Abstract
Childhood immune thrombocytopenia (ITP) is often considered a benign hematologic disorder. However, 30% of affected children will have a prolonged course and 5%-10% will develop chronic severe refractory disease. Until recently, the only proven therapeutic option for chronic severe ITP was splenectomy, but newer alternatives are now being studied. However, because immunosuppressive agents such as rituximab are not approved for use in ITP and the thrombopoietin receptor agonists are not yet approved in children, the decision to use alternatives to splenectomy needs to be considered carefully. This review describes the factors that should affect decisions to treat ITP at diagnosis and compares the options for the occasional child in whom ITP does not resolve within the first year.
Introduction
Childhood immune thrombocytopenia (ITP) affects 4-6 of 100 000 children per year. In 2010, the International Working Group proposed changes in the nomenclature and classification of ITP. The disorder is now more appropriately called immune thrombocytopenia rather than idiopathic thrombocytopenic purpura and is defined as a platelet count < 100 × 109/L not explained by any other cause.1 Newly diagnosed ITP refers to the time from onset of thrombocytopenia to 3 months later; whereas persistent ITP is from 3-12 months after diagnosis. Chronic ITP is defined as a platelet count that has been < 100 × 109/L for longer than 12 months. Refractory ITP now refers to severe ITP that persists after splenectomy. Before splenectomy, patients with ITP are divided into responders and nonresponders to the different treatment modalities.1 Presentation of ITP may be dramatic, with extensive soft tissue bruising and mucosal hemorrhage, but it is more often a self-limiting condition. Approximately one-third of children will have persistent/chronic ITP with ongoing thrombocytopenia more than 6 months after diagnosis, and 5%-10% will develop severe, chronic, and/or refractory disease.2 Other than splenectomy, there is limited experience in the management of the child with a severe and prolonged course of ITP. However, new agents are being explored in children that may offer relief from bleeding symptoms, increase quality of life, and allow for avoidance of splenectomy.
Treatment considerations for newly diagnosed ITP
Although there are no new treatment options for newly diagnosed ITP, changing trends in practice should be noted. Traditionally, drug therapy is prescribed to raise the platelet count and prevent serious and/or life-threatening bleeding, particularly intracranial hemorrhage (ICH) and other bleeding requiring transfusions. However, such bleeding is uncommon in children. Neunert et al, for the Intercontinental Cooperative ITP Study Group (ICIS), reported that the rate of serious bleeding upon diagnosis of ITP in children with platelet counts < 20 × 109/L was only 3%. Among the group who had minor or no bleeding at presentation, the rate of new serious bleeding (ie, gastrointestinal, oral, or nasal) in the first 28 days was only 0.6% irrespective of initial drug therapy.3 In addition, Psaila et al specifically evaluated potential risk factors for ICH and demonstrated that the majority of children who present with or develop ICH have platelet counts < 10-20 × 109/L, bleeding manifestations beyond petechiae and ecchymoses (particularly hematuria), and a high rate of reported head trauma.4
Since the early 1990s, the United Kingdom has adopted a minimalistic treatment approach to children with little or no bleeding at diagnosis of ITP, with only 16% of children now receiving drug therapy compared with 61% in 1995.5 The United States has not experienced this same trend, but it is now recommended to consider observation alone for patients without clinically significant bleeding regardless of platelet count.6 The decision to treat should not be based solely on platelet count, but also on the severity of the associated bleeding, reliability of family for close follow-up, and potential loss or gain of quality of life.
