Abstract
The achievement of complete hematologic remission (CR) is a prerequisite for cure in acute myeloid leukemia (AML). The conventional definition of CR, based on the morphologic recognition of ≤ 5% of leukemic blasts in the BM, does not provide sufficient insight into the quality of the response. Despite CR rates of 50%-80% (depending on age), the majority of patients with AML relapse within 3-5 years from diagnosis. Therefore, there is great need of more sensitive prognostic factors that can predict relapse. Minimal residual disease (MRD), defined as any measurable disease or leukemia detectable above a certain threshold (defined by the methodology applied), predicts failure to maintain a morphologic CR and affects survival negatively. AML is lagging behind acute lymphoblastic leukemia with respect to the implementation of MRD criteria for guidance during therapy. AML is particularly disadvantaged compared with acute lymphoblastic leukemia in that approximately half of AML patients lack a molecular target suitable for MRD monitoring. The detection of altered antigen (Ag) expression by leukemic myeloblasts is a valid alternative to DNA- or RNA-based MRD assays. Although associated with presenting prognostic factors (eg, cytogenetics and genotype), MRD represents the collective end result of all of the cellular mechanisms that determine a patient's response to a given therapy. Therefore, MRD has 2 potential roles in AML treatment: (1) as a posttherapy prognosticator used to assign patients to optimal postinduction/consolidation therapy, and (2) as an early surrogate end point for the evaluation of therapy efficacy.
The logistics of MRD assessment in AML
The detection of residual leukemic cells with techniques more sensitive and less subjective than morphology has added a new prognostic dimension to the treatment of AML. With few exceptions,1 the integration of MRD has superseded standard risk classifications based on age, WBC count, and cytogenetic and genotypic features. To date, MRD has rarely been used in the prospective risk stratification of AML patients, given that testing the clinical utility of MRD monitoring has been marred by inconsistencies in MRD thresholds, uncertainty on the choice of the most informative MRD time points, and the lack of standardized MRD assays. Therefore, clinical proof for improved outcome after MRD-directed therapy remains scarce. The interactions between MRD and presenting prognostic features continue to reveal an unforeseen complexity.2,3 Whereas genetic features such as unfavorable cytogenetics and poor-risk gene mutations are among the factors associated with an increased frequency of MRD after therapy, MRD status adds independent prognostic information within these established risk categories. Conversely, additional pretreatment factors that usually do not enter standard risk classification contribute to a patient's overall response to treatment (eg, resistance mechanisms, cell-cycle state, and DNA hypermethylation). Such uncategorized features associated with MRD occurrence and poor outcome include the frequency of cells with antigenic features of leukemic stem cells (LSCs), the drug efflux activity of P-glycoprotein (a multidrug resistance modulator),4 and the expression of Ag CD25 (the α-chain of the IL-2 receptor) by leukemic myeloblasts.3 All of these prognostic pretreatment parameters determine the likelihood of patients of being MRD+ after therapy.
