Abstract
The results of recent clinical trials for the management of limited-stage Hodgkin lymphoma have led to considerable debate, especially regarding the role of radiation therapy. This review highlights those recent trials and provides perspectives regarding their interpretation from a radiation oncologist and a hematologist. The trial protocol is available at http://www.nejm.org/doi/suppl/10.1056/NEJMoa1111961/suppl_file/nejmoa1111961_protocol.pdf.
Introduction
The results of recent randomized controlled trials (RCTs) testing therapeutic options for patients with limited-stage Hodgkin lymphoma have separately concluded that treatment with abbreviated chemotherapy plus involved-field radiation therapy (IFRT)1 and chemotherapy alone with doxorubicin (Adriamycin), bleomycin, vinblastine, and dacarbazine (ABVD)2 may both be acceptable options for these patients. Practice guidelines from the National Comprehensive Cancer Network (NCCN)3 also describe these alternatives as options. Because these trial results have led to considerable debate and disagreement, it is our goal to review this topic and provide context to assist practitioners and patients in their decision-making processes. Although we will not systematically review all available literature, we will describe the evolution of therapies for these patients and highlight data that most inform today's practices. We will then provide interpretations of these data from the perspectives of a radiation oncologist and a hematologist.
Background
The history of the treatment of Hodgkin lymphoma is remarkable. At the beginning of the 20th Century, it was one of the first cancers to demonstrate impressive responses to the “Roentgen Rays.”4 With technologic advances that permitted higher radiation doses and safer treatment to larger fields, pioneer radiologists, including Rene Gilbert,5 Vera Peters,6 and Henry Kaplan,7 demonstrated that Hodgkin disease was curable, especially when presenting as limited-stage disease. With the development of nitrogen mustard in the 1940s, Hodgkin disease proved to be a chemosensitive cancer as well.8 The introduction of newer agents and their combination into programs, such as nitrogen mustard, vincristine (Oncovin), prednisone, and procarbazine (MOPP) and ABVD by investigators, including DeVita9 and Bonadonna,10 proved that Hodgkin disease could be cured even in advanced stages.
The availability of 2 potent treatment modalities has provided great opportunity to develop effective treatments for all stages of disease. The most dramatic improvement in outcome for any cancer during the period 1960-1990 was for Hodgkin lymphoma. Effective treatments, long-term survival, and the demographics of patients with Hodgkin lymphoma, in which many patients are less than 40 years old at diagnosis, led to identification of potential late (3-40 years) complications, including most notably secondary leukemia and solid tumors, infertility, cardiovascular and pulmonary disease, but also hypothyroidism, soft tissue effects, and psychosocial effects.11
Until the mid-1990s, standard treatment for patients with favorable presentations of stage I or II Hodgkin lymphoma was with subtotal nodal irradiation (STNI) alone,12,13 which included sequential treatment to the cervical, axillary, mediastinal, and hilar lymph nodes followed by irradiation of the spleen (if present) and para-aortic nodes; 10-year relapse-free survivals were approximately 80%. Combining STNI with chemotherapy provided even better outcomes.14 15 However, as large cohorts treated with STNI were followed for more than 10 years, late risks, which were often fatal, resulted in a reevaluation of such aggressive therapies, particularly when patient demographics would otherwise be associated with expectation of long life expectancies. Thus, beginning in the late 1980s, research priorities have emphasized identification of effective management programs that would be associated with fewer late effects, including testing of chemotherapy in combination with IFRT, in which irradiation was restricted to the involved lymphoid region(s). Control arms of these RCTs incorporated STNI alone14–16 or chemotherapy plus STNI.17 These studies uniformly demonstrated superior outcomes with combined modality treatment (CMT) when compared with radiation therapy (RT) alone, and similar outcomes, with less potential toxicity compared with chemotherapy plus STNI. Based on these results, CMT consisting of chemotherapy that was often attenuated in duration or intensity followed by IFRT became a standard CMT treatment by the late 1990s, although STNI alone remained an option for treatment at many centers until the early 2000s.
Testing of chemotherapy alone for patients with limited-stage disease was first reported in the early 1970s. Initial experience was limited to MOPP or similar alkylator-intense therapies; results of RCTs were inconsistent,18–20 and many considered the associated sterility and leukemogenic risks to be unacceptable. Testing of epirubicin, bleomycin, vinblastine, and prednisone in an RCT was abandoned because of unacceptable rates of disease progression.21 A Cochrane analysis of clinical trials comparing predominantly alkylator-based chemotherapy alone with CMT, in which the RT component was generally involved-field treatment, showed superior tumor control and overall survival (OS) in patients treated with CMT.22 However, most of the aforementioned trials, including the majority that contributed to the meta-analysis, incorporated chemotherapy regimens now known to be inferior to ABVD and thus do not properly inform current decision-making.
