Abstract
The maturation and postnatal development of the human coagulation system was first studied and described more than 20 years ago. These older studies, supported by more recent data, confirm the significant and important differences in the physiology of coagulation and fibrinolysis in neonates and young children compared with older children and adults. Subsequently, significant differences were also described in the physiology of primary hemostasis and in global in vitro tests for hemostasis. These differences, which mostly reflect the immaturity of the neonatal hemostasis system, are functionally balanced. Healthy neonates show no signs of easy bruising or other bleeding diathesis and no increased tendency to thrombosis for any given stimulus compared with adults. Systemic diseases may affect hemostasis, predisposing ill neonates to increased hemorrhagic or thrombotic complications. The immaturity of the hemostasis system in preterm and very-low-birth-weight neonates may contribute to a higher risk for intraventricular hemorrhage. Therapies targeting the hemostasis system can be effective for preventing and treating these events. The concept of “neonatal coagulopathy” has an important impact on both the diagnosis and management of hemorrhagic or thrombotic events in neonates. For diagnosis of hemostasis disorders, diagnostic laboratories processing pediatric samples should use age-, analyzer-, and reagent-appropriate reference ranges. Age-specific guidelines should be followed for the management of neonates with hemostatic disorders.
Introduction
Hemostasis is a complex process that balances pro- and anticoagulant forces to protect the organism from uncontrolled bleeding secondary to vessel injury while at the same time preventing excessive clotting. At the site of vessel wall injury, adhesion, activation, and aggregation of platelets result in the formation of a platelet plug (primary hemostasis). Activation of the coagulation pathway results in the formation of covalently cross-linked fibrin that stabilizes the platelet plug (secondary hemostasis). Inhibitors of the coagulation cascade limit and confine the coagulation response, and activation of the fibrinolysis pathway results in the dissolution of fibrin clots to maintain and/or restore blood vessel patency.
The term “developmental hemostasis” was coined to reflect the fact that the hemostasis system is incompletely developed at birth and matures throughout infancy until adulthood. The aim of this review is to summarize our knowledge on the neonatal hemostasis system and to delineate the implications of age-related changes on the risk of thrombosis or bleeding in neonates. The approach to the diagnosis of coagulopathy in neonates is also reviewed.
Developmental hemostasis
Although the key components of the hemostasis system are present at birth, important quantitative and qualitative differences exist among preterm neonates, full-term neonates, children, and adults (Table 1). It has been thought that these age-related changes are not related to coagulation, but rather are part of normal physiological development.1
Neonatal hemostasis versus older children/adult hemostasis
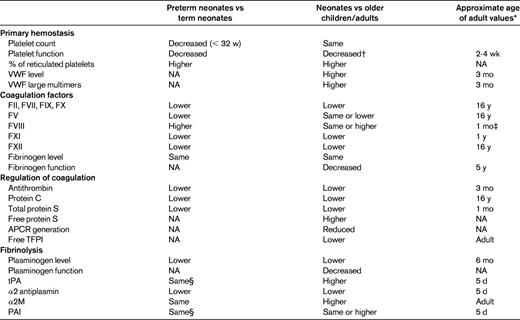
NA indicates not available; APCR, activated protein C resistance; and TFPI, tissue factor pathway inhibitor.
For reference values of coagulation and regulation of coagulation factors, see Monagle et al, 2006.14
*Maximum age reported.
†Decreased response was reported to agonists such as thrombin, collagen, epinephrine, and thrombin activation peptide as tested by flow cytometry.
‡Lower levels compared with adults are reported from 1 mo to 16 y of age.
§Higher levels in extremely preterm neonates on d 10 of life compared with older preterm or term neonates.
