Abstract
Salvage chemotherapy followed by high-dose therapy and autologous stem cell transplantation is the standard of treatment for chemosensitive relapses in diffuse large B-cell lymphoma. The addition of rituximab to chemotherapy has improved the response rate and failure-free survival after first-line treatment and relapses. Fewer relapses are expected, although there is no consensus on the best salvage regimen. The intergroup Collaborative Trial in Relapsed Aggressive Lymphoma (CORAL) set the limits for this standard of treatment after first comparing 2 salvage regimens: rituximab, ifosfamide, etoposide, and carboplatin (R-ICE) and rituximab, dexamethasone, aracytine, and cisplatin (R-DHAP). There was no difference in response rates or survivals between these salvage regimens. Several factors affected survival: prior treatment with rituximab, early relapse (< 12 months), and a secondary International Prognostic Index score of 2-3. For patients with 2 factors, the response rate to salvage was only 46%, which identified easily a group with poor outcome. Moreover, patients with an ABC subtype or c-MYC translocation responded poorly to treatment. More than 70% of patients will not benefit from standard salvage therapy, and continued progress is needed. Studies evaluating immunotherapy after transplantation, including allotransplantation, new conditioning regimens with radioimmunotherapy and other combinations of chemotherapy based on diffuse large B-cell lymphoma subtype, are discussed herein. Early relapses and/or patients refractory to upfront rituximab-based chemotherapy have a poor response rate and prognosis. A better biological understanding of these patients and new approaches are warranted.
Introduction
The development of rituximab, a chimeric anti-CD20 Ab, represented a revolutionary advance in the therapy of hematologic malignancies. The combination of rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) has produced significant survival benefits in elderly patients with untreated diffuse large B-cell lymphoma (DLCBL) compared with CHOP alone.1 The same immunochemotherapy regimen has resulted in an improved outcome in young, low-risk patients as defined by the age-adjusted International Prognostic Index (aaIPI).2 Despite these major advances, some patients will experience early treatment failure, a partial response, or a relapse after the initial chemotherapy. In this context, treatment is a major challenge. The initial approach to relapsed DLBCL management is to determine whether a patient is a candidate for high-dose therapy (HDT) and autologous stem cell transplantation (ASCT). In 1995, the PARMA trial evaluated salvage chemotherapy with a platinum- and cytarabine-based (DHAP) regimen alone or in combination with ASCT.3 Both the event-free survival (EFS) and overall survival (OS) were superior in the transplantation group compared with patients receiving chemotherapy alone. Based on these results, HDT/ASCT has become the standard of care in younger patients with chemosensitive relapsed or primary refractory aggressive lymphoma. However, in the rituximab era, the impact of chemotherapy on the final therapeutic results must be determined along with other prognostic factors. For example, how many patients require salvage treatments and is salvage with ASCT an efficient treatment?
What is the rate of relapse or failure according to aaIPI after first-line treatment?
In young patients (< 60 years) with no adverse prognostic factors, the expected relapse rate after first-line treatment for DLBCL is 10%.2 In the case of only one IPI factor, the rate of relapse varies from 20%-30% depending on whether the patient has received treatment with dose-intensive R-ACVBP (Adriamycin, cyclophosphamide, vindesine, and prednisone) or R-CHOP.4 Fewer data are available for patients with 2 or 3 aaIPI factors, but the relapse rate is estimated at between 25% and 35% after generally intensive treatment and ASCT.5,6 Finally, in patients between 60 and 70 years of age, the relapse rate was 40% in the R-CHOP study. Despite major progress, the number of patients eligible for salvage treatment and ASCT remains significant.
What are the results of salvage regimens before HDT/ASCT?
Only patients identified with a disease responsive to salvage regimens are eligible for HDT/ASCT. The addition of rituximab was expected to improve the complete remission and partial remission (CR/PR) rates and allow more patients to access HDT/ASCT. The initial studies incorporating rituximab in salvage regimens, mostly in rituximab-naive patients, were encouraging (Table 1).7–10
Rituximab-based salvage therapy in relapsing/refractory DLBCL
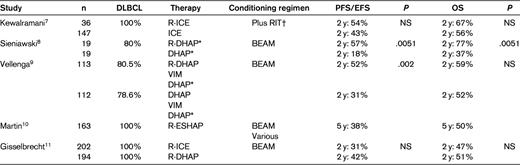
NS indicates not significant.
