leukemia who received MAC compared with results in recipients of MUD transplantations.27 Laughlin et al observed higher TRM and shorter LFS with CB compared with 6 of 6 HLA-matched MUD, whereas the LFS was similar to 5/6 HLA-matched MUD.28 None of those studies compared CB with PBPC transplantations.
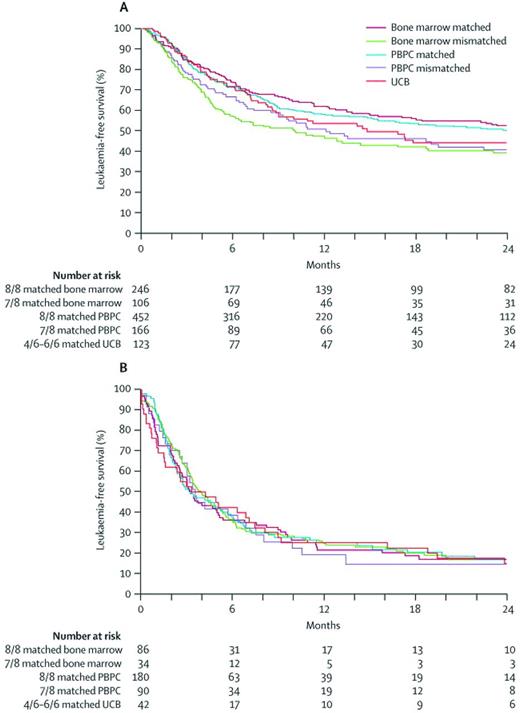
Recently, Eurocord and the CIBMTR performed a study comparing unrelated BM (n = 472) or PBPC (n = 888) transplantation with CB (n = 165) HCT in adults with acute leukemia.29 In that study, allele-level HLA typing was performed for the adult donors at A, B, C, and DRB1 loci and either 8/8 and 7/8 matched donors were included. All CB units were HLA-typed at the antigen level for the A and B loci, with allele-level typing for DRB1. CB units matched at 4-6/6 loci were included. CB recipients received a single unit containing a minimum of 2.5 × 107 total nucleated cells/kg of bodyweight at cryopreservation. Multiple regression analyses revealed higher TRM but lower relapse and GVHD rates with CB, leading to comparable LFS rates compared with other stem cell sources (Figure 2). The results of this study and others confirmed that CBT is feasible in adults when a CB unit has a higher number of cells and should be considered an option for allogeneic HCT in patients lacking an HLA-matched donor.
Probabilities of LFS by hemapoietic stem cell source and donor-recipient HLA match. The probabilities of LFS by hematopoietic stem cell source and donor-recipient HLA match for patients in remission at transplantation (A) and patients who were not in remission at transplantation (B) are shown. Reprinted with permission from Eapen et al.29
Probabilities of LFS by hemapoietic stem cell source and donor-recipient HLA match. The probabilities of LFS by hematopoietic stem cell source and donor-recipient HLA match for patients in remission at transplantation (A) and patients who were not in remission at transplantation (B) are shown. Reprinted with permission from Eapen et al.29
Double CBT
The relatively low number of progenitor cells present in a single CB unit resulting in delayed hematopoietic recovery and an increased rate of engraftment failure initially limited the use of CBT in adults. The majority of adults do not have access to a single CB unit containing the recommended nucleated cell dose of 2.5 × 107/kg.30 To overcome the cell-dose limitation, Barker and the University of Minnesota investigators pioneered the use of double CBT (dCBT), sequentially infusing 2 CB units instead of 1 after conditioning therapy.31,32 These investigators initially reported the safety and feasibility of dCBT in 21 adults with hematological malignancies after MAC HCT.31 The results were encouraging, with all patients engrafting neutrophils in a median of 23 days (range, 15-41). Interestingly, by day 21 in more than 80% of the patients, only 1 of the 2 CB units was detected and was responsible for the long-term hematopoiesis in those patients. In other studies, dominance of the “winning” unit as early as day 12 has also been reported.33,34 Ramirez et al showed that, in the myeloablative setting, CD3+ cell dose was the only factor associated with unit predominance, but in the nonmyeloablative setting, CD3+ cell dose and HLA match were independent factors associated with unit predominance.35 Although these findings suggest that immune reactivity may have a role in unit predominance, the biological mechanisms responsible for single-donor predominance after dCBT remain incompletely understood and are under investigation by many groups.
Another important observation after dCBT is the higher incidence of acute GVHD. MacMillan et al reported a higher rate of grade II-IV acute GVHD in recipients of dCBT (58%, n = 185) compared with those of single CB units (39%, n = 80) due to an increased rate of grade II skin GVHD.36 The rates of grade III-IV GVHD were the same for both groups and the 1-year TRM was significantly lower after dCBT compared with single CBT (24% vs 39%).
