Abstract
The term “unexplained anemia” appears frequently in a request for a hematology consultation. Although most anemia consultations are fairly routine, they occasionally represent challenging problems that require an amalgam of experience, insight, and a modicum of “out-of-the-box” thinking. Problem anemia cases and pitfalls in their recognition can arise for one of several reasons that are discussed in the cases presented herein. “Anemias beyond B12 and iron deficiency” covers a vast domain of everything that lies beyond the commonly encountered anemias caused by simple deficiencies of 2 currently major hematologically relevant micronutrients. However, even these deficiencies may be obscured when they coexist or are not considered because of misleading distractions. They may also be mistakenly identified when other less common nutrient deficiencies occur. I present herein case examples of such situations: a young patient with pancytopenia and schistocytes who was responsive to plasmapheresis, but in whom pernicious anemia was not suspected because of ethnicity and age; a bicytopenic patient with anemia and myelodysplastic features caused by copper deficiency after gastric reduction surgery; and a patient with BM hypoplasia and a dimorphic blood smear who was found to have paroxysmal nocturnal hemoglobinuria. These “pearls” represent but 3 examples of the many varieties of problems in anemia diagnosis and are used to illustrate potential pitfalls and how to avoid them.
Introduction
The term “unexplained Anemia” is found in a sizeable proportion of consultation requests received by the hematology service in a typical academic medical center or multispecialty medical group. The majority of anemia consultations pose no particular difficulty for a practicing hematologist. Occasionally, however, there are cases that lie beyond the typical presentations of common anemias prevalent among patients in hospital or clinic populations. Generally, problem anemia cases and pitfalls in their recognition arise for one of several reasons shown in Table 1: Several well-worn mantras regarding clinical diagnosis have been passed down the generations of medical teaching. The maxim of “thinking horses when you hear hoofbeats” is a sound general paradigm for teaching cost-effective medical practice and is usually accomplished by the judicious use of laboratory testing according to some reflex-driven algorithm. However, the exercise of identifying an occasional “zebra” in the herd is often an arcane art that is based on a combination of experience, awareness, and intuition. A rational approach that does not abjure the horse-zebra metaphor is the recognition that if, after the application of pattern recognition and appropriate use of resources, an answer is still not forthcoming, then out-of-the-box thinking is required. What separates the good from the exceptional among anemia sleuths is knowing when and how to start looking for the zebras. This presentation focuses on some of the clues that should alert the hematology consultant to the possibility that there may be something unusual about a particular case that begs divergence from what might on the surface appear ordinary and may in fact be extraordinary. Common reasons for enigmas in anemia diagnosis include the simple occurrence of the rare or exotic (eg, paroxysmal nocturnal hemoglobinuria [PNH]), the appearance of an anemia caused by some new and often iatrogenic factor (eg, copper deficiency after gastric reduction surgery for morbid obesity), and the coexistence of more than one cause of anemia, sometimes even mitigating the more classical features of both (combined B12 deficiency and thalassemia trait or iron deficiency). These and other related examples are explored herein using illustrative case histories against the backdrop of conventional approaches to anemia diagnosis starting from the routine complete blood count (CBC) and exploring some of the possible confounders that litter the landscape of standard algorithms.
A well-established but fairly robust approach to anemia diagnosis takes as the preliminary pivot point of its decision tree the useful RBC index of mean cell volume (MCV). Using MCV, anemia is classified into the 3 major silos of microcytic, normocytic, and macrocytic anemia. The title of this presentation focuses primarily on what traditionally would occupy much of the space that constitutes the major treatable causes of microcytic (iron deficiency) and macrocytic (vitamin B12 deficiency) anemias. Before the implementation of folic acid fortification of the food supply in the United States and many other countries beginning in the mid-1990s, folate deficiency eclipsed B12 deficiency as the most likely remediable cause of macrocytic anemia. This is still true in Europe and in much of the rest of the world where there is still no mandatory folic acid fortification. Folic acid fortification is a prime example of how a change in the prevalence of certain types of anemia can occur as a result of a public health measure such as fortification of the food supply with a hematinic nutrient.