Trials evaluating combined modality therapy
Six trials inform decisions to use chemotherapy plus IFRT (Table 1). The first was the H8F Trial of the European Organization for Research and Treatment of Cancer (EORTC)16 for “favorable” patients (based on considerations of age, sex, stage, mediastinal disease, B-symptoms, erythrocyte sedimentation rate [ESR], and histologic subtype). The H8-F compared STNI with 3 cycles of MOPP-ABV combined with IFRT.16 The 5-year event-free survival (EFS; 98% vs 74%) and 10-year OS (97% vs 92%) were superior in the CMT arm. For “unfavorable” patients, the H8-U compared 6 cycles of MOPP-ABV plus IFRT with 4 cycles of MOPP-ABV plus IFRT and with 4 cycles of MOPP-ABV plus STNI. No significant differences among the treatment arms were detected for either EFS or OS. The authors concluded that STNI alone could no longer be recommended in stage I or II disease and that a reduced volume of radiation fields, from STNI to IFRT, did not compromise outcome.
Clinical trials of CMT in stage I or II Hodgkin lymphoma
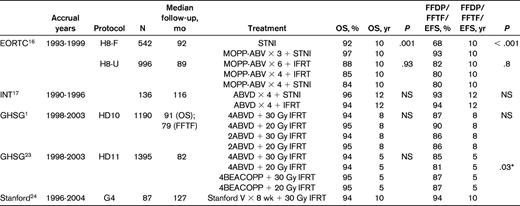
FFDP indicates freedom from disease progression; INT, Istituto Nazionale Tumori; and NS, not significant.
*Comparison of PFS for 4ABVD + 20 Gy IFRT vs 4ABVD + 30 Gy IFRT.
The remaining 4 trials incorporated CMT in all treatment arms and tested radiation field size and/or dose, and types and duration of chemotherapy. In a trial conducted at the Istituto Nazionale Tumori in Milano, Italy, patients were treated with 4 cycles of ABVD and the RT was randomized between STNI and IFRT17 ; 29% of patients had unfavorable characteristics (bulky disease, pulmonary hilar disease, E-lesions, or B-symptoms). With a median follow-up of 116 months, no differences were detected in 12-year freedom from progression (93% vs 94%) EFS (87% vs 91%) and OS (96% vs 94%) between the STNI and IFRT arms, respectively. The 6 deaths among the 136 patients in this trial included 3 from Hodgkin lymphoma, 1 cardiovascular event (during ABVD treatment), 1 hepatitis, and 1 acute leukemia. There were 3 secondary cancers in the STNI arm and none in the IFRT arm. The authors concluded that ABVD followed by IFRT could be considered an effective and safe modality in early Hodgkin disease with either favorable or unfavorable presentation.
Two complementary trials were conducted by the German Hodgkin Study Group (GHSG): HD10 and HD11. Both include patients with stage I or II disease, but only patients without risk factors, which were ESR > 50, ESR > 30 with B-symptoms, extranodal lesions, more than 2 sites of disease, and presence of a large mediastinal mass, were included in HD10. The remainder of “unfavorable” patients, including those with either B-symptoms (29% of patients) or bulky mediastinal adenopathy (19% of patients) was included in HD11. Patients with both B-symptoms and large mediastinal adenopathy were treated as advanced disease on the HD12 study.
The HD10 trial involved 4-armed randomization with a factorial analysis and compared 2 versus 4 cycles of ABVD and 20 versus 30 Gy IFRT; the trial was designed according to noninferiority principles with a primary endpoint of freedom from treatment failure (FFTF).1 With a median follow-up of 79 months, the arm that included 2 cycles of ABVD and 20 Gy IFRT was considered to be noninferior with respect to FFTF and was associated with less severe toxicity and was thus concluded by the authors to be optimum therapy. The 8-year FFTF on this arm of the trial was 86% and 8-year survival was 95%. Among the 1190 patients randomized, there were 10 deaths resulting from Hodgkin lymphoma, 12 from toxicity of treatment, 11 from secondary cancer, and 9 from cardiovascular causes.
The HD11 trial was of a similar design and compared 4 cycles of bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and procarbazine (BEACOPP) given at baseline doses with 4 cycles of ABVD, followed by 20 Gy versus 30 Gy IFRT.23 The primary endpoint was also FFTF. At a median follow-up of 82 months, the 5-year FFTF was inferior in the 4 ABVD + 20 Gy arm (81% vs 85%-87%), although no difference in 5-year survival (94%-95%) was observed; the 4 ABVD + 30 Gy arm was concluded to be noninferior to the BEACOPP regimens and was recommended as the treatment of choice because of the greater toxicity of BEACOPP; among these 356 patients, the 5-year FFTF was 85% and survival was 94%; there were 7 deaths from Hodgkin lymphoma, 5 from treatment toxicity, 3 from secondary cancers, and 5 from cardiovascular causes.