For the children who do require drug therapy, primary options remain corticosteroids, IVIg, and anti-D Ig. The most recent change in the use of these medications relates to anti-D Ig. Anti-D has been given a black box warning by the US Food and Drug Administration (FDA) for rare but serious and potentially life-threatening intravascular hemolysis. Anti-D is still recommended as a safe and effective first-line therapy for Rh+ children without splenectomy who do not present with anemia, but it is no longer licensed or available in some countries.6–9
Treatment options for nonresponders and/or patients with chronic ITP
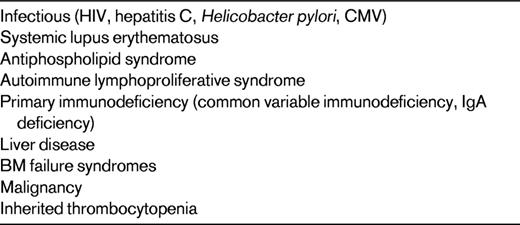
If a child has persistent severe bleeding symptoms that have not responded to conventional-dose corticosteroids, IVIg, or anti-D or has had persistent and marked thrombocytopenia for longer than 6-12 months, other considerations are necessary. The initial step is to ensure that the diagnosis is correct.10 Table 1 shows alternative causes of thrombocytopenia that may not respond to traditional first-line ITP therapy. Second, treating physicians need to determine whether the severity of symptoms warrants aggressive therapy. Unlike adults with ITP, many children with chronic disease will not have major bleeding symptoms and will likely recover within an additional 12-24 months. Therefore, continued observation may be the only therapy indicated. The dilemma for pediatric hematologists is to determine the best second-line therapy for the symptomatic children, with the goals being increase of platelet count, resolution of bleeding, and increased quality of life.
The available treatments take into consideration all possible mechanisms for thrombocytopenia in ITP. The primary cause for thrombocytopenia is immune destruction of platelets in the reticuloendothelial system, and splenectomy is a proven modality to achieve a long-term improvement of platelet count.11 However, other approaches have been shown in small numbers of children to suppress either B-cell or T-cell dysfunction and the resultant antiplatelet Ab production.12 The best-studied medication is rituximab, as described further below (see “Rituximab”). Finally, platelet production is decreased in the BM of patients with ITP, likely due to Abs against megakaryocytes.10,11,13 This supports the use of thrombopoietin (TPO) receptor agonists, as described more fully in a later section. These next sections will discuss the mechanism of action and the risks and benefits of these modalities.
Splenectomy
Mechanism of action and efficacy
Splenectomy remains the most predictable method for achieving a durable response in ITP. Nearly 85% of patients undergoing splenectomy will have an immediate platelet response and close to 70% will maintain them at 5 years. Splenectomy is successful because it removes the primary site of platelet destruction and a site of antiplatelet Ab production. Unfortunately, the ability to predict response to splenectomy is not well defined. There are mixed results in studies suggesting that prior response to corticosteroids and IVIg may be predictive.14–16 There is also no reliable radionuclide imaging test to recognize which patients may have sequestration of platelets in the liver that would diminish the response to surgery.17
If relapse occurs, it is generally within in the first 2 years after splenectomy. At this point, the patient should have evaluation for and removal of accessory spleen. If there is no spleen to remove, then alternative therapies such as rituximab or TPO receptor agonists should be considered, because they have been shown to be equally efficacious in splenectomized and nonsplenectomized patients.17
Safety