*Plus RIT at bulky disease.
†The choice of conditioning regimen depended on physician decision and the clinical trial.
A clear demonstration of the impact of rituximab is provided by a prospective randomized trial exploring the potential benefits associated with the addition of rituximab to platinum-based salvage regimens. In a study conducted by the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) group, 239 patients with relapsed or refractory DLBCL received a salvage regimen consisting of DHAP-VIM-DHAP with or without rituximab followed by ASCT.9 The analysis of the 225 evaluable patients showed that after 2 courses of chemotherapy, CR/PR was obtained in 54% of the patients in the DHAP arm and 75% in the R-DHAP arm (P ≤ .01). Posttransplantation PR and CR were obtained in 50% and 73% of the patients, respectively (P = .003). A marked difference in favor of the R–DHAP arm was observed at 24 months for failure-free survival, at 50% compared with 24% (P < .001), respectively, but not for OS, at 52% compared with 59% (P = .15), respectively. A Cox regression analysis demonstrated a significant effect of rituximab treatment on failure-free survival and OS when adjusted for time since upfront treatment, age, performance status, and secondary aaIPI. However, < 5% of the patients had been exposed to rituximab previously.
Is there an optimal salvage chemotherapy regimen?
The Collaborative Trial in Relapsed Aggressive Lymphoma (CORAL) intergroup trial compared a combination therapy consisting of rituximab, ifosfamide, etoposide, and carboplatin (R-ICE) with rituximab, dexamethasone, aracytine, and cisplatin (R-DHAP). Patients who were DLBCL CD20+ at the time of the first relapse and patients remaining refractory after first-line therapy were randomized between the R-DHAP and R-ICE groups. Responding patients received BEAM and ASCT and were randomized between observation and rituximab maintenance for 1 year. An analysis was conducted on the first 396 randomized patients (R-ICE, n = 202; R-DHAP, n = 194).11 The median age was 55 years. In 225 patients, a relapse after > 12 months was observed after initial CR. In 166 cases, patients did not achieve initial CR (ie, were refractory) or had early relapses at < 12 months. A group of 244 patients (63%) received immunochemotherapy with rituximab as the first-line treatment. At the time of relapse, 226 patients had a secondary aaIPI score of 0-1 and 149 patients had an aaIPI of 2-3. The overall response rate was 63%; 38% of patients achieved CR. There was no difference between the response rates in the R-ICE (63.5%; 95% confidence interval, 56%-70%) and R-DHAP (62.8%; 95% confidence interval, 55%-69%) groups or in the mobilization-adjusted response rates (52% vs 54%, respectively). The factors that affected the response significantly (P < .0001) were as follows: refractory/relapse at < 12 months, with response rates of 46% versus 88%, secondary aaIPI > 1 (52% vs 71%), and prior exposure to rituximab (51% vs 83%). There were no statistically significant differences between the patients achieving CR/PR just before ASCT. Patients with prior exposure to rituximab had more refractory disease and adverse prognostic factors.
Based on this first randomized study on relapses, there is no difference in the response rates and the ability to mobilize stem cells between the 2 commonly used regimens. A similar study under the National Cancer Information Center (NCIC) sponsorship comparing R-GDP (gemcitabine, dexamethasone, and platinum) with R-DHAP has been completed and results are awaited. There is obviously a need to incorporate new agents to improve the response rates of salvage regimens. Several phase 1/2 clinical trials are currently under way. For example, one trial has combined inotuzumab ozogamicin, a humanized anti-CD22 Ab conjugated to cytotoxic calicheamicin, with rituximab, gemcitabine, and oxaliplatin (R-Gem-Ox)12 and another trial has added lenalidomide to various regimens such as R-DHAP.
What are the clinical characteristics of patients failing salvage treatment?