The final trial is the Stanford G4 study. This was a single arm trial of Stanford V chemotherapy plus 30 Gy IFRT.24 A large intergroup trial failed to detect differences between Stanford V and ABVD in the CMT setting for those with bulky stage I or II and stage III or IV disease.25 The G4 trial for stage I or II disease was conducted at Stanford and the Northern California Kaiser Hospitals. Patients with B-symptoms or large mediastinal adenopathy were excluded. All patients received 8 weeks of Stanford V chemotherapy followed by 30 Gy “modified” IFRT, that is, unless involved, the upper neck and lower mediastinum were not treated in conjunction with other portions of those fields. A total of 87 patients were treated, and the median follow up was 10.6 years. The 10-year freedom from disease progression and OS are both 94%. There were 4 deaths: 2 from transplantation-related complications, one from metastatic colon cancer, and one from swine flu. The authors concluded that this regimen was safe and highly effective.
Trials evaluating chemotherapy alone
Because treatment with ABVD is associated with superior outcomes compared with alkylator and epirubicin-based therapies, RCTs testing ABVD alone provide the basis of comparison with treatment that includes RT. The results of 4 RCTs comparing ABVD alone2,26,27 or cyclophosphamide, vincristine, procarbazine, and prednisone (COPP)-ABV,28 in which cyclophosphamide is substituted for nitrogen mustard, with CMT in cohorts that include patients with limited-stage disease, have been previously summarized. These consistently noted superior disease control of approximately 7% with combined modality treatment; no differences in OS were detected29 (Table 2).
Randomized trials comparing ABVD or regimen of equivalent efficacy alone with treatment that includes RT
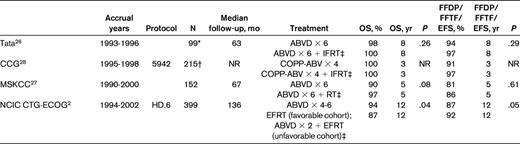
FFDP indicates freedom from disease progression; Tata, Tata Memorial Hospital, Mumbai; CCG, Children's Cancer Group; MSKCC, Memorial-Sloan Kettering Cancer Center; and NR, not reported.
*Includes stage I or II patients only.
†Includes group 1 patients only;
‡Control therapy.
The final analysis of one these trials, the National Cancer Institute of Canada (NCIC) Clinical Trials Group (NCIC CTG) Eastern Cooperative Oncology Group (ECOG) HD.6 trial has been recently reported.2 In this trial, patients with nonbulky stage I or IIA disease were randomized to receive ABVD alone or radiation-based treatment. A risk-categorization schema was used to stratify patients into favorable and unfavorable cohorts: those with any one of age ≥40 years, ESR ≥ 50, mixed cellularity or lymphocyte-depleted histology, and/or 3 or more disease sites were placed into the unfavorable risk group. For the control arm, those in the favorable-risk cohort received STNI alone, whereas the unfavorable-risk cohort patients received 2 cycles of ABVD followed by STNI. Experimental arm therapy was the same for both risk groups: 4-6 cycles of ABVD, with the number of cycles dependent on rapidity of response documented by computed tomographic imaging. The trial included 405 patients, including 399 eligible patients. The primary outcome was 12-year survival. With a median follow up of 11.3 years, the 12-year survival was superior in patients randomized to receive chemotherapy alone (94% vs 87%; hazard ratio [HR] = 0.05; P = .04); in contrast, 12-year freedom from progressive disease (FFPD) was inferior (87% vs 92%; HR = 1.91; P = .05). The difference in OS was attributed to fewer deaths from causes other than progressive Hodgkin lymphoma in those allocated to ABVD alone (6 vs 20), whereas deaths from progressive Hodgkin lymphoma were similar (6 vs 4). There were 10 deaths from second cancers among those assigned to radiation and 4 in those randomized to ABVD alone. In subset analyses, no differences in either 12-year FFPD (89% vs 87%; HR = 0.88; P = .82) or 12-year survival (98% vs 98%; HR = 1.09; P = .95) were detected between the ABVD alone and radiation therapy groups among the favorable cohorts. In the unfavorable cohorts, 12-year FFPD was inferior in those allocated to ABVD (86% vs 94%; HR = 3.23; P = .006), whereas 12-year survival was superior (92% vs 81%; HR = 0.47; P = .04). The authors concluded that treatment with ABVD alone is associated with superior long-term OS because it is associated with fewer deaths from other causes.