The clinical significance of MRD
As a posttherapy prognosticator, MRD status has variable prognostic power depending on the time of assessment.2 Although rapid tumor clearance after therapy initiation is of critical value,5–8 some investigators have found that postconsolidation MRD levels carry superior prognostic power.9,10 Therefore, there are distinct approaches to the use of the information provided by MRD. One relies on MRD detection early on, after induction therapy, to refine risk stratification, which otherwise relies mostly on pretreatment parameters. Early evaluation of disease response may also be of particular interest in the context of novel, investigational agents. Risk-directed therapy not only aims at improving outcome, but also at averting excessive toxicities by not exposing patients unnecessarily to additional treatment. The other approach uses serial monitoring of MRD during hematologic remission in an effort to prevent the development of clinical relapse by reacting to increasing MRD levels with preemptive therapy. Although limited, prospective data available in AML support the clinical relevance of MRD-guided risk stratification.8,9 To date, the efficacy of preemptive therapy has only been proven in acute promyelocytic leukemia (APL)11 ; however, recent data in non-APL AML are also promising.12
It is still a matter of debate what is the best method to use to measure MRD. The various methods are compared based on their sensitivity, specificity, and applicability to the largest number of patients. Although MRD is quantified as a continuous variable reflected by the percentage of blasts among all normal nucleated cells, the absolute levels of leukemia transcripts or mutated genes or their log change with treatment, thresholds are regularly defined for clinical decision-making. Therefore, patients are classified in a dichotomous manner as high/positive MRD (above a given threshold) or low/negative MRD (below a given threshold). Whereas this may not always be biologically sound, it facilitates the use of MRD results in guiding therapeutic decisions. The chosen threshold determines both sensitivity and specificity of an MRD assay.13 Sensitivity reflects the assay's ability to classify patients as high MRD (ie, when the number of leukemic blasts in a tissue is higher than the set threshold); specificity reflects the assay's accuracy in defining patients with low MRD (ie, when the number of leukemic cells in a tissue is lower than the set threshold). MRD thresholds with prognostic relevance are highly dependent on methodology (molecular or immunophenotypic), MRD target, and trial design and, unless standardized, are difficult to compare across reports. Using multicolor antibody (Ab) combinations, the limit of detection with multiparameter flow cytometry (MPFC) can reach 10−4 (0.01%) depending upon the leukemia-associated immunophenotype (LAIP) used and sample quality, a level comparable to that achieved by RT-PCR methodology for measuring molecular targets. The few direct comparisons of the 2 methodologies published in AML have demonstrated that quantitation of molecular targets and MPFC were equally effective in identifying patients at high risk of relapse.14,15
Although RT-PCR for leukemia fusion transcripts is a very sensitive way to detect MRD, the clinical significance of a positive result (specificity) can vary with the time of assessment during the clinical course. The detection of low transcript levels in core-binding factor (CBF) leukemia patients in long-term remission curtails the clinical significance of PCR positivity in this AML subtype.9,14 The persistence of MRD in a presumably cured AML patient may reflect successful immune surveillance or the presence of the MRD target in LSCs that requires additional genetic hits for transformation and progression to overt disease. Corbacioglu et al recently revised the view on MRD in CBFβ/MYH11+ AML.16 Using the standardized Europe Against Cancer quantitative RT-PCR protocol, CBFβ/MYH11 copy ratios at diagnosis and after induction therapy did not affect outcome; however, achievement of MRD− in at least 2 specimens during or up to 3 months after consolidation predicted for long-term remission. Another unique situation is encountered in APL patients treated with all-trans retinoic acid and chemotherapy, in which persistent PML/RARα transcripts at the end of consolidation therapy carry a significant relapse risk. After induction therapy, however, the presence of PML/RARα in apoptotic, differentiating leukemic cells interferes with the interpretation of MRD.2,11 And yet, recommendations for the use of MRD in APL change when arsenic trioxide (ATO) is introduced in frontline therapy. After induction with single-agent ATO, any molecular MRD is associated with a risk of relapse.17 Therefore, the prognostic value of MRD needs to be assessed in the context of each therapy.
Another target for PCR-assays for MRD in AML are recurrent gene mutations, whereby most experience has been gained with internal tandem duplications of FLT3 (FLT3-ITD) and mutated NPM1 (NPM1MUT).9 Changes in mutation status between presentation and relapse may limit the clinical applicability of these markers18 and prompts the search for new, more stable mutations, such as of DNMT3A.19 Clonal instability implies that gene mutations should be reestablished at the time of relapse, not only for the purpose of MRD determination but especially before continuing therapy targeting those mutations. There is the possibility, however, that lack of longitudinal stability of gene mutations reflects insufficient sensitivity of currently used methodologies.9 Systematic assessment of MRD by next-generation sequencing (NGS) offers a novel platform with increased sensitivity, which may soon find its way into clinical diagnostic laboratories. NGS might be particularly useful for MRD targets like FLT3-ITD, which is plagued by mutational shifts between diagnosis and relapse, by multiclonality at presentation leading to the outgrowth of a clone at relapse different from that dominant at diagnosis, and by variable insertion sites and lengths among patients.20 Bachas et al recently provided evidence for the expansion of a minor oligoclonal LSC fraction present at initial diagnosis as the cause of mutational shift at relapse.21 High-sensitivity testing, possibly with NGS, may detect molecular aberrations at presentation in many more patients than hitherto appreciated, rendering those patients eligible for targeted therapy.