Only 206 ASCTs were performed in the CORAL study, which had an dropout rate of 50% mainly due to tumor progression.11 Despite salvage therapy, only approximately half of patients actually proceed to transplantation, a figure that identifies one of the key limitations to current salvage practice. There was no significant difference between the R-ICE and R-DHAP groups with respect to 3-year EFS (26% vs 35%, respectively; P = .6) or OS (47% vs 51%, respectively; P = .5). The 3-year EFS was affected by the following factors: prior treatment with rituximab (21%) versus none (47%; P < .0001), early relapse at < 12 months (20%) versus > 12 months (45%; P < .0001), and secondary aaIPI 2-3 (18%) versus 0-1 (40%; P = .0001). In the Cox model, all of these parameters were significant (P < .0001) for EFS, progression-free survival (PFS), and OS, but not the treatment arm. Because early relapse (< 12 months) was the main prognostic factor, we more closely examined the population with a relapse at > 12 months to determine whether prior exposure to rituximab affected the outcome. There was no difference in EFS or OS between the 2 subgroups with or without rituximab exposure. At the other end of the spectrum, the population of relapsed patients with prior rituximab exposure and relapse at < 12 months had a 46% response rate, with a 3-year PFS rate of 23% and a median PFS of 5 months (Figure 1). The same negative impact of rituximab was found in other nonrandomized studies.10
PFS according to failure from diagnosis by prior rituximab therapy in DLBCL (CORAL study). Failure from diagnosis was < 12 months.
PFS according to failure from diagnosis by prior rituximab therapy in DLBCL (CORAL study). Failure from diagnosis was < 12 months.
Due to the introduction to the armamentarium of a very efficient mAb for first-line treatment, we are now observing patients with early relapse who are more refractory to any available treatment. Currently, salvage therapy using HDT/ASCT is inefficient and requires improvement. One may even question whether it is helpful, because no randomized study has been modeled after the PARMA trial. In the European Group for Blood and Marrow Transplantation (EBMT) registry, we found 470 relapsed DLBCL patients, but only those in CR after salvage and pre-ASCT were evaluated.13 The median duration of the first remission was < 12 months in 49% of the patients; 119 patients received rituximab in the first-line treatment and 351 did not. The 5-year OS was 63% and the 5-year PFS was 48%. The duration of post-ASCT PFS was longer than that before ASCT in 289 patients. When each patient was used as his/her own control, PFS after ASCT was longer than PFS before ASCT (P < .001). This difference in favor of post-ASCT remission was significant for patients with or without rituximab exposure. In patients responding to a salvage regimen, even those in good PR, HDT/ASCT remains the best choice of treatment.14
Is determining the biological characteristics of patients helpful for adapting treatment?
Among the 394 patients included in the CORAL study, histological material was available for a total of 249 patients at the time of diagnosis (n = 189 cases) and/or relapse (n = 147 cases). The samples were analyzed by immunohistochemistry to establish cell of origin using CD10, BCL6, MUM1, FOXP1, and BCL2 expression and by FISH for BCL2, BCL6, and c-MYC break points.15 One interesting finding was the similar biological characteristics between diagnosis and relapse biopsies in 87 pair-matched patients.