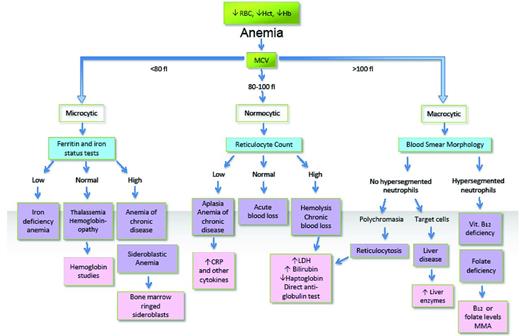
A comprehensive and generally useful algorithmic approach to anemia diagnosis is shown in Figure 1. Although applicable in the majority of anemias, slavish adherence to such a pathway frequently fails and may actually mislead a clinician faced with a puzzling anemia. The cases presented illustrate this caveat, describing unusual presentations of patients with normocytic anemias in whom the true diagnosis was or could have been missed because they fell into the space “beyond B12 and iron deficiency,” or at least what is considered typical in those deficiencies. Just as the truisms that not all microcytic anemias are caused by iron deficiency and not all macrocytic anemias by B12 (or folate) deficiency hold, so too do the obverse maxims that not all iron deficiency is associated with microcytosis and not all B12 deficiency with macrocytosis.
MCV-based algorithmic approach to anemia diagnosis (does size matter?).
MCV-based algorithmic approach to anemia diagnosis (does size matter?).
Apart from the pitfalls, there are a few pearls that are worth remembering when it comes to anemia diagnosis. These relate to the use of numbers available from a CBC or from scrutiny of the RBC volume histograms available on most modern automated blood-counting instruments. An example involves distinguishing iron deficiency from other causes of microcytic anemia. Before proceeding to tests assessing iron status and hemoglobin analysis (Figure 1), there are some indicators that can be derived from a CBC and used to distinguish a thalassemia trait from iron deficiency. Some are numerical or computational, others dependent on examination of RBC population distributions.
The normal RBC volume distribution is near Gaussian, apart from a slight tailing at the upper end of the distribution caused by the minor population of larger reticulocytes normally comprising approximately 1% of circulating erythrocytes. The SD of this distribution expressed as a coefficient of variation (SD/MCV × 100) is now generated in most automated blood-counting instruments and is termed the RBC distribution (RDW), which is normally less than 14.5%. Elevation of the RDW represents a quantitative measurement of anisocytosis, which may be of some utility in distinguishing various causes of microcytic anemia. However, the utility of this approach is somewhat limited and may require some refinement, as discussed further below. Divergence from a normal RBC distribution may be either quantitative (increased RDW) or qualitative, as occurs when the RBC population is bimodal or dimorphic. The causes of dimorphic anemia are shown in Table 2. RBC dimorphism may be discerned on the blood smear by an astute trained observer. However, more subtle degrees of RBC dimorphism are only apparent through examination of RBC distribution curves (either volume or hemoglobin concentration). These underutilized but highly informative datasets are generated during the performance of a routine CBC by most standard automated blood-counting systems, whether based on electrical aperture impedance or light scatter technology. The information is displayed on a screen readily available to the machine operator, but rarely examined in the hurly-burly of a busy routine hematology laboratory, where machine throughput attains the staggering rate of 120 blood counts each hour. In the interests of “efficiency,” avoidance of confusion through data overload, and regulatory restrictions regarding approved versus nonapproved use of instrument-generated data for diagnosis, voluminous amounts of valuable data are suppressed and rarely, if ever, are inspected by laboratory scientists and hematologists, let alone their clinical colleagues. Therefore, many a dimorphic or skewed distribution blood picture passes unnoticed unless a “smear review” is requested by the ordering physician or generated by a test-driven reflex algorithm. Even if not diagnostic, potentially available and low-cost information may be obtained that might provide useful clues to consider complex or exotic possibilities and to pursue additional, more sophisticated testing in the differential diagnosis of puzzling anemias.
Several discriminant functions have been described that further refine the distinction between common causes of microcytic anemia, such as the one described by Green and King,1 which incorporates the notion that for a given level of anemia, microcytosis is more marked (lower MCV) and the RBCs more uniform in size (lower RDW) in thalassemia minor than is the case for iron deficiency. This is a low-cost and immediately available measurement with good predictive value to direct further testing if necessary and affordable.