The treatment of limited-stage Hodgkin lymphoma: a radiation oncologist's perspective
Is radiation therapy alone ever an option for the treatment of early-stage Hodgkin lymphoma?
The use of radiation therapy alone for the treatment of classic Hodgkin lymphoma was abandoned more than a decade ago. Clinical trials comparing STNI and CMT demonstrated an improved EFS for CMT.14–16 However, STNI may have equivalent outcomes to the use of chemotherapy alone. The NCIC CTG HD.6 trial compared ABVD chemotherapy alone (4-6 cycles) and STNI alone in the favorable cohort of patients with nonbulky stage I or II disease.2 The 12-year EFSs were 89% and 86% (P = .64), respectively, and the 12-year survivals were 98% (P = .95) in both arms of the trial. However, the superiority of CMT demonstrated in the EORTC,16 GHSG,15 and Southwest Oncology Group14 trials has led to an exclusion of STNI alone as a management option for patients with classical Hodgkin lymphoma in guidelines of the European Society for Medical Oncology30 and NCCN.3
The situation is different for lymphocyte predominant Hodgkin lymphoma. Outcomes of treatment using limited radiation therapy alone for patients with stage I or limited stage II disease have been excellent. The GHSG evaluated retrospectively patients treated on their sequential trials with extended-field RT (EFRT), CMT, or IFRT and found no differences in outcome related to the intensity of therapy.31 Both the European Society for Medical Oncology30 and NCCN3 guidelines recommend limited radiation (IFRT) alone for patients with stage I disease and include it as an option for patients with stage II disease. Given the paucity of patients with this diagnosis (only ∼ 11% of patients in the NCIC CTG trial),32 it is unlikely that a RCT will ever be conducted to test treatment options for this subtype of Hodgkin lymphoma.
What extent of irradiation is indicated in the setting of combined modality therapy?
Clinical trials of CMT have incorporated RT fields of varying extent. Involved field irradiation was used in these trials as long ago as 1974,33 but its adequacy compared with EFRT or STNI in the CMT setting was proved only in the RCTs initiated in the 1990s. One of the first of these17 randomized patients between IFRT and STNI after 4 cycles of ABVD. The early reports of these results influenced the first version of the NCCN Practice Guidelines for Hodgkin Disease,34 published in 1999, which recommended that IFRT was the appropriate extent of irradiation for patients with stage IA or IIA disease treated with combined modality therapy. Larger RCTs that were published subsequently by the EORTC16 and the GHSG15 confirmed these conclusions, and IFRT was accepted as the standard in combined modality therapy programs.
What about chemotherapy alone for nonbulky stage I or II Hodgkin lymphoma?
The largest trial testing chemotherapy alone is the NCIC CTG HD.6 trial.2 The results of this trial show a good outcome for patients treated with ABVD alone. In the favorable cohort, there was no significant difference in outcome between treatment with ABVD or STNI. In the unfavorable cohort, the 12-year OS after ABVD treatment was 92% and the freedom from disease progression was 86%. The OS in the radiation-containing regimen (ABVDx2 plus STNI) was only 81%, despite the superior freedom from disease progression (94%). Although the results with ABVD alone are commendable, care must be taken in interpretation of the results of radiation-based therapies included in this trial. The authors of the study admit that the extent of radiation used was outdated and that this was likely to have contributed to the excess deaths. The NCIC CTG trial used RT that violates current standards with respect to both volume and dose of irradiation in the CMT setting.
What criticisms or concerns does the NCIC CTG trial raise for radiation oncologists?
As noted, the current standard for radiation volume in combined modality therapy programs is IFRT. STNI exceeds the volume of IFRT by 3- to 5-fold; and most importantly, IFRT is associated with lower doses to the breasts, lungs, and heart in nearly all cases. All of the irradiated patients in the NCIC CTG trial also were treated to their clinically uninvolved spleens and para-aortic nodes, exposing them to risks of infection, cardiac disease, and secondary malignancy. Involved field irradiation for these patients would never have included those volumes. All women in this trial who were irradiated received irradiation to bilateral axillary nodes, exposing them to a risk of breast cancer, although it is probable that no more than 25% of women would have had either axilla irradiated with IFRT and the reduction in mean breast tissue dose would be ∼ 65%.35 All irradiated patients in this trial had treatment to the entire mediastinum, exposing them to cardiac risks, whereas this would not have been the case for patients treated with IFRT who had an uninvolved mediastinum.
The risk for radiation-related cancer is proportional to the volume irradiated. It has been shown, for example, that irradiation of smaller volumes is associated with a lower risk for breast cancer in women,36 and so patients irradiated in this trial were at greater risk for radiation-related complications than if they had been treated with IFRT. Likewise, the risk for cardiac complications increases as the volume of heart irradiated increases.37
The radiation dose used in this trial (35 Gy) is higher than is used in contemporary combined modality therapy. Doses of 20-30 Gy have been shown to be adequate in patients with stage I or II disease.1,23 Higher doses probably increase the secondary cancer risk and definitely increase the cardiac risks.