Before splenectomy, it is important to determine that the patient does not have an underlying disease giving rise to secondary ITP.6,7,10 For example, performing splenectomy in patients with primary immunodeficiency may be deleterious. In children, additional considerations for delaying splenectomy include the particularly high rates of spontaneous remission. Exceptions would include emergency bleeding or severely diminished quality of life due to unresponsive disease. Because children younger than 5 years of age are more susceptible to overwhelming infection from encapsulated organisms, it is recommended to delay splenectomy until after that age.10 However, with the advent of more effective vaccines against Haemophilus influenza, Neisseria meningitidis, and Streptococcus pneumoniae and the use of prophylactic penicillin, the risk of death from these organisms has been reduced substantially.6 Children undergoing splenectomy should be vaccinated against all encapsulated organisms 2 weeks before surgery if possible and receive booster immunizations every 5 years. Prophylactic penicillin or an equivalent antibiotic should be prescribed to all children < 5 years of age and to older children for at least 2 years after surgery because that is the highest-risk period for overwhelming infection.10
Laparoscopic splenectomy is considered safe. However, bleeding is possible, particularly if platelet counts are less than 20 × 109 at the time of surgery. In 2 large pediatric case series on the outcome of laparoscopic splenectomy (390 children, 83 with ITP), no deaths were reported. Perioperative bleeding occurred in 1%-5%, and conversion to open splenectomy occurred 1.7%-5%.18,19 Portal vein thrombosis in the immediate postoperative period has also been reported in children.17,18 In addition to the long-term risk of sepsis, postsplenectomy patients have to be monitored for late thrombotic events. Although the rate of venous thrombosis, atherosclerosis, and pulmonary hypertension is unknown for patients undergoing splenectomy specifically for ITP, these complications are well described after splenectomy in other hematologic diseases such as hereditary spherocytosis and thalassemia.7,17,20
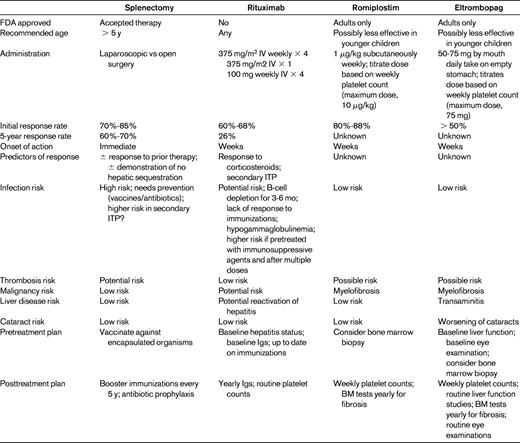
Even though splenectomy is a relatively safe and effective treatment for severe and nonresponsive ITP, it is an irreversible procedure that places young children at high susceptibility for complications for many years. Table 2 compares the risks and benefits of the splenectomy to the newer agents described in the following sections.
Rituximab
Mechanism of action
Rituximab depletes B cells by binding to the CD20 antigen surface markers. By removing the autoreactive B-cell clones, autoimmunity can be eradicated.21 In addition, rituximab has been shown to increase numbers of T-regulatory cells and prevent the activity of Th1-autoreactive cells specifically against GPIIb/IIIa.12 Although it is not FDA approved for the treatment of ITP, we have more than 5 years of experience in the use of rituximab as a second-line option and numerous additional reports of its safety and efficacy in malignant and rheumatologic diseases.
Efficacy
There are no randomized trials for rituximab in children with ITP, but in 4 cohort studies and 10 case reports, there is evidence that children with chronic ITP have an overall response rate of 61%.21 The first long-term follow-up study for both adults and children with ITP described an initial response rate of approximately 60%.22 In children, nearly 60% of the initial responders maintained platelet counts > 50 × 109/L for longer than 1 year and had a greater than 80% chance of maintaining the response at 2 years. An estimated 26% of children will have a 5-year durable response. One theory for the lack of sustained response to rituximab is the persistence of autoreactive B cells in the germinal centers and the BM.12 In addition, the majority of plasma cells are preserved in patients receiving anti-CD20 Ab therapy.12
The good initial response rate of 68% (33%-100%) in children with primary ITP was confirmed by Liang et al in a systematic review of children only.23 Patients with secondary ITP had an even better response at 64%-100%. The median time to response was 3 weeks, with a median duration of response of 12.8 months. Liang et al pointed out that there is no “standard” dose for rituximab in children. Although the most frequently administered dose remains 375 mg/m2 for 4 consecutive weeks, there are small series of children who had similar response rates with a single dose of 375 mg/m2 or after lower doses regimens of 100 mg/dose for 4 weeks.23,24 Because late relapses are possible after achieving remission with rituximab, ongoing follow-up is necessary.22 Repeated doses of rituximab can be considered for relapsed patients.