Platelet number and volume are relatively similar in neonates compared with adult values. Samples from more than 47 000 neonates demonstrated that the platelet count increases with gestational age (GA) from a lower-limit (5th percentile) platelet count of 104 200 × 109/L for those at 32 weeks GA to a platelet count of 123 100 × 109/L for late preterm and term neonates.2 In the same study, the platelet count normally increased during the first 9 weeks after birth up to 750 000 × 109/L.2 Differences were described in the kinetics of thrombopoiesis between preterm neonates, term neonates, and adults.3,4
Platelet ultrastructure in neonates does not differ from that in adults. Platelet surface glycoproteins are present on neonatal platelets, but their expression and response to agonists are different from those of adults.5 Neonatal platelets have a decreased response to agonists, decreased granule secretion, and decreased expression of fibrinogen-binding sites. The decreased platelet response persists for the first 2-4 weeks after delivery.6
Despite platelet hyporeactivity in neonates, in vivo global assays of platelet function, such as bleeding time and the platelet function analyzer (PFA-100), do not show platelet dysfunction.6–8 Furthermore, global in vitro testing for hemostasis using thromboelastography and rotating thromboelastometry show accelerated coagulation and strong clot firmness9,10 (Table 2). This inconsistency may be explained by the role of the VWF in neonatal hemostasis. Neonates have a higher concentration of plasma VWF levels and a greater percentage of large VWF multimers.11,12 The higher hematocrit levels in neonates may also explain this inconsistency, because a higher hematocrit level is associated with a shorter bleeding time.7
Screening laboratory tests for hemostasis: neonates versus adults
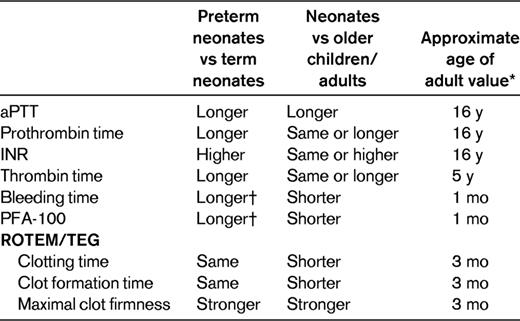
INR indicates International Normalized Ratio; ROTEM, rotating thromboelastometry; and TEG, thromboelastography.
*Maximum age reported.
†In samples drawn in the first 7-10 d of life.
For the procoagulant factors, the differences in neonates are mainly quantitative (Table 1).11,13,14 Both full-term and preterm neonates are born with low levels of most procoagulant factors, including all of the contact activation factors and vitamin K–dependent factors. The reduced plasma concentration of coagulation factors in neonates may be the result of decreased production and/or accelerated clearance15 and are probably explained by reasons unrelated to hemostasis, such as the function of these proteins in angiogenesis, inflammation, and wound repair.16 Low levels of factor II (FII), FVII, FIX, and FX could not be explained solely by vitamin K deficiency, because low levels were also measured in neonates who received vitamin K prophylaxis at birth. The low levels of contact activation factors contribute to the normally prolonged activated partial thromboplastin time (aPTT) in neonates (Table 2).
Levels of the major anticoagulant proteins are also low at birth. Antithrombin levels in the first 3 months of life are lower than those observed in many adults with antithrombin deficiency and recurrent thrombosis. Furthermore, plasma concentrations of protein C and protein S at birth are very low. However, because protein S in neonates is completely present in the free, active form, the functional activity of protein S in neonates is similar to that in adults.1 Conversely, alpha 2 macroglobulin (α2M), an important inhibitor of thrombin in neonatal plasma, is elevated in neonates, being up to twice the level in adults. It is suggested that α2M in neonates partly compensates for the low levels of antithrombin and may increase the interaction of protein S with activated protein C.17
The qualitative differences in neonatal procoagulant and anticoagulant factors were reviewed recently.15 Posttranslational modifications affect the structure of hemostatic proteins and most probably affect the function of these proteins.18 Fetal fibrinogen was shown to have fewer fractions of high-molecular-weight fibrinogen and decreased content of sialic acid and phosphorus compared with adult fibrinogen. These biochemical differences may explain the slower polymerization of fibrin clot from fetal fibrinogen compared with that of fibrin from adult fibrinogen.19 Although no human studies are yet available, neonatal forms of protein C and antithrombin have been described in animal fetuses.15
Plasminogen, the major protein in fibrinolysis, has both lower levels and decreased activity in neonates compared with adults. In neonates, 5 times the amount of tissue plasminogen activator (tPA) is required to activate plasminogen compared with activation of plasminogen in adults.20 Elevated plasma levels of tPA and plasminogen activator inhibitor (PAI) also reflect the reduced fibrinolytic activity in neonates. The increased levels of tPA and PAI found on the first day of life contrast markedly with values obtained from cord blood, which are significantly lower than adults levels. Indeed, samples from cord plasma show an increased fibrinolytic index, as measured by the clot formation and lysis assay (CloFAL).21 The change in fibrinolytic activity immediately after birth may depend on circulatory and respiratory adaptation to extrauterine life.