Rodrigues et al reported a significantly lower risk of relapse at 1 year after dCBT compared with single CB in a study of 104 adult patients with lymphoid malignancies (13% vs 38%).37 Recently, a study prospectively comparing single CBT versus dCBT in adult patients (assignment was based on the cell dose in the primary unit) confirmed previous findings of a lower relapse rate after dCBT versus single CBT (30.4% vs 59.3%).38 The possible enhancement of graft-versus-malignancy may be due to greater alloreactivity when 2 CB units are infused, but this finding requires confirmation and further mechanistic studies.
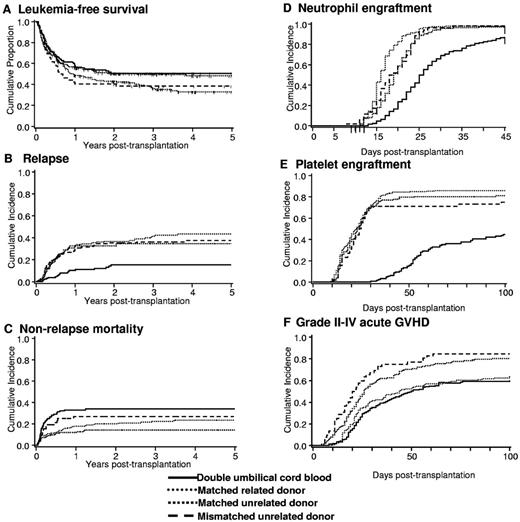
The risks and benefits of dCBT (n = 128) relative to those observed after transplantations with MRD (n = 204), MUD (n = 152) or 1-antigen–MMUD (n = 52) after MAC in leukemia patients were reported by the University of Minnesota in collaboration with the Fred Hutchinson Cancer Center.39 Risk of relapse was significantly lower in recipients of dCBT (15%) compared with MRD (43%), MUD (37%), and MMUD (35%) (Figure 3). However, TRM was higher for dCBT (34%) vs MRD (24%) and MUD (14%). LFS after dCBT was comparable to that observed after MRD and MUD transplantation, supporting the use of 2 partially HLA-matched CB units when patients lack an HLA-matched donor. Whether dCBT is preferable to single CBT when the cell dose in one unit is acceptable is not known. The ongoing Blood and Marrow Transplant Clinical Trials Network (BMT-CTN) 0501 study (www.clinicaltrials.gov number NCT00412360) has randomized children with leukemia to single CBT versus dCBT for MAC HCT and the results are eagerly awaited.
Clinical outcomes after myeloablative conditioning HCT by donor source. Clinical outcomes after dCBT, MRD, MUD, and MMUD transplantation. (A) LFS. (B) Relapse. (C) Nonrelapse mortality. (D) Neutrophil engraftment. (E) Platelet engraftment. (F) Grade 2-4 acute GVHD. Reprinted with permission from Brunstein et al.39
Clinical outcomes after myeloablative conditioning HCT by donor source. Clinical outcomes after dCBT, MRD, MUD, and MMUD transplantation. (A) LFS. (B) Relapse. (C) Nonrelapse mortality. (D) Neutrophil engraftment. (E) Platelet engraftment. (F) Grade 2-4 acute GVHD. Reprinted with permission from Brunstein et al.39
CBT after RIC regimens
The development of reduced-intensity conditioning (RIC) regimens was particularly important in extending HCT transplantation to adults. Barker et al reported that the fludarabine, cyclophosphamide, and low-dose total body irradiation (TBI) regimen40 was well tolerated, with rapid neutrophil recovery, a sustained donor engraftment rate of 94%, and a low incidence of TRM. Rocha et al from the Eurocord Registry also reported similarly encouraging results using a RIC regimen of fludarabine, Endoxan, and TBI, with a 1-year TRM of 24% and DFS of 50%.41 Ballen et al used the RIC regimen of fludarabine, melphalan, and rabbit antithymocyte globulin and reported a 1-year DFS of 67%.42 Multiple studies supporting the use of RIC CBT in patients who would not be able tolerate more intensive preparative regimens have subsequently been reported32,38,41–49 and are summarized in Table 1.
RIC regimens in CBT
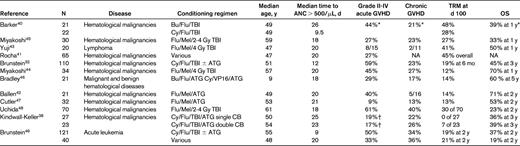
ANC indicates absolute neutrophil count; Bu, busulfan; Flu, fludarabine; Gy, Gray; Mel, melphalan; Cy, cyclophosphamide; TBI, total body irradiation; ATG, antithymocyte globulin; VP16, etoposide; CB, cord blood; TRM, transplant-related mortality; OS, overall survival; and NA, not applicable.