In this trial, there were 23 deaths among the 139 patients in the ABVD plus STNI group. This included 9 deaths from secondary cancer, although it is not reported which, if any, of these were in the irradiated field or whether they would have been within an involved field. There were 2 deaths from cardiac events (identical to the ABVD arm), 3 from infection, and one each from Alzheimer disease, drowning, suicide, respiratory failure, and unknown. There were no miscellaneous causes of death in the ABVD group and none in the group of patients treated with STNI alone. The impact of these deaths resulted in a 12-year OS for ABVD plus STNI of only 81%. The number of patients who developed any second cancer was 23 in the radiation therapy groups and 10 in the ABVD group. Among the 23 cancers in the radiation therapy group, location relative to the radiation fields is not noted; however, 6 were in the pelvis and unlikely to have been irradiated.
The risk that radiation oncologists perceive is that these results will be interpreted incorrectly to imply a negative impact for radiation therapy, in general, when they only apply to the way that RT was used in this trial. An analogy would be to criticize the use of any chemotherapy for early-stage Hodgkin lymphoma based on experience with MOPP, with its leukemic and sterilizing potential. Just as there are differences between outdated and current chemotherapy, there are differences between outdated and current radiation therapy. Subtotal nodal radiation therapy is outdated. Many of the risks associated with radiation therapy in this trial would not be risks with contemporary radiation therapy.
Another risk that radiation oncologists perceive is that these results will be translated into clinical practice for patients with bulky stage I or II Hodgkin lymphoma. This trial only addressed patients with nonbulky disease. Patients with bulky disease are at greater risk for relapse, and clinical trials of the EORTC and GHSG, which incorporate radiation therapy, should be used to inform treatment practice for these patients.
What is the current standard for radiation therapy in combined modality therapy?
Involved field irradiation implies treatment to the entirety of a lymphoid region. For example, when the supraclavicular area is involved, IFRT includes treatment to the entire cervical region, including submandibular, cervical, and supraclavicular nodes. This field includes the submandibular salivary glands, the entire length of the carotid arteries, and a good portion of the thyroid. Variations on the IFRT concept have been incorporated selectively into clinical trials. In the Stanford G4 trial, modifications were introduced into the involved field concept.24 In the presence of supraclavicular disease, the upper half of the neck was not irradiated unless clinically involved, and a margin (2-5 cm) beyond involved nodes in the mediastinum was used, rather than treatment to the entire mediastinum. A retrospective analysis of similar modifications of the involved field concept shows no compromise of outcome in combined modality therapy.38 Recently, clinical trials have used even more limited fields, referred to as involved node radiotherapy (INRT). The concept of involved node irradiation (INRT) was developed initially in the EORTC/GELA39 and has been adopted by other clinical trials groups.
Involved node radiotherapy is attractive because the irradiated volume is less and a smaller volume of radiation treatment must be associated with less risk for late effects. Preliminary data confirming this concept have been published.40 Although the EORTC/GELA have adopted INRT in their clinical trials, the GHSG has chosen to test the concept. In the GHSG HD17 trial, patients who are treated with combined modality therapy are randomized to treatment with IFRT or INRT.
What does the future hold for the management of patients with early-stage Hodgkin lymphoma?
In this era of personalized medicine, treatment is likely to become more adaptive to the individual clinical situation. It will not be “one size fits all.” Some clinical treatment guidelines already endorse the use of either chemotherapy alone or combined modality therapy. The NCIC CTG trial incorporated adaptive therapy in defining the number of cycles of chemotherapy to be used (4-6) based on the rapidity of a complete response. However, more than 60% of patients were treated with 6 cycles of ABVD according to this trial design. The doses of doxorubicin and bleomycin included in 6 cycles of ABVD are not without risk.41 Ideally, if IFRT can be effective in preventing relapse, it could be refined even further (eg, INRT) and incorporated selectively, with the prospect of reducing the number of cycles of chemotherapy from 4-6 to as few as 2, and thereby reducing the potential late risks of chemotherapy, as has been advocated by the GHSG.1
Identification of those patients most likely to benefit from the addition of radiation therapy remains a challenge. A promising technique is interim positron emission tomography (PET) imaging, following 2 or 3 cycles of chemotherapy, to identify patients with a slow or inadequate response to chemotherapy. Currently, clinical trials in the GHSG (HD16), EORTC-GELA (H10), and in the United Kingdom (RAPID Trial) are testing this concept.