Several studies have investigated predictors of response to rituximab. Patel et al determined that age, sex, prior duration of ITP, and previous response to therapy did not predict response to rituximab.22 Although previous splenectomy did not affect the overall response rates, those who had undergone the procedure tended to relapse earlier. Children with only partial responses to alternative therapies initially relapsed more frequently after rituximab than those with complete responses.22 The most recent publication by Grace et al found in a multivariate analysis that previous response to corticosteroids and history of secondary ITP predicted better response.25 Those investigators theorized that there is an overlapping biology of response between glucocorticoids and rituximab in how they target both the B-cell and T-cell arms of the immune system.
Although there are no randomized trials comparing rituximab with splenectomy for long-term treatment of ITP, 2 randomized trials explored rituximab use in adults before splenectomy. The first study comparing rituximab + dexamethasone to dexamethasone alone for newly diagnosed ITP demonstrated a higher rate of sustained responses in those receiving rituximab.26 The second trial compared rituximab with placebo in patients nonresponsive to first-line therapy. Interestingly, the placebo-controlled study demonstrated no statistical difference in rate of treatment failures, and the overall response rates for both groups was still close to the expected 60%.27 These trials and a recent meta-analysis of adults who received rituximab before splenectomy suggest that surgery could be delayed and maybe avoided altogether for patients likely to go into remission.28
Safety
The most common adverse reaction to rituximab occurs at the first infusion, after which fever, serum sickness, and hypotension have been reported. Serum sickness occurs more frequently in children than adults and slowing the infusion and premedication with antihistamines or corticosteroids may minimize these side effects.21 The severity of reactions decreases with subsequent doses.17,21–23
Infection is a concern after administration of any immunosuppressive agent. During the 3-6 months before reconstitution of B cells, there have been reports in children of pneumonia, varicella, meningoencephalitis, and reactivation of hepatitis C.23 Because most plasma cells do not have CD20 on their surface, humoral immunity should be spared and production of Igs maintained after rituximab administration. However, there is a concern for hypogammaglobulinemia, particularly after repeated treatments, which has been reported in 1 child and 2 adults.22,23 Due to the risk of hypogammaglobulinemia, it is recommended to check baseline Igs before administration of rituximab and then yearly. If levels are abnormal, supplemental IVIg is indicated.22 Because the majority of vaccinations are given to young children, it is important to consider how administration of rituximab will affect their immunogenicity. Some experts recommend that vaccines be given before rituximab or delayed until the B cells have recovered.21
Neutropenia, progressive multifocal leukoencephalopathy, hematologic malignancies, and acute respiratory distress syndrome have also been described with rituximab use.21 Most reports of toxicity are not in ITP patients, but in older adults and in young children with autoimmune hemolytic anemia, combined variable immunodeficiency, and other disorders requiring additional immunosuppressive agents.21
Recombinant TPO and TPO receptor agonists
Mechanism of action
Endogenous TPO regulates platelet production by increasing the number, ploidy, and maturation of BM megakaryocytes.13 TPO is made in the liver, and its synthesis is consistent over time unless there are reductions in the number of functioning hepatocytes. Because TPO is cleared by binding to platelet surface receptors, when platelet production is reduced, less TPO is bound and TPO levels increase. In ITP, there is only a small, if any, increase in TPO.13
TPO receptor activation on megakaryocyte precursors leads to increased number and maturation of cells and prevents apoptosis, thereby increasing platelet production. This antiapoptotic effect of TPO likely plays an important role in ITP, in which BM megakaryocytes and precursors are increased in number but undergo apoptosis mediated by antiplatelet IgG.13
In 1994, 5 institutions cloned and purified TPO, and in 1995, 2 first-generation recombinant human TPO agents entered clinical trials. Recombinant human TPO was a full-length glycosylated protein identical to endogenous TPO, and the second agent was a pegylated recombinant human megakaryocyte growth and differentiating factor identical to the first 163 amino acids of the native protein. Both products had half-lives of 40 hours and increased platelet counts in humans. Unfortunately, several subjects developed Abs to endogenous TPO and subsequently new thrombocytopenia.13
Since then, 2 new TPO receptor agonists (romiplostim and eltrombopag) have been studied extensively and are approved for the treatment of chronic ITP in adults who have had an insufficient response to corticosteroids, IVIg, and splenectomy.