The maturation of the hemostasis system in utero is reflected in hemostatic differences between preterm and term neonates (Tables 1 and 2).1,9,22–24 The differences in platelet activity and in global assays of platelet function are detected in samples drawn in the first week to 10 days of life.3,7,25,26 Coagulation and fibrinolysis differences have also been found between small-for-GA and appropriate-for-GA term and preterm neonates.27,28
Clinical implications of developmental hemostasis
Despite the quantitative and qualitative deficiencies of multiple hemostatic factors, healthy neonates have normal hemostasis. Although often characterized as “immature,” the neonatal hemostatic system is nevertheless functionally balanced with no tendency toward coagulopathy or thrombosis. Healthy neonates show no signs of easy bruising or other bleeding diathesis, and no increased tendency to thrombosis for any given stimulus compared with adults. Illnesses that disrupt the hemostatic system may predispose neonates to hemorrhagic or thrombotic complications. Studying the effects of neonatal illness on hemostasis values is important both for understanding the risk for thrombosis/bleeding events in ill neonates and for exploring the most effective intervention that may be used to treat or prevent these events.
Neonatal thrombosis
Neonates have the highest risk of developing thrombosis compared with infants and children, likely promoted by sepsis, inflammation, hypotension, hypoxia, and the use of intravascular catheters in small-caliber and umbilical vessels.29,30 Deficiency of α2M, protein C, protein S, and antithrombin in ill neonates may explain their increased risk of thrombotic events.31 Nevertheless, because most cases of neonatal thrombosis occur with normal age-appropriate levels of inhibitors of coagulation proteins, the indication for measuring plasma levels of protein C, protein S, and/or antithrombin should be discussed on a case-by-case basis. In vitro data suggest that transfusion of adult platelets into neonatal blood results in stronger clot firmness and shorter clotting, potentially increasing the thrombosis risk.32 Currently, this in vitro observation has not been tested in vivo and thus cannot be translated into practical recommendations.