*The results were presented for the whole group.
†Reported only grade III-IV acute GVHD.
Recently, CIBMTR compared the outcomes in adults with acute leukemia receiving dCBT (n = 161) and 8/8 (n = 313) or 7/8 (n = 111) HLA-matched PBPCs after RIC regimens between the years 2000-2009.49 This analysis demonstrated comparable outcomes of dCBT patients only if they were treated with TBI 200 cGy, cyclophosphamide and fludarabine (TCF). Higher TRM and lower OS and LFS were observed in recipients of dCBT if they were treated with alternative regimens. Noteworthy observations included: (1) TRM was not significantly different after dCBT-TCF and PBPC transplantations despite lower neutrophil recovery with dCBT, and (2) risk of relapse was not found to be different between stem cell sources after RIC HCT, although relapse incidence has been reported to be lower with dCBT in patients with acute leukemia after MAC.39 These results with single-center studies supported the use of RIC CBT as a valuable strategy for broadening the application of transplantation therapy to those patients with leukemia and malignant disorders previously excluded on the basis of age, comorbidities, and the absence of an HLA MUD.
To study the reproducibility and the wider applicability of encouraging results after RIC dCBT and compare them with HLA-haploidentical related donor BM, the BMT-CTN completed 2 parallel phase 2 trials studying RIC alternative donor HCT.50 Fifty patients were treated in each study; all patients received an RIC regimen of fludarabine, cyclophosphamide, and low-dose TBI. Nonrelapse mortality was higher after CBT (24% for CB vs 7% for haploidentical), but the relapse rate was higher after haploidentical HCT (31% for CB vs 45% for haploidentical). The 1-year progression-free survival was comparable, at 46% for CBT and 48% for haploidentical HCT.
Novel strategies to improve CBT
Strategies under investigation to overcome the cell-dose limitation include the transplantation of ex vivo–expanded CB units,51 direct intra-BM injection,52 coinfusion of with a haploidentical T cell–depleted graft,53,54 the systemic addition of mesenchymal stem cells (MSCs),55 and the use of agents to enhance the homing of CB to the BM.56
Improving CB engraftment
Ex vivo expansion of CB progenitors before infusion is being studied as a way to shorten time to engraftment and reduce the rate of engraftment failure. Robinson et al showed that coculture of CB cells with MSCs would yield to a 10- to 20-fold increase in total nucleated cells, a 7- to 18-fold increase in committed progenitor cells, and a 16- to 37-fold increase in CD34+ cells.57 Based on those results, a clinical trial was initiated to evaluate the ex vivo coculture of CB mononuclear cells with either third-party haploidentical family member BM derived MSCs or off-the-shelf Stro3+ MSCs from Mesoblastd patients (www.clinicaltrials.gov number NCT00498316). The results with either source of MSCs was similar, with prompt engraftment of neutrophils in 15 days and platelets in 40 days.58 Long-term engraftment was provided by the unexpanded unit in the majority of patients by 11 months after transplantation. These results provide the rationale for a multinational randomized trial beginning soon that will compare unmanipulated dCBT with dCBT in which one of the units is expanded in MSC cocultures.
Similarly promising results have been reported by Delaney et al using Notch-mediated ex vivo expansion system for human CD34+ CB progenitors, with a decrease in the median time to neutrophil recovery by more than 1 week compared with results reported after infusion of 2 unmanipulated units.59 The expanded cells contributed almost exclusively to initial myeloid engraftment observed at 1 week, demonstrating an enhanced capacity of the expanded cell graft to provide rapid myeloid recovery. Furthermore, all but one evaluable subject engrafted before day 21 regardless of whether the expanded cell graft persisted in vivo.
A pivotal trial sponsored by Gamida Cell Ltd evaluated the ex vivo expansion of a fraction of a single CB unit using growth factors in conjunction with the copper chelator tetraethylenepentamine.60 Preliminary analysis revealed faster engraftment and improved survival compared with historical control recipients of single CBT reported to the international registries.51 A more definitive analysis of these data is in progress. In preclinical studies, CB CD34+ cells cultured ex vivo with growth factors (SCF, thrombopoietin, IL-6, and FMS-related tyrosine kinase 3) and nicotinamide (pyridine-3-carboxamide) displayed increased migration toward SDF-1 and enhanced homing to BM compared with untreated CB.61 A pilot clinical trial led by Horwitz et al is in progress to evaluate this strategy in the dCBT setting (www.clinicaltrials.gov number NCT01221857). Preliminary analysis has shown rapid engraftment (10 days for neutrophils and 30 days for platelets) with, interestingly, sustained engraftment coming from the expanded unit in the majority of patients evaluated to date (personal communication with Dr Horwitz).