Efficacy
Romiplostim is a TPO mimetic formed from 4 14–amino acid peptides. The 4 chains increase its stability and effectiveness over single-chain peptides. Because it has no homology to TPO, the risk of immunogenicity seen with early agents is diminished. Only one report of a transient neutralizing Ab against romiplostim itself has been reported.29 In early animal and human studies, the response to romiplostim was found to be dose dependent, and the effect begins on day 5 and diminishes by day 28 after a single subcutaneous dose.
Romiplostim has been FDA approved since 2008, and the majority of patients have had a very good response with an initial dose of 1 μg/kg given subcutaneously each week.30,31 Nearly all patients receiving romiplostim were able to discontinue or dose reduce other immunosuppressive therapies and required fewer rescue treatments. An open-label study of romiplostim in nonsplenectomized patients demonstrated a reduced incidence of treatment failure, which was defined as platelet count < 20 × 109/L, bleeding events, or need for splenectomy.30
In children, there has been one randomized clinical trial of romiplostim and 3 recent case series. The trial was a phase 1/2 study in children diagnosed with ITP for at least 6 months.32 The primary outcome was safety, but efficacy was assessed as well. Outcome measures included the total number of weeks the patients had a platelet count > 50 × 109/L, number of bleeding events with scores of grade 2 or higher on the Buchanan and Adix scoring system, and the percentage of patients with platelet count higher than 50 × 109/L or greater than 20 × 109/L from baseline for 2 consecutive weeks. Twenty-two children were enrolled (5 received placebo and 17 romiplostim).32 Romiplostim increased platelet count in 88% of patients and platelet counts were maintained at > 50 × 109/L for a median of 7 weeks in the treatment group and 0 weeks in placebo group. The average weekly dose was between 1 and 10 μg/kg. The 2 nonresponders in the treatment group had had prior splenectomy. Twenty of the original 22 children are in the open-label continuation study that is ongoing. A second report from this trial studied health-related quality of life in the 22 enrolled children by using the Kids ITP Tools quality of life scores. The results of this pilot study suggest that using romiplostim decreased the parental burden from the disease.33 Improvement in health-related quality of life has also been demonstrated in adults taking TPO agonists.34 Three more recent case series also demonstrated some efficacy of off-label use of romiplostim in children with acute and chronic ITP, but the response was variable, with younger children having a less robust response.35–37
Eltrombopag is a small, nonpeptide TPO mimetic that has no homology to endogenous TPO so it is not immunogenic. It binds to the transmembrane region of the TPO receptor rather than the TPO-binding site. Therefore eltrombopag does not compete with endogenous TPO and any effect it has is additive.38 Pharmacokinetics are linear and dose dependent, with the half-life being 21-30 hours. It is orally administered with the starting dose of 50 mg daily.13 Polyvalent cations such as calcium, aluminum, magnesium, and zinc also reduce the absorption of eltrombopag, so this drug cannot be given within 4 hours of any foods or medications containing these minerals.13 This dietary limitation may diminish its utility in children even though it is given by the preferred oral route rather than as an injection.