Among the many challenging cases that arise from time to time, in this presentation, 3 recently encountered “pearls” have been selected that illustrate one or more of the important principles regarding vigilance for the unusual, awareness of potential pitfalls, and the application of broad insights into possible confounders that can obscure the correct underlying diagnosis.
Case 1
A 22-year-old African-American woman was seen at an outside referring hospital with fever, abdominal pain, and vomiting, with a recent history of intermittent bright red blood in the stool during the preceding 3 months. Laboratory findings were: hemoglobin 81 g/L, platelets 51 × 109/L, lactate dehydrogenase (LDH) 12 375 IU/L, blood urea nitrogen 2.1 nmol/L, and creatinine 60 μmol/L. There was no record of any examination of a blood smear while the patient was receiving care at the referring hospital. Based on a clinical suspicion of thrombotic thrombocytopenic purpura (TTP), the patient was treated with 12 plasma volume exchanges. The patient's plasma volume based on a nomogram for weight was 2.8 L. She showed marked symptomatic improvement after plasmapheresis.
Three months later, the patient was seen at our hospital with similar symptoms and pancytopenia (RBCs 1.93 × 1012/L, WBCs 3.0 × 109/L, platelets 114 × 109/L) with marked anisopoikilocytosis (RDW: 24.3%), normal MCV (89.5 fL), and a markedly increased LDH (4428 U/L). Total bilirubin was 24 μmol/L. Based on her recent history and resolution of symptoms in response to plasmapheresis, she was presumed to have relapsed TTP. The patient was given 2 units of RBCs and 7 plasma volume exchanges. However, in addition to schistocytes, hypersegmented neutrophils were noted on her blood smear and examination of a BM aspirate smear showed marked megaloblastosis with giant myeloid precursors. Plasma B12 was 57 pmol/L (reference range, 156-672 pmol/L); plasma methylmalonic acid was 800 nmol/L (reference range, 0-370 nmol/L). ADAMTS13 activity was normal (84%).
During one week of hospitalization, the patient received plasmapheresis and showed symptomatic and hematologic improvement. Her laboratory findings were as follows: WBCs 4.68 × 109/L, hemoglobin 840 g/L, MCV 92 fL, platelets 175 × 109/L, and LDH 247 U/L. After the presumptive diagnosis of B12 deficiency, plasmapheresis was discontinued and vitamin B12 replacement was started. The patient remained asymptomatic with normal platelet counts and the hemoglobin concentration returned to normal. Iron studies were normal.
Subsequent testing for anti-parietal cell and anti-IF Abs were negative. However, a novel test for detecting vitamin B12 malabsorption developed in our laboratory that uses C14-labeled cyanocobalamin detected by accelerator mass spectrometry2 was carried out as part of an institutional review board–approved research study protocol and confirmed the presence of profound vitamin B12 malabsorption.
The absence of macrocytosis in a case of florid megaloblastic anemia and B12 deficiency, which was responsive to B12 replacement without concurrent iron deficiency, was suspected to be due to possible underlying thalassemia trait, considering the patient's ethnicity. This was confirmed by the PCR finding of a single α-globin gene deletion (ie, the patient was a silent carrier).
Case 1 discussion
The original diagnosis of TTP was considered likely based on the constellation of clinical findings including fever, abdominal pain, thrombocytopenia, and hemolytic manifestations with a markedly elevated LDH and clinical response to plasmapheresis. Although severe deficiency of the VWF cleaving protease ADAMTS13 occurs in patients with inherited TTP, there is no absolute criterion for the diagnosis of acquired TTP. The sensitivity of finding low levels of the VWF cleaving protease ADAMTS13 is not well established.3 The initial response to plasmapheresis in our patient was likely due to the B12 in the plasma infusion of 52 jumbo units (400 mL each) containing a total estimated amount of 10 μg of B12. This is approximately 5 times the amount of the daily B12 requirement, which would have been sufficient to allow partial temporary hematologic remission from the B12 deficiency with correction of the thrombocytopenia.