Increased sensitivity for MRD monitoring is particularly important post allogeneic hematopoietic stem cell transplantation (HSCT), when presence or rise in MRD is likely to be predictive of relapse.22 Especially in patients who lack specific molecular markers, quantitative analysis of chimerism (ratio of donor- to recipient-derived hematopoiesis) serves as a reliable indicator of imminent relapse, particularly if mixed chimerism is assessed in CD34+ cells.22 Because of the rapid dynamics of AML relapse, the higher the sensitivity of detection of increasing mixed chimerism, the better the chances for early intervention.23 Chimerism-based pre-emptive intervention with azacitidine has been shown to substantially delay relapse,24 allowing for donor lymphocyte infusions or a second HSCT.
Conversion of MRD− to MRD+ during CR would be a useful predictor of relapse, if prospective studies yield evidence for improved outcome when MRD is treated rather than frank hematologic relapse. The decision to treat pre-emptively is a challenging one, particularly when HSCT is planned. Therapies alternative to HSCT, such as combined anti-CD33 Ab, chemotherapy, donor lymphocyte infusion and cessation of immune suppression, delayed but did not prevent relapse.25 In contrast, anti-CD33 Ab was efficacious in eradicating molecular relapse in APL, where conversion from MRD− to MRD+ incontrovertibly leads to clinical relapse, if left untreated.11 It was recently shown that early intervention with ATO prevented progression of molecular to hematologic relapse.11
Variations in the kinetics of leukemic cells affect the usefulness of MRD monitoring for the detection of relapse and determine the frequency of optimal MRD assessments during CR.23 With respect to the potential need for frequent MRD sampling, the question of whether peripheral blood (PB) could replace bone marrow (BM) is important when considering patient comfort and cost. In AML with slow relapse kinetics, (eg, CBFβ/MYH11+ AML), sampling intervals may be longer,23 although Corbacioglu et al warned against too infrequent MRD testing in this disease.16 Due to variable, but generally faster, doubling times of the PML/RARα clone, more frequent sampling is necessary. The growth rate of NPM1MUTFLT3-ITD+ leukemic cells is double that of NPM1MUT/FLT3 wild-type (NPM1MUTFLT3WT) blasts,23 suggesting that relapse kinetics in AML depend on the complex genetic makeup of individual patients. Therefore, suggestions regarding the frequency of MRD sampling cannot be generalized, even within one genotype, but instead need to be determined for the unique combination of molecular features in every patient.
MPFC for MRD detection
The advantage of MPFC-based MRD-assays is that they determine accurately the number of leukemic cells and are applicable to the majority of AML patients. With common methodologies, only approximately 50% of AML patients have a suitable molecular target. Levels chosen to distinguish MPFC MRD+ from MRD− patients range from 0.035%-1%, with most studies using 0.1%.2,5,8,9,14,15,26,27 Leung et al, who studied both AML and acute lymphoblastic leukemia (ALL) patients with MPFC, found no added clinical benefit in reducing MRD levels in AML to those defining MRD positivity in ALL (<0.01%).26 The potentially higher prognostic MRD threshold in AML has important implications: (1) sensitivities of current MPFC MRD assays suffice and (2) reducing MRD levels in AML to those significant prognostically in ALL may not be necessary for improved clinical outcome. One significant drawback of most prognostic MRD levels to date is that they were derived retrospectively without validation in prospective clinical trials.