Improving CB homing to BM
Another strategy to improve engraftment is to correct the decreased fucosylation of CB cell-surface molecules, which is thought to impair homing of CB-derived progenitor cells to the BM.62 In murine models, Robinson et al showed that CB CD34+ cells treated with fucosyltransferase-VI led to more rapid and higher levels of engraftment.56 A clinical trial in which recipients will receive dCBT in which one of the CB units will be fucosylated before infusion was opened recently (www.clinicaltrials.gov number NCT01471067). In future trials, combining CB expansion with fucosylation may produce maximally rapid hematopoietic recovery in the patients.
Prostaglandin E2 (PGE2) has been shown to enhance hematopoietic stem cell homing, survival, and proliferation.63 Based on these data, Cutler et al are conducting a clinical trial to evaluate the ex vivo treatment of 1 of 2 CB units with dimethyl PGE2 before infusion (www.clinicaltrials.gov number NCT00890500). After optimizing the procedure, it appears that engraftment of neutrophils is prompt (17 days), with dominance of the dimethyl PGE2–modulated unit documented in the majority of recipients.64
Broxmeyer et al have shown that inhibition of the CD34+ CB cell-surface protein CD26/dipeptidyl peptidase enhanced their engraftment in sublethally irradiated NOD/SCID mice.65,66 They are conducting a pilot clinical trial of systemic sitagliptin, a United States Food and Drug Administration–approved CD26/dipeptidyl peptidase inhibitor for diabetes, to evaluate the potential for enhancing engraftment in recipients of single CBT (www.clinicaltrials.gov number NCT00862719).
CB immune cells to improve outcome
CBT is associated with delayed immune reconstitution and a higher risk of morbidity and mortality due to viral and other infections after transplantation. GVHD remains a problem, particularly in the dCBT setting.36 Relapse is also of concern, particularly in high-risk patients with disease before transplantation67 To address these major obstacles to the widespread use of CBT, there are several exciting clinical studies in progress to evaluate several different CB-derived immune cells to improve survival.
CB-derived virus-specific cytotoxic T lymphocytes
Hanley et al developed a strategy using AD5f35pp65-transduced CB-derived antigen-presenting cells to generate autologous CB T cells specific for CMV and adenovirus.68 Based on this approach, a clinical trial led by Bollard et al at Baylor College of Medicine has been testing the use of CB-derived multivirus-specific T cells targeting EBV, CMV, and adenovirus for the prevention and treatment of viral infections after CBT (www.clinicaltrials.gov number NCT01017705).
CB-derived NK cells
Resting CB natural killer (NK) cells are reported to have significantly less cytotoxicity compared with peripheral blood NK cells.69 However, after cytokine stimulation, the cytotoxicity of CB NK cells can be rapidly increased to levels comparable to peripheral blood NK cells.70
A phase 2 trial using T cell–depleted dCBT with posttransplantation IL-2 is being conducted by the University of Minnesota investigators in refractory AML patients to investigate NK-cell expansion and function in vivo (www.clinicaltrials.gov number NCT01464359).
CB-derived Tregs
Regulatory T cells (Tregs) are a subset of CD4+ T cells that coexpress CD25 (IL-2Rα chain) and high levels of Foxp371 and are dependent on IL-2. Tregs represent a novel cell-based approach for potentially reducing the risk of GVHD.
Brunstein et al expanded Tregs obtained from a third CB unit and infused them into 23 patients undergoing dCBT.72 No severe Treg-related acute toxicities were observed and accrual to the study continues, with refinements in the Treg-generation procedure.
Conclusions
CB is used increasingly as a source of allogeneic hematopoietic support for patients who need HCT and do not have access to an HLA-matched donor. To overcome the limitation of low cell doses in single CB units, dCBT has been adopted for many patients and is associated with outcomes comparable to those with other donor sources. There have been new strategies under development to improve engraftment with ex vivo expansion or homing and to enhance immune reconstitution with the infusion of CB-derived NK cells and cytotoxic T lymphocytes with antiviral and antileukemic specificities. Tregs are being evaluated to reduce the incidence of GVHD. Prospective, multicenter clinical trials are needed to determine the efficacy of these promising technologies that are likely to improve outcome for CBT patients.
Disclosures
Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Betul Oran, MD, MS, The University of Texas MD Anderson Cancer Center, Department of Stem Cell Transplant and Cellular Therapy, 1515 Holcombe Blvd, Unit #423, Houston, TX 77030; Phone: 713-745-2820; Fax: 713-794-4902; e-mail: boran@mdanderson.org.