There are several causes of vitamin B12 deficiency. However, the most common cause of clinically evident vitamin B12 deficiency is the disease pernicious anemia. Typically, pernicious anemia is more common among the elderly, with estimates of the prevalence of the disease ranging from 1%-2% among subjects over the age of 65.4,5 However, among some ethnic groups, including those of African and Hispanic origin, pernicious anemia has an earlier age of onset and has an unexplained high prevalence in younger women,5,6 as has also been noted in other diseases of autoimmune origin.7 Not all patients with pernicious anemia display macrocytic RBC indices. There are several reasons for this, but usually the masking of macrocytosis results from the coexistence of some cause of microcytosis, such as iron deficiency or thalassemia trait. Even individuals with single gene deletion α-thalassemia, although they are usually normocytic, have MCVs that lie at the lower end of the normal range, such that megaloblastic macrocytosis can be blunted and even obscured in a patient with pernicious anemia.5 Because of the high allele frequency of both α- and β-thalassemia genes in the African-American population, these genetic disorders have been described as the most frequent hematological disorder encountered in this population.
As this case demonstrates, B12 deficiency with masked macrocytosis caused by concomitant α-thalassemia and thrombocytopenia with associated hemolysis can masquerade as TTP, which in this case was responsive to plasmapheresis because there was sufficient B12 in the donor plasma to induce temporary symptomatic relief. Relapse of initial symptoms prompted further workup and revealed profound B12 deficiency and the underlying α-thalassemia silent carrier genotype. Fortuitous response of B12 deficiency to nonspecific treatment can also occur in other situations. In a patient with asymmetric neurological symptoms originally diagnosed as multiple sclerosis and responsive to adrenocorticotropic hormone and corticosteroids, vitamin B12 deficiency caused by pernicious anemia was subsequently identified. The neurological symptoms responded to B12 replacement. The original response to corticosteroids and adrenocorticotropic hormone was attributed to partial remission of the underlying autoimmune gastritis.8 Cases of B12 deficiency like these, with varied clinical and hematologic presentations, underline the need to maintain a high level of vigilance for the protean manifestations of this eminently treatable but otherwise potentially devastating disease.
Case 2
A 46-year-old obese woman with a history of gastric bypass surgery presented with progressive fatigue and numbness of her hands and feet. She had been taking Chinese herbal medicines and iron supplements. There was no other history of medication use or occupational exposures. Her physical examination was unremarkable, with no adenopathy or hepatosplenomegaly noted. Initial laboratory results showed moderate normocytic anemia with leukopenia (hemoglobin 68 g/L, WBC 1.4 × 109/L, platelets 147 × 109/L, and MCV 83 fL. Additional laboratory results included a reticulocyte count of 1.1%, reduced for the degree of anemia, with normal iron, ferritin, vitamin B12, and RBC folate levels. A BM examination showed slight hypercellularity for age, with mild erythroid and myeloid dysplastic changes, including vacuolated erythroid precursors. No ring sideroblasts were observed. Further laboratory testing revealed a serum copper < 1.57 μmol/L (reference range, 13-24 μmol/L), ceruloplasmin < 18 mg/L (reference range, 170-540 mg/L), and serum zinc 39 μmol/L (reference range, 9-20 μg/dL). The patient gave no history suggesting a possible source of excess zinc other than her history of consumption of Chinese herbal medications, but was unable to produce any further details or containers of the medication. She denied use of any other nutritional supplements or unusual dietary patterns. She did not wear dentures. The patient was given copper supplementation in the form of copper sulfate 1 mg IV weekly. Her anemia, leukopenia, and mild sensory neuropathy resolved within 3 months.