The analysis of immunophenotypic aberrancies with MPFC as a measure of MRD presents with its own challenges. In AML, unlike ALL, multiple LAIPs are detected regularly on subsets of blasts in individual patients at diagnosis.2,3 Comparisons of paired presentation/relapse samples often show selective LAIP changes. Such immunophenotypic shifts will not affect the utility of MPFC for MRD detection, provided that as many independent LAIPs as possible are monitored per patient.2,3,28 This approach also reduces the likelihood of false-positive MRD results due to the potential presence of LAIP Ag combinations at low frequencies in normal BM, BM after chemotherapy, or after growth factor administration.3,29 Although both specificity and sensitivity of detection of many LAIPs is limited by background, claims that MRD by MPFC will consistently produce low-level positivity in normal or treated BM13 are unfounded. Six (or more)–color technology and comprehensive Ab combinations, including Abs to LSCs and multiple lymphoid Ags, allow for the construction of LAIPs with the highest specificity.2,3,9 Granted, depending on the expertise of the flow cytometry operator, Ag expression patterns may be misinterpreted. In this regard, standardized protocols and automated data file analyses may be particularly useful for MRD detection in AML.30 In experienced laboratories, a positive MRD result by MPFC rests on the identification of only 20 clustered abnormal events. Because leukemic cells are quantified in relation to other cells in the specimen, the smallest abnormal cell cluster that can be reliably called MRD depends on the total number of cells analyzed. With 200 000 cells acquired, a 20-cell cluster represents a sensitivity of 1 in 104 (0.01%). The lower the number of abnormal cells present, the higher the number of cells required to be analyzed. As a result, if the sample quantity is limited, the sensitivity of the MRD assay will be lowered.2,3,13,30 This stresses the importance of sample quality for accurate MRD evaluation.
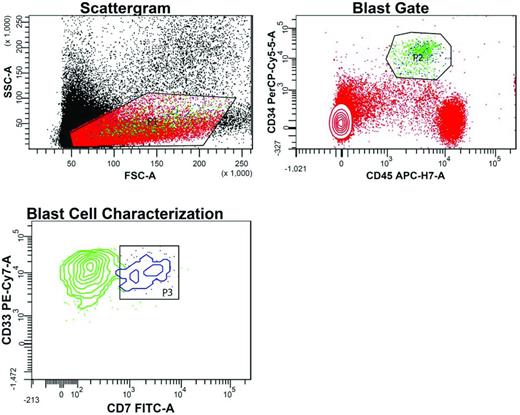
Abs suitable for MRD detection (1) distinguish leukemic blasts from normal myeloid precursors; (2) detect lineage-foreign markers (eg, lymphoid-affiliated Ags such as CD7 and CD19), (3) detect altered density of myeloid or lineage-uncommitted Ags compared with normal myeloid precursors (eg, CD33 and CD11a), or (4) detect asynchronous expression of Ags, (eg, CD123 and CD34). Figure 1 illustrates the usefulness of CD7 expression for detecting MRD in a patient treated for AML.
Flow cytometric detection of MRD after induction chemotherapy in the BM of an AML patient led by the patient's LAIP. In the Scattergram, a gate (P1) is set around cells with low forward scatter (FSC) and side scatter (SSC), reflecting small to intermediate size and low granularity, respectively. In the blast gate, cells (green dots) within P1 with high CD34 and intermediate CD45 expression are selected away (P2) from normal lymphocytes (red dots with high CD45 expression) and erythroid precursor cells (red dots lacking CD45). Gating based on the typically reduced intensity of staining with the pan-leukocyte marker CD45 facilitates the detection of leukemic cells. In the blast cell characterization contour plot, MRD is detected within the CD34HIGHCD45WEAK gate based on the dual expression of CD33 and CD7 (P3, blue cluster). These cells with the patient's LAIP features account for 0.013% of all nucleated cells, a common denominator for MRD definition. The remaining green cells in this plot represent normal myeloid precursor cells (CD33+CD7−) caught in P2.
Flow cytometric detection of MRD after induction chemotherapy in the BM of an AML patient led by the patient's LAIP. In the Scattergram, a gate (P1) is set around cells with low forward scatter (FSC) and side scatter (SSC), reflecting small to intermediate size and low granularity, respectively. In the blast gate, cells (green dots) within P1 with high CD34 and intermediate CD45 expression are selected away (P2) from normal lymphocytes (red dots with high CD45 expression) and erythroid precursor cells (red dots lacking CD45). Gating based on the typically reduced intensity of staining with the pan-leukocyte marker CD45 facilitates the detection of leukemic cells. In the blast cell characterization contour plot, MRD is detected within the CD34HIGHCD45WEAK gate based on the dual expression of CD33 and CD7 (P3, blue cluster). These cells with the patient's LAIP features account for 0.013% of all nucleated cells, a common denominator for MRD definition. The remaining green cells in this plot represent normal myeloid precursor cells (CD33+CD7−) caught in P2.