The impact of developmental hemostasis on antithrombotic therapy should also be considered.18 For example, neonates may require a higher concentration of tPA to successfully induce fibrinolysis and lower doses of antifibrinolytics to prevent fibrinolysis.33 The poor correlation between the aPTT test and anti-Xa levels in neonates and infants treated with unfractionated heparin (UFH) may imply that the anti-Xa level and not the aPTT test should be used to monitor UFH therapy in children < 1 year of age.34 A recent study showing high thrombus resolution in neonates and infants < 6 months of age treated with UFH, despite the delay in attaining therapeutic anti-Xa levels questions this recommendation and raises a question on the required therapeutic dose of UFH in this age group.35 These examples and others highlight the importance of using age-specific guidelines for the management of neonatal thrombosis.34
Neonatal bleeding
Neonates with severe congenital bleeding disorders are more vulnerable for bleeding, especially intracranial hemorrhage, compared with older children and adults.36 Asphyxiated neonates develop thrombocytopenia, decreased platelet survival, decreased platelet function, and an increased risk for disseminated intravascular coagulation.37 Septic neonates may develop thrombocytopenia and coagulopathy secondary to liver failure and/or disseminated intravascular coagulation. It is speculated that the deficiency in the ability of neonates to increase their megakaryocyte size may contribute to the predisposition of sick neonates to develop prolonged and severe thrombocytopenia.4 Prolonged clotting time and reduced clot firmness in neonates with complex congenital heart disease38 and prolonged bleeding times in those with low hematocrit and those receiving ampicillin7,39,40 may increase bleeding risk in these children. Bleeding time was shown to be increased by 3.6 seconds for every 1% decrease in hematocrit in these neonates.7
The contribution of the immaturity of the hemostasis system to the occurrence of intraventricular hemorrhage (IVH) in very-low-birth-weight (VLBW) neonates is unclear. Preterm and VLBW neonates are at increased risk for IVH, especially in the first week of life, as a result of immaturity of the cerebral circulatory system.41 The higher bleeding tendency in preterm and VLBW neonates may also contribute to this risk. A higher bleeding tendency, found mostly in the first 10 days of life, is correlated with the risk period for IVH.7,23 The role of therapies targeting the hemostasis system for the prevention or the treatment of IVH in preterm and VLBW neonates is currently being debated.42
Laboratory diagnosis of hemostatic disorders in neonates
Laboratory diagnosis of hemostatic disorders in neonates may be difficult to establish because of the need to adapt laboratory tests to the smaller sample volumes obtained. Sample integrity is a major problem in pediatric coagulation studies, and attention to using repeat samples is important to avoid erroneous results.18 Overdiagnosis and misdiagnosis are both common when age-, analyzer-, and reagent-specific reference reagents are not used.
Recently, the Perinatal and Pediatric Hemostasis Subcommittee (SCC) of the International Society on Thrombosis and Hemostasis (ISTH) published consensus recommendations for laboratories reporting pediatric samples for hemostasis tests. The main recommendation is that all diagnostic laboratories processing pediatric samples should use age-, analyzer-, and reagent-appropriate reference ranges.43 The following age-appropriate reference ranges were recommended; neonates, 1 month-1 year of age, 1-5 years of age, 6-10 years of age, and 11-16 years of age. Addressing the labor-intensive effort needed for development of local reference ranges, the committee stated that hemostasis test results can be compared across laboratories providing the population, reagents, and analyzer are identical.
Currently, age-appropriate reference ranges have been established for platelet counts, coagulation screening tests, and coagulation and anticoagulation proteins in preterm and term neonates.2,11,13,14,24 Age-dependent references have also been established for global in vitro hemostasis testing such as PFA-100, thromboelastography, and rotating thromboelastometry.8,10,44 Term and preterm neonatal reference ranges for the percentage of reticulated platelets were established and can be used for interpretation of this test in thrombocytopenic neonates.3 The consensus paper pointed to the need for developing reference values in very young, VLBW, and extremely sick premature infants and for new devices and hemostasis assessment techniques.43
Finally, the interpretation of diagnostic laboratory results in neonates may also be misleading and should be approached with caution. A laboratory test result outside of the 95% confidence limit of healthy neonates is not sufficient to define a disease. The diagnosis of thrombophilia or of a bleeding disorder in neonates should be based on the presence of a positive clinical phenotype, family history, and of reproducible abnormal laboratory results.18 Overdiagnosis and misdiagnosis of hemostatic disorders in neonates may lead to the administration of wrong and potentially harmful treatments for many years. Therefore, insisting on both clinical correlation and on repeated abnormal laboratory results is extremely important in this population.
Disclosure
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Shoshana Revel-Vilk, MD, MSc, Pediatric Hematology/Oncology Department, Hadassah Hebrew-University Hospital, POB 12000, Jerusalem, Israel 91200; Phone: 972-26777408; Fax: 972-26777833; e-mail: shoshanav@hadassah.org.il.