Case 2 discussion
The possible differential diagnosis in this patient included myelodysplastic syndrome or vitamin B12 deficiency, although the clinical features were not typical for either condition. The MCV in the low normal range was atypical for both conditions, yet the bicytopenia and dysplastic BM changes were highly suggestive of myelodysplasia, and the associated sensory deficits in the upper and lower extremities placed B12 deficiency on the list of possible diagnoses. However, the history of gastric bypass surgery necessitated the measurement of serum copper and the findings of hypocupremia pointed to copper deficiency. Gastric bypass surgery for morbid obesity is associated with an increased risk of hematological disorders. The incidence of anemia after bariatric surgery has been reported to be as high as 74% and has been mostly ascribed to iron deficiency. Bariatric surgery can, however, also lead to other severe micronutrient- and vitamin-deficient states.9–11 In addition to vitamin B12 deficiency, notable among these is copper deficiency, which can have protean hematological manifestations with or without associated neurological complications.12,13 Although often macrocytic, the MCV in anemia associated with copper deficiency can vary from microcytic through normocytic to macrocytic. In a large series of reported cases, MCV ranged from 70.3-114.1 fL.12 Leukopenia is reported in more than 50% of cases and, occasionally, there may also be thrombocytopenia.13 BM findings are also variable, but the constellation of features described may mimic those of a myelodysplastic syndrome, particularly because of the not-infrequent finding of increased BM iron with ring sideroblasts.14–16 Cytoplasmic vacuolization of both erythroid and myeloid precursor cells is a fairly common finding that is associated with a marked left shift in myeloid precursors, resulting in a decreased number of late and terminally differentiated forms and giving the appearance of a myeloid arrest. Hemosiderin staining of plasma cells is an occasional curious and unexplained finding in this disorder. Another potentially perplexing clinical conundrum may arise in a patient with macrocytic anemia caused by copper deficiency and associated with dorsal and lateral column spinal cord dysfunction or other neurological deficits. This picture closely mimics the classical picture of vitamin B12 deficiency associated with demyelinating combined degeneration of the spinal cord.17
Iatrogenic factors, both surgical and medical, largely account for the upsurge in the prevalence of clinical copper deficiency with anemia, elevating what was once an obscure cause of nutritional deficiency anemia to red flag status, thus serving as an important admonition for the avoidance of misdiagnosis or missed diagnosis of this condition with its attendant litigation risks. In addition to bariatric gastric reduction surgery, other iatrogenic factors include excessive consumption of zinc. This has been reported through the overuse of zinc-containing denture adhesives.18,19
In our patient, there was no clear history of ingestion of this metal. The source of the excess zinc is presumed to be the herbal nostrum that she had been taking. High zinc concentrations have been reported in certain Chinese herbal products,20 as have been other heavy metals including lead.21,22 This case illustrates the importance of considering some of the less common micronutrient deficiencies when faced with an unexplained anemia, particularly if there is a clue in the history, such as gastric reduction surgery, that points to such a possible deficiency.
Case 3
A 31-year-old woman was transferred from an outside hospital for evaluation of Coombs-negative hemolytic anemia associated with cytopenias and BM hypoplasia. Before transfer, there had been concern for possible TTP because of associated thrombocytopenia and the presence of schistocytes on the blood smear, for which she had been given 4-5 apheresis treatments with no improvement. She gave a history of vaginal bleeding and on admission was found to have a hemoglobin concentration of 37 g/L, platelets 45 × 109/L, WBC 4.0 × 109/L, and MCV 91.1 fL. The blood smear contained a mixed dimorphic population of RBCs with occasional schistocytes. Review of the BM aspirate and biopsy showed trilinear hypoplasia with 30% cellularity, predominantly affecting granulocytic and megakaryocytic lineages. Iron stores were reduced. Cytogenetics showed a normal 46 XX female karyotype. The patient received RBC and platelet transfusions and was started on iron, folate, and B12 supplementation to assist with BM production. She was monitored for several days to determine whether she would become transfusion dependent. Her counts slowly recovered and hemoglobin and platelet count stabilized with a corrected reticulocyte count of 2.8%. A flow cytometric panel for PNH was positive, with PNH clones identified on granulocytes (56.9%), monocytes (58.1%), and a minor component of erythrocytes (type III, 1.279%). In the setting of multiple transfusions, these results supported the diagnosis of PNH.