The NCIC CTG trial emphasizes the importance of late effects, which can only be assessed in long-term follow-up. Clinical trials in Hodgkin lymphoma are unlike those for patients with solid tumors. Long follow-up (> 10 years) is essential, and acknowledgment of this reality must be a factor in planning new clinical trials. This reality needs to be considered by funding agencies.
The treatment of limited-stage Hodgkin lymphoma: a hematologist's perspective
Evidence has been described supporting 2 treatment alternatives for patients with nonbulky stage I or IIA Hodgkin lymphoma: CMT with 2-4 cycles of ABVD plus IFRT, best exemplified by the GHSG HD10 1 and HD1123 trials, and chemotherapy alone with ABVD, primarily based on results of the NCIC CTG/ECOG HD.62 trial. These alternatives have not been directly compared in RCTs; and although new evidence will emerge from RCTs testing chemotherapy alone in conjunction with response-adapted strategies that include PET,42 those results are likely to be 2 to 4 years away and to initially emphasize disease control outcomes over the first 5 years of follow-up. In the absence of data that permit evidence-based recommendations based on the highest levels of evidence, practitioners and patients currently face decision-making processes that require synthesis of other information. In this section, a synthesis will be provided to argue that treatment with ABVD alone is reasonable, appropriate and, for many patients, may be preferred. This information will be presented in 4 tiers: reports of primary results, interpretation of other trial-specific conclusions, integration of hypothesis-generating data, and additional context and supposition. These tiers will extend from evidence to speculation. Among trials listed in Table 2, results of HD.6 will be emphasized as this trial includes the largest sample size with the most homogeneous adult population and is associated with the longest follow-up. The role of the author as principal investigator of that trial is declared so that any perception of a nonfinancial conflict of interest43 is apparent.
Results associated with the primary trial objective of the HD.6 trial form the basis of the first tier; this objective was to compare the 12-year survivals of patients with nonbulky stage I or IIA Hodgkin lymphoma treated with ABVD alone with patients given treatment that included STNI.2 At 12 years, OS was 94% among those assigned to ABVD alone and 87% among those allocated to receive STNI, with differences because of more deaths in the STNI arm from causes other than progressive Hodgkin lymphoma or early treatment complication (20 vs 6). The HD.6 trial illustrates the dilemma of evaluating long-term OS as the primary endpoint in a limited-stage Hodgkin lymphoma trial: therapeutic advances will undoubtedly occur in the interim. In this case, advances include recognition that STNI is excessive, outdated, and probably contributed to the results and conclusions. Although scientific reporting standards mandate careful descriptions of the comparative outcomes associated with a primary objective, it is recognized that these specific results, although important, are not the deliverables from HD.6 that most directly inform decision-making about today's best practices.
Instead, 3 results from HD.6 and associated conclusions form a second tier of evidence and best inform today's decision-making. These findings relate to disease control and survival outcomes of patients assigned to ABVD alone and to the topic of surrogate outcomes. The observed 12-year FFPD associated with ABVD was 87%. These results were inferior to those observed in the HD.6 control arm (92%) and might be assumed to be inferior to those associated with modern CMT. Logical extensions might then suggest that treatment that includes IFRT will have fewer late effects than observed with HD.6 control arm therapy, meaning that modern CMT may be associated with superior disease control, a reduced need for subsequent therapy, fewer late effects, and OS that is as good or better than observed with ABVD alone. However, important existing data do not support these assumptions. Specifically, results of the GHSG HD10 trial,1 which are reported with a median follow-up of 79 months, include 8-year disease control outcomes of FFTF of 86% and progression-free survival of 87% and do not suggest superiority over the 12-year disease control outcomes observed in the HD.6 trial. Furthermore, eligibility criteria for HD10 appear to be more restrictive than those of HD.6, as patients with more than 2 disease sites were ineligible. Cross-trial comparisons are associated with substantial risks because of differences in patient populations, outcome assessment, and analysis. However, practitioners and patients need to consider HD10 results relative to those of HD.6. These comparisons question the assumption that today's CMT provides substantially better long-term disease control and reduced eventual needs for subsequent-line therapy as compared with ABVD alone. Cross-trial comparisons between GHSG HD11 and HD.6 are more complex. With HD11, the 5-year FFTF associated with 4 cycles of ABVD and 30 Gy IFRT was 85%.23 Although this disease control outcome is similar to the 12-year outcomes of HD.6, the HD11 trial included patients with B-symptoms (29%) or bulky disease (20%) and represents a higher-risk group. There is a need to better understand the comparative outcomes of patients without those risk factors.