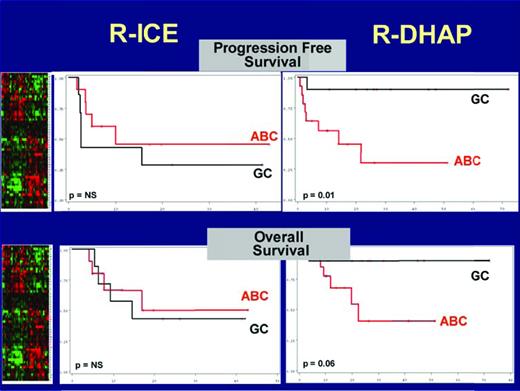
When treatment interaction was tested, the GCB-like DLBCL based on the Hans algorithm was significantly associated with a better PFS in the R-DHAP arm.16 In a multivariate analysis, independent prognostic relevance was found for the interaction between GCB/non-GCB Hans phenotype and treatment (P = .04), prior rituximab exposure (P = .0052), secondary aaIPI (P = .039), and FoxP1 expression (P = .047). Confirmation was obtained by gene-expression profiling in a subset of 39 patients (Figure 2). In a univariate analysis, the presence of c-MYC gene rearrangement was the only parameter significantly correlated with both a worse PFS (P = .02) and a worse OS (P = .04). Of the 161 patients analyzed, 28 (17%) presented with a MYC+ DLBCL, targeted as either a simple hit (25%) or complex hits (n = 75%), including MYC/BCL2, MYC/BCL6, and MYC/BCL2/BCL6.17 The outcomes of patients with MYC+ DLBCL were significantly worse than those with MYC− DLBCL, with the 4-year PFS rate at 18% versus 42% (P = .0322), respectively, and the 4-year OS rate at 29% versus 62% (P = .0113), respectively. The type of treatment (RDHAP or RICE) had no impact on survival, with the 4-year PFS rate at 17% versus 19%, respectively, and the 4-year OS rate at 26% versus 31%, respectively. These findings underline the need to study the effects of new treatments according to DLBCL subtypes.
OS and PFS according to the treatment and GC/ABC classified by the gene predictor based on gene signatures.34 Gray lines indicate patients with a GCB profile (n = 19, 51%). Red lines indicate patients with an ABC profile (n = 18, 49%). Patients with GCB-like DLBCL treated with R-DHAP had a significantly better PFS and OS than patients with ABC-like DLBCL treated with R-DHAP. Patients treated with R-ICE had poor survival regardless of the molecular subtype. Adapted by Catherine Thieblemont from Thieblemont et al.15
OS and PFS according to the treatment and GC/ABC classified by the gene predictor based on gene signatures.34 Gray lines indicate patients with a GCB profile (n = 19, 51%). Red lines indicate patients with an ABC profile (n = 18, 49%). Patients with GCB-like DLBCL treated with R-DHAP had a significantly better PFS and OS than patients with ABC-like DLBCL treated with R-DHAP. Patients treated with R-ICE had poor survival regardless of the molecular subtype. Adapted by Catherine Thieblemont from Thieblemont et al.15
Rituximab for posttransplantation maintenance/consolidation
After transplantation, the rate of progression in the CORAL study was 39% at 3 years. The focus should now be on preventing relapses. Post-ASCT rituximab maintenance has been evaluated as a means to reduce minimal residual disease. Two institutions have independently reported improvements in disease-free survival and OS rates with the use of rituximab after ASCT. In the first study,18 concurrent administration of rituximab was introduced at a dose of 1000 mg/m2 before the collection of peripheral blood stem cells for a purging effect and after transplantation on days 1 and 8. The disease-free survival rate after a median follow-up of 20 months was 67% (compared with 43% in a historical control group). The 2-year OS rate was 80%, an improvement compared with the 53% in historical controls who underwent ASCT without rituximab. In another study,19 rituximab was given once weekly at weeks 4-8 after salvage ASCT (and repeated if needed over 4 weeks at month 6) to 21 patients with relapsed or refractory large-cell lymphoma. After a median follow-up of 30 months, the EFS rate was 81% and the OS rate was 85%. It should be noted that there was an increased risk of prolonged neutropenia complicated by infection and hypogammaglobulinemia.