Characteristic immunophenotypes that are associated with recurrent genetic lesions, also termed surrogate marker profiles,3,31 allow a highly focused approach to MRD assessment (eg, CD19+CD11a−CD56+/− AML1/ETO+ AML or CD2+ CBFβ/MYH11+ AML). Unfortunately, to date, reliable surrogate profiles are rare and do not comprise common gene mutations in AML, such as FLT3-ITD. NPM1MUT AML typically lacks CD34 and CD133 expression3 ; this relationship between genotype and immunophenotype is useful for MRD testing, even if it is not found in all cases. The disappearance of characteristic Ag profiles at relapse could signify the loss of the defined molecular clones.3,21
Independent from the initial LAIP is the “different-from-normal” approach, which uses a standard Ab panel with every patient and recognizes leukemic cells based on their characteristic antigenic patterns that differ sufficiently from those expected in normal hematopoietic elements in the various myeloid cell lineages to allow them to be recognized even when present at very low levels.2,3,27 Pioneered in the Children's Oncology Group, this methodology is allegedly advantageous to LAIP-MPFC in monocytic leukemias, which present with few aberrant antigenic targets for LAIP-guided monitoring.
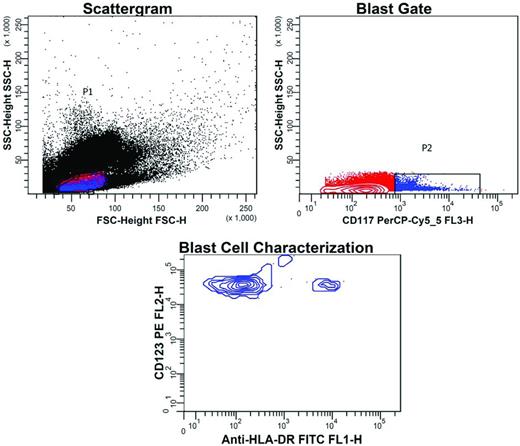
MPFC also provides a means to detect LSCs: their frequency at diagnosis predicts for the occurrence of MRD3 and their presence before and after therapy is associated with relapse (“stem cell MRD”).3,32–34 The persistence of LSCs after therapy and outgrowth at relapse may explain treatment failure in MPFC-MRD− AML,9,21 because routine MRD Ab panels fail to detect LSCs. Contained within the CD34+CD38− cell compartment, LSCs in AML, but not APL, differ from normal hematopoietic stem cells by high expression of several Ags, including CD123 and C-type lectin-like molecule-1 (CLL-1).3,33 Figure 2 exemplifies MRD monitoring based on the LSC phenotype CD123+CD117+CD34+HLA-DR−CD38−. Figure 3 shows the usefulness of CD25, another LSC Ag.
Flow cytometric detection of LSCs in a patient treated for AML. BM from a patient treated for AML was tested for MRD. Cells with the typical immunophenotype of LSCs had been demonstrated at this patient's presentation: CD34+CD123+CD117+HLA-DR−CD38−. After therapy, sequential gating for the presence of this phenotype was performed and detected 0.02% of these cells. In the scattergram, gate P1 was drawn around agranular cells (low side scatter, SSC) of small size (low forward scatter, FSC). In the blast gate, a second gate (P2) was drawn around cells with CD117 expression (blue dots), whereas the population of red cells in the first gate was CD117−. In the blast cell characterization contour plot, blast cells within the CD117+ cell population were demonstrated by expression of CD123, but negativity for HLA-DR. The small cell cluster with high HLA-DR expression represents some normal stem cells.