Cross-trial comparisons of OS are the second result for consideration in this tier. The 12-year OS observed with ABVD alone in HD.6 was 94%. The 8-year OS observed with the recommended arm of HD10 was 95%, and the 5-year OS observed with the recommended arm of HD11 trial was 94%; these results do not yet reflect sufficient durations of follow-up necessary to understand the frequency of late effects. Recognizing that cross-trial comparisons lack methodologic rigor and that the GHSG HD11 population includes higher-risk patients, the duration of follow-up associated with HD.6 suggests that the reported survival results are robust and are unlikely to be inferior to those observed in HD10 or HD11.
A third result and conclusion from HD.6 relates to the use surrogate outcomes and includes quite powerful findings. Whereas the risk of disease progression (FFPD) with chemotherapy alone was almost twice that associated with treatment that includes STNI (HR = 1.91), the risk of death was reduced by half (HR = 0.5). These results refute use of disease control measures as an accurate proxy for OS in this patient population. The magnitude of the discrepancy between FFPD and OS is undoubtedly exaggerated by late effects associated with STNI, and less extreme discordance may be observed with modern CMT. However, accounting for this principle is necessary when evaluating any treatment strategy for these patients and mandates that longer-term follow-up of RCTs testing modern CMT be reported to better inform decision-making processes.
In a third tier of information are hypothesis-generating data; 2 examples will be explored. First is the concept of response-adapted therapy, which is based on the premise that patients with more rapid complete responses to ABVD represent a favorable prognostic group that might be spared more extensive treatment. In the ABVD alone cohort and among those evaluable for response after 2 treatment cycles (90% of patients), those with a complete or unconfirmed complete remission (CR/CRu) had superior long-term disease control (94% vs 81%; HR = 0.28; P = .02) and a trend to superior OS (98% vs 92%; HR = 0.17; P = .06) compared with those not achieving a CR/CRu status. Conduct of HD.6 preceded use of PET, and no patients underwent this evaluation after 2 treatment cycles. These data prompt hypotheses to test whether PET can be added to evaluations used in HD.6 to provide even better delineation of prognosis among those achieving a CR/CRu with conventional evaluation (ie, the 94%) and/or to determine who among those not achieving a CR/CRu with conventional evaluation have achieved a biologic state of remission (eg, evaluations detect residual fibrosis) and thus have an excellent prognosis (ie, those within the 81%).42
The second finding relates to the prognostic categorization schema used in HD.6, which included age at randomization of 40 years or older as a risk factor. Although now considered outdated, HD.6 data suggest that an important interaction may exist. In the control group, those assigned to the favorable cohort and receiving STNI alone had inferior 12-year FFPD compared with those in the unfavorable cohort who received CMT (87% vs 94%) and yet their 12-year OS was superior (98% vs 81%). Such extreme differences were not seen in the experimental arm, in which 12-year FFPD (89% vs 86%) and OS (98% vs 92%) were observed in the favorable and unfavorable cohorts, respectively. These observations require more detailed exploration to assess for an interaction between age, treatment, and risk of death from causes other than Hodgkin lymphoma. Previous data show that, while in comparison with populations without Hodgkin lymphoma, young age at treatment is associated with an increased relative risk of late effects (because of the uncommon occurrence of these events in the young control population), absolute excess risk is greater among older patients in whom the prevalence of diseases associated with late effects are more common.44 Thus, the importance of late effects may first become apparent in older patients. If confirmed, this hypothesis would also suggest that, although the HD.6 trial is associated with a prolonged median follow-up by clinical trials standards, this duration is too short to evaluate the risks of late-effects in patients treated at a young age.