There are 2 randomized placebo-controlled phase 3 trials demonstrating efficacy. The RAISE trial in adults demonstrated an increased platelet count and decreased need for rescue treatment compared with placebo. This effect was seen regardless of splenectomy status.38 The other trial demonstrated a 59% response rate for platelet counts > 50 × 109/L at 6 weeks compared with 16% in the placebo group.31 In the open-label expansion study, 86% of patients achieved a platelet count > 50 × 109/L, which remained in the target range for more than 50% of the time on medication.12 The second trial, PETIT, a randomized clinical trial of eltrombopag in children, is currently in progress. The initial results in 15 patients divided among 3 age cohorts were presented at the European Hematology Association in June 2012. Fifty percent of children with bleeding symptoms at the start of the study had a reduction of events. The results suggest that children may require higher median doses than adults at 70-75 mg in all age groups.39
Safety
After more than 5 years of collecting data on patients receiving romiplostim and eltrombopag, it can be seen that TPO receptor agonists are well tolerated and reduce the need for rescue therapy and ongoing immunosuppression. Adverse events during administration include headache, nasopharyngitis, and fatigue with romiplostim and nausea and vomiting with eltrombopag.12
In the open-label extension studies, thromboembolic events were reported with both agents. Although it is difficult to assess the strength of the prothrombotic nature of these agents due to the uncontrolled design of these extension studies, thrombosis is a major concern with their long-term use. There did not appear to be a correlation between platelet count increase and thrombosis, but it is recommended that the target platelet range be limited to 50-100 × 109/L to minimize risk.12
There is also concern for myelofibrosis formation after prolonged stimulation of megakaryocytes with TPO receptor agonists. This was demonstrated clearly in mouse models, and there are reports of reversible BM reticulin deposits in patients on romiplostim.12 In a recent study of BM biopsies, Bioicchi et al demonstrated an increase from baseline in cellularity and megakaryocyte hyperplasia in all patients. In addition, there was a significant increase in reticulin (grade MF-1) seen in the BM at a mean duration of 2.7 years on therapy with TPO receptor agonists.40 Fortunately, there was no collagen present, the BM function remained normal, and no patient had chromosomal or molecular abnormalities identified. However, because the TPO agents likely stimulate the same pathways that can lead to myeloproliferative neoplasms, including the JAK2 pathway, monitoring for BM changes is likely needed until there is more definitive information about the risk of transformation to malignancy.
With eltrombopag specifically, there is a concern for liver toxicity and dose reduction is required for use with hepatic insufficiency.12,13 Fortunately, the transaminitis resolves with discontinuation. Another side effect that has been observed in animal studies of eltrombopag and also reported in humans was the development or worsening of cataracts and lens changes.13,30,31 Neither TPO agent should be given to pregnant or nursing teens or adults becaue it is likely to be transmitted to the fetus or milk through FcRn receptors.12 Finally, the main concern for the practical use of TPO receptor agonists is the indefinite duration of treatment required and the need to see patients frequently to titrate the dose. Relapse rates are high in patients who have interruption of therapy.12
The off-label use of TPO agents will likely benefit those children with ITP hoping to delay or avoid splenectomy. These agents are also a potential bridge for treating nonresponding symptomatic children while awaiting spontaneous remission, although studies in such settings have not been performed. In addition, these agents will not replace corticosteroids or IVIg as initial therapy or rescue medications because it takes an average of 2 weeks to see a response.
Other agents
There are multiple studies of other immunosuppressive agents used in chronic refractory ITP in both children and adults. Although they are not new, these agents include azathioprine, cyclophosphamide, danazol, dapsone, cyclosporine, sirolimus, and mycophenolate mofetil. These have been used as single agents or in combination. However, the published evidence for their efficacy, particularly in children, is controversial and limited and their use should be reserved for patients refractory to all other therapies, including splenectomy.10,12
Conclusion
Childhood ITP is generally a self-limiting, benign hematologic condition in which drug therapy may not be needed. However, we have multiple options to consider for those children with severe bleeding at presentation or those who develop prolonged symptomatic disease. Each has a unique side effect profile and the final decision regarding which modality to choose will depend on family/patient preference, quality of life, and evidence of durable response. The new TPO agents are a promising alternative to conventional therapies that need to be further explored in children with problematic ITP.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: Recombinant TPO and rituximab in children.
Correspondence
Janna M. Journeycake, MD, MSCS, Department of Pediatrics, University of Texas Southwestern Medical Center at Dallas, 5323 Harry Hines Blvd, Dallas, TX 75390-9063; Phone: 214-456-8556; e-mail: janna.journeycake@childrens.com.