Flow cytometric detection of LSCs in a patient treated for AML. BM from a patient treated for AML was tested for MRD. Cells with the typical immunophenotype of LSCs had been demonstrated at this patient's presentation: CD34+CD123+CD117+HLA-DR−CD38−. After therapy, sequential gating for the presence of this phenotype was performed and detected 0.02% of these cells. In the scattergram, gate P1 was drawn around agranular cells (low side scatter, SSC) of small size (low forward scatter, FSC). In the blast gate, a second gate (P2) was drawn around cells with CD117 expression (blue dots), whereas the population of red cells in the first gate was CD117−. In the blast cell characterization contour plot, blast cells within the CD117+ cell population were demonstrated by expression of CD123, but negativity for HLA-DR. The small cell cluster with high HLA-DR expression represents some normal stem cells.
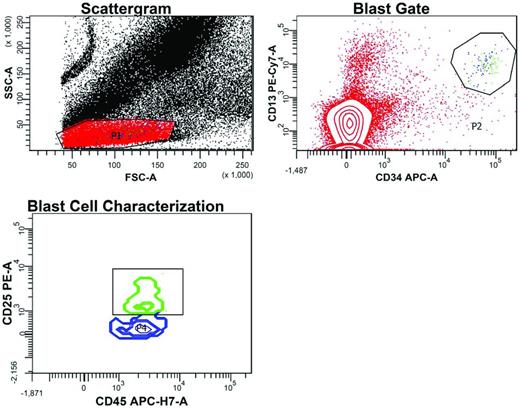
Flow cytometric detection of MRD after induction chemotherapy in the peripheral blood of an AML patient led by the patient's LAIP, which included CD25. In the scattergram, a gate (P1) is set around cells with low forward scatter (FSC) and side scatter (SSC), reflecting small to intermediate size and low granularity, respectively. In the blast gate, cells within P1 with high CD34 and high CD13 expression are selected away (P2) from CD34−CD13− cells (red dots), representing predominantly lymphocytes. Some CD34−CD13+ cells are monocytes caught in P1. In the blast cell characterization contour plot, MRD is detected within the CD34+CD13+ gate based on the expression of CD25 and intermediate CD45 staining (green cluster). These cells with the patient's LAIP features (CD34+CD13+CD45WEAKCD25+) account for 0.003% of all nucleated cells, a common denominator for MRD definition. The blue CD25− cluster represents normal myeloid precursor cells (CD34+CD13+CD45WEAKCD25−) caught in P2.
Flow cytometric detection of MRD after induction chemotherapy in the peripheral blood of an AML patient led by the patient's LAIP, which included CD25. In the scattergram, a gate (P1) is set around cells with low forward scatter (FSC) and side scatter (SSC), reflecting small to intermediate size and low granularity, respectively. In the blast gate, cells within P1 with high CD34 and high CD13 expression are selected away (P2) from CD34−CD13− cells (red dots), representing predominantly lymphocytes. Some CD34−CD13+ cells are monocytes caught in P1. In the blast cell characterization contour plot, MRD is detected within the CD34+CD13+ gate based on the expression of CD25 and intermediate CD45 staining (green cluster). These cells with the patient's LAIP features (CD34+CD13+CD45WEAKCD25+) account for 0.003% of all nucleated cells, a common denominator for MRD definition. The blue CD25− cluster represents normal myeloid precursor cells (CD34+CD13+CD45WEAKCD25−) caught in P2.
Association of MRD with presenting features
MRD is one of few posttreatment prognostic factors, others being the number of chemotherapy cycles needed to achieve CR and blood count recovery after induction. Although presenting standard prognostic features (ie, cytogenetics and gene mutation status) predict for the occurrence of MRD after therapy, MRD improves standard risk-classification independently because it reflects physiologic mechanisms not comprised in established prognostication, including multidrug resistance drug-efflux pumps and apoptosis. The practical implication of this information is that accurate risk allocation in AML has to wait at least until the MRD status of a patient is known after induction.