The final tier includes the greatest degree of speculation, emphasizes health service delivery, and has implications for adopting new RT technologies. Data from the GHSG trials illustrate that caution is needed when interpreting other clinical trials data, especially when these are from nonrandomized and limited-site trials. When tools of critical appraisal are applied to the HD10 and HD11 trials, it is apparent that each was rigorously conducted and reported. The HD10 trial was conducted at 329 sites across 5 countries.1 When outcomes are considered relative to those reported in other trials that have included higher-risk patients,16,17,45,46 it is perhaps surprising that, with a median follow-up of 79 months, the 8-year FFTF associated with the recommended arm is only 86%. Although again associated with substantial risks of cross-trial comparisons, these results are, at best, similar to those described for other trials evaluating higher-risk patients. Rather than a critique of the HD10 trial, these data may demonstrate methodologic strength and reflect high degrees of generalizability47 and better representation of what might be observed with population-based evaluations. This line of reasoning includes several facets. First, the HD10 and HD11 trials included rigorous radiation therapy quality assurance measures.48 Among the 60% of cases evaluated in HD10, coverage of the target volume was assessed as inadequate in 43%; in the 67% of cases assessed in HD11, coverage was deemed inadequate in 48%. These data suggest that practice variation exists. An argument can thus be forwarded that delivery of RT to patients with Hodgkin lymphoma is complex, variation in quality can exist and may affect outcomes, especially when chemotherapy is limited to 2 treatment cycles. Quality may relate to a relation referred to as “volume-outcomes,”49 in which quality of care is shown to be superior in treatment centers with greater numbers of patients with the condition of interest. This relation was demonstrated when RT practices for patients with Hodgkin lymphoma were evaluated in another era with the 1988-1989 Patterns of Care Study,50 and is also evident in more recent evaluations of cancer care delivery,51 including RT for patients with head and neck cancer.52
Issues of practice variation and quality will intersect with methodologic details when trials of different designs are conducted at single institutions or a small number of centers. Risks associated with conclusions derived from single-arm trials, which lack a control population, are well recognized. Often these trials are conducted at single institutions, and specific risks, such a referral-filter bias,53 may exist. Recent data suggest that even RCTs conducted at single centers may yield results associated with greater effect sizes than observed with multicenter trial conduct.54 Although these authors emphasized that their results suggest risks of bias associated with single-center trials, the contribution of a volume-outcome relation is plausible, especially when the treatment intervention is complex. Although speculative, these considerations should promote caution when interpreting results of single-site evaluations of new technologies, such as conformal, intensity-modulated, stereotactic body, and proton RT.55,56 At the level of individual practitioner and patient decision-making, it is important to understand the experience of the treatment center in administering RT to patients with Hodgkin lymphoma, especially if new technologies are implemented.
This synthesis of information is intended to show a series of considerations demonstrating that ABVD alone is an acceptable treatment option for patients with nonbulky stage I or IIA Hodgkin lymphoma. High-quality evidence demonstrates that ABVD alone is superior to past treatment approaches that included EFRT and that disease control endpoints cannot be assumed to be reliable surrogates for OS. Lower level evidence from cross-trial comparisons suggests that ABVD alone is associated with comparable efficacy to modern CMT in providing long-term disease control and at least comparable long-term OS. Evidence exists to promote the hypothesis that response-adapted approaches, including with PET, are priorities for evaluation, late-effects may first become evident in older patients and reflected by increases in absolute excess risk, and that longer-term follow-up is needed to better evaluate the magnitude of reduction in frequency of late effects associated with IFRT compared with EFRT or STNI. Based on more speculative information, potential volume-outcome relations and appraisal of potential biases that influence evaluations of single-arm and/or single-center clinical trials should be considered when determining local treatment practices, especially when adopting new technologies.
Finally, physicians and patients should enter into shared decision-making processes. Factors to be considered will include those related to the health status of the patient, including extent and location of disease sites and comorbidities. In addition, after sufficiently understanding the pros and cons of treatment alternatives, patients may have different preferences.57 Healthcare providers are obliged to provide an opportunity for these preferences to be explored by each patient. Based on the information described in this section, treatment with ABVD alone should be considered an appropriate option and may be preferred by many patients.
Conclusions
In conclusion, we have witnessed in our lifetimes dramatic improvements in the treatment of Hodgkin lymphoma, a disease that previously had been considered to be fatal. This progress has been the result of detailed clinical observations, carefully conducted clinical trials, the refinement of existing treatments, the development of novel therapies, and the collaboration of specialists across the breadth of medicine. Although outcomes are excellent, further advances can be anticipated. Functional assays, genetic profiling, and the introduction of biologic therapies all hold promise for the future and may enable us to be more selective in defining treatment for individual patients. The existing debate will become moot when these advances are realized.
This article was selected by the Blood and Hematology 2012 American Society of Hematology Education Program editors for concurrent submission to Blood and Hematology 2012. This article is reprinted with permission from Blood. 2012; Volume 120.
Disclosures
Conflict-of-interest disclosure: R.M.M. has received honoraria from Celgene regarding his role on the Independent Response Committee of a clinical trial and from Lilly regarding his role as Chair of an Independent Data Safety Monitoring Committee. R.M.M. is Director of the NCIC CTG, which has received research funding from Amgen Canada, Ariad Pharmaceuticals, Astex Therapeutics, AstraZeneca, Boston Biomedical Inc, Bristol-Myers Squibb, Celgene, Geron Corp, GlaxoSmithKline, Janssen-Ortho, Lilly, Merck Frosst Canada, Novartis, Oncolytics Biotech, Oncothyreon, Orthobiotech, Pfizer, Roche, Sanofi-Aventis, and Schering Canada. R.T.H. declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Richard T. Hoppe, Stanford University, Department of Radiation Oncology, 875 Blake Wilbur Drive, Rm G-228, Stanford, CA, 94305; Phone: 650-723-5510; Fax: 650-725-8231; e-mail: rhoppe@stanford.edu.