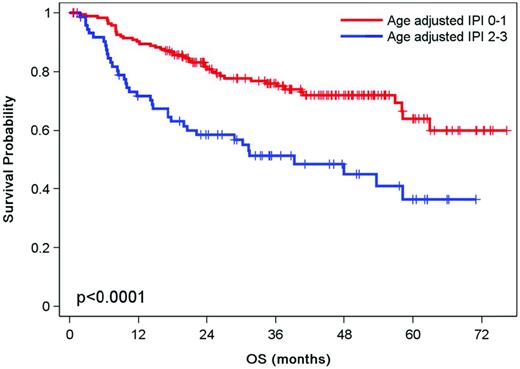
The second question addressed in the CORAL study was whether additional therapy with rituximab improves PFS. Patients were randomized after transplantation to observation or rituximab given at a dose of 375 mg/m2 every 2 months for 1 year.20 The 4-year post-ASCT EFS rates were 52% and 53% for the 122 patients with rituximab and the 120 patients in the observation group, respectively (P = .7). Several factors affected in univariate analysis EFS posttransplantation (P < .05), including relapsed disease within 12 months (46% vs 56%, respectively), secondary aaIPI > 1 (37% vs 61%, respectively), and prior treatment with rituximab (47% vs 59%, respectively) (Figure 3). However, the Cox model revealed that only a secondary aaIPI of 2-3 remained significant (P < .001) for EFS, PFS, and OS. The relapse rate posttransplantation remains over 40% despite adjuvant treatment with anti-CD20. The introduction of other immunomodulatory agents constitutes an alternative that remains to be established in a randomized study.
OS according to the aaIPI at the time of relapse in DLBCL after transplantation.
OS according to the aaIPI at the time of relapse in DLBCL after transplantation.
Improving the conditioning regimen
A further attempt to develop a more effective therapeutic strategy for relapsed DLBCL patients consists of the combination of radioimmunotherapy (RIT) with standard chemotherapy regimens. After an initial report of the use of high-dose iodine-131 and ASCT,21 several studies have used myeloablative RIT with promising results. The development of radiolabeled immunotherapies such as 90Y-ibritumomab tiuxetan has capitalized on the targeting ability of Abs to deliver therapeutic doses of radiation to disseminated tumor sites with limited radioprotection. In a phase 2 study, 90Y-ibritumomab tiuxetan combined with BEAM was superior to a historical control in the salvage of patients with high aaIPI scores.22 To further increase the therapeutic potential of RIT, a dose escalation study for 90Y-ibritumomab tiuxetan with BEAM and ASCT has been performed.23 The delivered RIT dose could safely reach 70 mCi, twice the standard dose, in 44 patients. Careful dosimetry was required to avoid toxicity rather than weight-based dose escalation. More recently, a randomized study compared 90Y-ibritumomab tiuxetan and BEAM chemotherapy versus BEAM alone in 22 and 21 patients, respectively, followed by ASCT. The 2-year OS rates were 91% and 62% after the Z-BEAM and BEAM treatments, respectively (P = .05).24 The investigators concluded that Z-BEAM was safe and possibly more effective than BEAM alone in the era of rituximab. Almost simultaneously, the results of the randomized comparison between 2 conditioning regimens were reported: rituximab BEAM versus iodine-131/tositumomab BEAM followed by ASCT.25 For the 113 patients treated with Rituxan-BEAM, the 2-year PFS rate was 49%, which was similar to the 2-year PFS rate (48%) of the 111 patients treated with 131-iodine/tositumomab BEAM. One of the principles of a conditioning regimen with ASCT is to use drugs such as alkylating agents at the maximum tolerated dose for nonhematologic toxicities. It should be noted that the RIT dose used in the RIT-BEAM treatment was a standard dose with limited myelosuppression, which might explain its lack of superiority. If new developments are to be made in this field, a regimen with a higher RIT dose or with other noncompeting antigens such as CD22 should be implemented.
Is there a place for reduced-intensity allografts?