Cytogenetic and increasingly molecular features at diagnosis stratify AML patients into favorable, intermediate, and unfavorable risk. It is generally accepted that favorable-risk patients do well without aggressive therapy, whereas unfavorable-risk patients require HSCT and further prognostic stratification may therefore appear to be meaningless. However, favorable- and intermediate-risk patients who achieve MRD− do substantially better than those who remain MRD+ after therapy, warranting the reallocation of patients to favorable or unfavorable categories based on MRD status.9 MRD levels are significantly higher in AML with unfavorable compared with favorable cytogenetics.4 Regardless, within each cytogenetic risk class, MRD levels carry prognostic significance,5,9 leading to the suggestion that all patients who fail to achieve MRD− after consolidation should be considered for HSCT irrespective of their cytogenetic risk category.9 The pediatric groups now use combined cytogenetic-molecular MPFC MRD information to stratify patients after induction into low-risk (favorable gene mutations or MRD−) and high-risk (adverse cytogenetic abnormalities, high ratio of FLT3-ITD to FLT3-WT, or MRD+), offering the latter HSCT in first remission.35 In pediatric APL, MRD− after 3 treatment courses makes children eligible for reduced anthracyclines (reduced cardiotoxicity) together with ATO, whereas high-risk and MRD+ standard-risk patients are treated equally, with higher anthracycline exposure.35
Favorable-risk CBF-AML patients have a lower likelihood and high-risk FLT3-ITD+ AML patients a higher likelihood of being MRD+ after induction than do patients with other genotypes.8 Nonetheless, among CBF leukemia patients, those with sustained MRD+ have a high relapse risk,14,16 and MRD+FLT3WT patients fare as poorly as MRD+FLT3-ITD+ patients.9 Likewise, NPM1MUT and NPM1WT AML patients do not differ in outcome when stratified by MRD status after consolidation.9 NPM1MUT transcript levels after therapy are lower in NPM1MUTFLT3WT than NPM1MUTFLT3-ITD+ AML,36,37 which is consistent with the better prognosis of the former. These data suggest that the various genetic lesions present in individual patients affect the incidence of MRD.
The choice of tissue for MRD determination in remission
Hemodilute BM aspirates yield lower MRD levels if the contaminating PB contains fewer residual blast cells. In APL, MRD conversion occurs earlier in the BM than in the PB.11 For molecular MRD targets with slow relapse kinetics, such as CBFβ/MYH11 and NPM1MUT (only when FLT3-ITD−), PB testing may suffice23 provided that MRD sampling is done frequently for the period in which most relapses occur.16 In NPM1MUT AML, MRD− results in PB despite continued MRD+ BM tests increase with prolonged therapy.37 With MPFC, the PB contains distinctly fewer residual blasts than BM at all time points.9,28 However, MRD information from the PB can have prognostic significance.28,30 Despite lower sensitivity, MRD MPFC assays in PB have higher specificity due to the absence of normal immature myeloid cells, which can complicate MRD analysis in BM. With gene overexpression as the MRD target, the normal physiologic background is lower in the PB than in the BM, thus interfering less with MRD interpretation.2,9,23
Clinical trials aimed at eradicating or preventing MRD
It is generally accepted that assessing the response to therapy is essential for the treatment of all patients with AML, irrespective of standard genetic risk. However, without standardized MRD assays and validated prognostic MRD thresholds, MRD-guided clinical trials in AML remain rare. The expectation of MRD-driven, individualized therapy is that current therapeutic strategies are efficacious in eradicating MRD. Evidence to this respect in AML is still lacking. In the pediatric AML02 trial, MRD levels did not differ whether induction included high- or low-dose cytarabine, being paralleled by similar relapse and survival parameters.8 In each treatment arm, the presence of high MRD (≥ 1%) was an independent adverse prognostic factor for survival. The benefits of allogeneic HSCT in MRD+ pediatric patients8,38 or adult AML patients39 are questionable, even though a St Jude study26 suggested that improvements in HSCT over time have diminished the negative effects of MRD. Further reduction of MRD before transplantation in that study did not impact outcome, a finding that contrasts with observations in ALL.
Current strategies in eliminating MRD are disappointingly limited. Clofarabine and cytarabine have been tested in pediatric AML patients in CR with MRD > 0.1% (NCT01158885). This drug combination was used previously in adult AML (NCT00863434), although data on this are not yet available. Despite the beneficial effect of clofarabine on intracellular concentrations of cytarabine triphosphate, the active form of cytarabine, the inefficiency of cytarabine against LSCs does not make it an ideal drug in MRD eradication.40 In the GIMEMA AML1310 trial (NCT01452646), patients 60 years of age or younger with intermediate genetic risk are stratified according to postconsolidation MPFC-MRD levels and receive risk-adapted therapy (autologous vs allogeneic HSCT).