Unlike ASCT, allogeneic SCT (alloSCT) generates an allogeneic GVL effect that reduces the likelihood of disease relapse after transplantation. It needs to be pointed out that previous studies, including the large Center for International Blood and Marrow Transplant Research (CIBMTR) report,26 suggested a very modest GVL effect (if any) in aggressive histology lymphomas. Standard myeloablative conditioning regimens were associated to unacceptable treatment related deaths and age limitations. The advent of reduced-intensity conditioning (RIC) regimens has renewed interest in alloSCT, which reduces nonrelapse mortality while maintaining a GVL effect and therefore allows the treatment of elderly patients and/or patients with comorbidities. Currently, the major role of RIC allogeneic transplantation (RIC-allo) is in the treatment of patients who have failed an autograft or in whom an autograft is not possible. Although RIC-allo has only been used for a few DLBCL patients, the results suggest that it may be beneficial. In previously published studies of RIC-allo, the rates of relapse at 2 or 3 years ranged from 33%-79%27 ; the stringency of patient selection is likely to be a major reason for such discrepancies. The use of RIC-allo in 48 consecutive patients with DLBCL (18 transformed from follicular lymphoma), 69% of whom had failed a previous autograft, was also reported,28 with an OS rate of 47% at 4 years. The French Society of Marrow Transplantation and Cellular Therapy reported 68 patients29 who had received a median of 2 regimens of therapy before RIC-allo, and 54 (79%) had previously undergone ASCT. Before allotransplantation, 32 patients (47%) were in CR. With a median follow-up of 49 months, the estimated 2-year OS, PFS, and cumulative incidence of relapse rates were 49%, 44%, and 41%, respectively. The 1-year cumulative incidence of nonrelapse mortality was 23%. Given the poor prognosis of this subset of patients when treated with conventional therapy, these results suggest that RIC-allo is an attractive therapeutic option for patients with high-risk DLBCL. An exciting finding of this BM transplantation study is that a history of anti-CD20 therapy before allogeneic transplantation did not affect the incidence of disease progression or relapse significantly (2-year PFS, 40% vs 48%, P = .59). The results of all of these studies can be considered encouraging and are confirmed by EBMT registry data.30 In a study of 101 patients who had relapsed after ASCT, the 3-year PFS and OS rates after transplantation were 41% and 52%, respectively. A comparison of RIC and myeloablative conditioning regimens before alloSCT revealed no significant differences in PFS or OS. However, there was a trend of lower unrelated mortality with RIC-allo. The 2 main factors affecting outcome were relapses < 12 months after ASCT and the quality of response before the transplantation. Improvement of the response remains the key issue.31 The introduction of RIT in a nonmyeloablative conditioning regimen has been proposed in phase 2 studies32,33 to improve the antitumor effect without increasing toxicity significantly.
With a better definition of the prognostic factors and the poor results obtained with HDT/ASCT in early relapse, high secondary aaIPI, and the ABC subtype, we can now discuss whether, in the case of a response, these patients should be transplanted directly with RIC-allo or ASCT or treated with a tandem approach of ASCT followed by RIC-allo. Nevertheless, it should be pointed out again that RIC-allo should not be proposed to refractory patients. The evaluation of such an approach remains an unsolved challenge.
Conclusions
If the introduction of rituximab represents major progress in the treatment of B-cell lymphoma, patients failing to respond early to rituximab combination chemotherapy generally have a poor prognosis and may be candidates for more experimental and innovative treatments or trials testing significant improvements of the existing tools. In total, only 30% of relapsed patients will benefit from HDT/ASCT. Identifying an efficient second-line treatment for DLBCL remains a key issue. Most regimens yield the same response rate and there are no differences between the 2 most widely used salvage treatments, R-ICE and R-DHAP. Based on the main prognostic factors, 2 prognostic groups can be identified. A good-prognosis group, with an 80% response rate and a 60% PFS rate, remains accessible to standard salvage HDT/ASCT. In the second group, patients with poor prognoses, with a 40% response rate and a 25% PFS rate, are candidates for alternative approaches. Any progress can be detected readily in a poor-prognosis patient. Improvements in salvage therapy will require the introduction of new drug combinations targeting the pathways involved in the biology of the different subtypes of DLCBL. Improvements in the existing situation may be directed toward the prevention of post-ASCT relapses using new immunomodulators or RIC-allo, which remains experimental. Enrollment in large, randomized studies will be more problematic in the absence of significant progress in response rates.
Disclosures
Conflict-of-interest disclosure: The author has received research funding from Baxter and Roche and has consulted for Roche. Off-label drug use: Rituximab after transplantation.
Correspondence:
Christian Gisselbrecht, Professor of Hematology, Hospital Saint Louis, Paris Diderot University, Paris, France; Phone: +33-1-40-05-46-96; Fax: +33-1-34-29-54-93; e-mail: christian.gisselbrecht@gmail.com.