Novel therapies are needed to treat MRD+ AML after chemotherapy more successfully; for example, in the form of immunotherapy.41 Two ongoing trials in adult AML are testing this hypothesis: NCT01347996 is assessing the effects of maintenance therapy with histamine dihydrochloride (Ceplene) and low-dose IL-2 on MRD and NCT01041040 (LAM07), which is being conducted by the PETHEMA Foundation, stratifies patients before first consolidation according to karyotype and molecular findings at presentation and ≥ 0.1% MRD at the end of induction. Consolidation treatment is tailored for each risk group, the anti-CD33 Ab gemtuzumab ozogamicin is given in addition to chemotherapy in the intermediate- and high-risk group, and allogeneic HSCT in first remission is saved for high-risk patients. Similar in design to the pediatric AML02 trial,8 this is the first trial in adult AML that responds to MRD-positivity with targeted immunotherapy. Therapeutic vaccination with dendritic cells, which is intended to generate antileukemic T-cell responses, is another investigational approach with great potential for eradicating MRD.42 A randomized trial of epigenetic priming with the hypomethylating drug decitabine (NCT01177540) also holds promise. Preemptive therapy with the DNA-methylation inhibitor 5-azacytidine in 10 NPM1MUT AML patients in molecular relapse yielded promising results.43 Given the multifaceted physiologic processes that affect a patient's response to treatment, such as immunosuppressive features of leukemic blasts and DNA hypermethylation, investigational protocols that address some of these variables should be strongly encouraged.
Another application of MRD is its role as an efficiency biomarker, which indicates whether a response has occurred in patients treated with a particular therapy. In fact, MRD was discussed as a surrogate end point for treatment efficacy in new drug approval for ALL at a Food and Drug Administration (FDA)– and American Society of Clinical Oncology (ASCO)–sponsored workshop on MRD as a surrogate end point in ALL held in Silver Spring, MD, on April 18, 2012. However, before deciding that drug activity can be measured in terms of molecular and/or immunophenotypic responses, proof has to be provided for the concept that MRD levels after therapy actually reflect the outcome observed subsequently with various treatment strategies. The other prerequisite is the availability of standardized MRD assays that can pass the requirements set forth by the FDA for integral markers in their Investigational Device Exemption reviews.44
Perspectives
Theoretically, we could be ready to treat every patient with AML individually according to risk allocation, which must include MRD levels. This approach would intensify treatment or switch chemotherapy to an MRD-targeting strategy in MRD+ patients and reduce treatment intensity in MRDLOW/− patients. In pediatric AML, MRD has been added to conventional risk stratifications, but not so in adult AML (with the exception of APL). Randomized trials assigning patients in morphologic CR to MRD-adapted treatment arms are a rarity rather than the norm. Recently, the National Cancer Institute (NCI) has initiated an effort to standardize MPFC-MRD measurements among the leukemia reference laboratories of the 3 adult cooperative groups. Although seemingly small, this is a step of the utmost importance in the right direction.
Acknowledgments
The author thanks Xerxes Vevai and Kerrie O'Shea for excellent work in her laboratory, which produced the MRD data discussed in this manuscript; Dr Letizia Foroni from the Imperial Molecular Pathology Laboratory (Hammersmith Hospital, London, United Kingdom), for expert reading of the manuscript; and the Eastern Cooperative Oncology Group (ECOG), whose support over the past 25 years has fostered her work in the acute leukemias. The author apologizes to those investigators whose work was cited as part of a review due to restrictions in the number of references. Work from the author's laboratory was supported by the National Cancer Institute of the National Institutes of Health (CA021115 and CA114737).
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Elisabeth Paietta, PhD, Montefiore Medical Center, North Division, 600 E 233rd St, Bronx, NY 10466; Phone: 718-920-9520; Fax: 718-920-1161; e-mail: epaietta@earthlink.net.