Case 3 discussion
PNH is a disease of many faces. Although uncommon, it has captivated hematologists for the past 130 years since its recognition as an entity separate from paroxysmal cold hemoglobinuria. The history of development of a basic understanding of the clinical manifestations of PNH, with its protean presentations and the gradual development of classical laboratory tests, are generally well known and have been comprehensively and lucidly reviewed previously.23,24 Milestones in the path toward the elucidation of the underlying defect in this once enigmatic hemolytic disease, its episodic nature, and the mystique of its typically nocturnal character included the recognition that hemolysis was precipitated by acidification of the blood plasma and was complement dependent. This gave rise to the laboratory tests such as the Ham test and the sugar water test (sucrose lysis) that formed the linchpins for diagnosis of PNH for decades. In the mid-1990s, a better understanding of the molecular basis for the disease began to emerge after the recognition that members of a class of cell membrane proteins that normally function as complement regulatory proteins were missing from PNH erythrocytes.25,26 This led to the introduction of flow cytometric testing to detect the absence of CD55 and CD59.27 These and other missing membrane proteins share in common the property of normally being connected to the cell surface by a glycosylphosphatidylinositol tether.28 PNH cells are deficient in the important complement regulatory proteins CD55 and CD59, as well as other members of this group of surface membrane proteins, including acetyl-cholinesterase and alkaline phosphatase. That somatic mutations in the phosphatidylinositol glycan class A (PIG-A) gene on the active X-chromosome of an early hematopoietic stem cell is responsible for the defective glycosylphosphatidylinositol-anchor protein was discovered a few years later.29,30 More recently, a humanized mAb that inhibits terminal complement activation was shown to independently alleviate the hemolysis and thrombotic tendency in patients with PNH, although it has no effect on the BM hypoplasia.31–33 Eculizumab is a humanized mAb against C5 that inhibits terminal complement activation through blocking the cytolytic component of the attack complex.
Several other seminal observations were made about the clinical features of PNH during the years leading up to the ultimate elucidation of the fundamental molecular underpinnings of this intriguing disease. These included the increased risk of thrombosis, the frequent association of PNH with BM failure, and the recognition that PNH is a clonal disorder affecting multilineage components of the hematopoietic system. The clonal nature of the disease and the side-by-side chimeric coexistence of both the abnormal PNH clone and normal hematopoietic elements are enigmatic and as-yet-unexplained features of the disorder and account in large part for the multifaceted, variegated, and metamorphosing clinical presentation and course of this fascinating disease.28 Central remaining unsolved questions about PNH surround the issue of why the disease, although clonal in nature, can present with so many polymorphic phenotypes. No doubt some of the answers reside in the propensity for dominance of some clonal variants compared with others, but what determines that selective growth advantage is unknown, as is the reason that the growth of the PNH clone can wax and wane. Does the PNH clone gain ascendancy opportunistically in the face of immune-mediated or other BM injury, or does it contribute in some way to the collapse of its normal polyclonal neighbors? Given the trajectory of growth in our knowledge about PNH, answers to these questions will be forthcoming. For now, what is central to a clear grasp of the fundamental approach to PNH in clinical practice is to be alert to the possible clues to the disease that extend beyond the more obvious presentations inherent in the very moniker of the disease. Not all PNH patients experience frank hemoglobinuria, and hemoglobinuria may not be apparent at all stages of the disease. However, longstanding intravascular hemolysis inexorably exhausts iron stores through the effects of cumulative hemosiderinuria. This leads to the unusual finding of depleted BM iron in the face of hemolysis (which usually shifts iron from the erythron into stores). Indeed, correction of iron depletion may actually exacerbate hemolysis through a burst of erythroid output in response to treatment, which would include the PNH clone and result in sudden expansion of its younger progeny that are more susceptible to hemolysis.34 In patients with unexplained BM failure, with unexplained thrombosis, or with the paradoxical combination of hemolysis associated with iron deficiency or precipitated by iron therapy, and in patients with hemolysis associated with BM aplasia, PNH should be considered and appropriate flow cytometric-based testing for the disease should be carried out. As important as this admonition always was, it has become even more compelling since the option to use specific mAb-based treatment to ameliorate many of the more serious complications of the disease has become available.
General concluding remarks
The title of this chapter, “Anemias beyond B12 and iron deficiency,” was chosen to convey that there is much beyond the commonly encountered anemias caused by typical deficiencies of these micronutrients. The features of these deficiencies may be obscured as might occur when 2 or more deficiencies collide either by chance or through common etiology. An example of this is seen in case 2, in which the anemia, although multifactorial, was in large part due to gastric reduction surgery that caused iron and copper deficiency. Features suggesting myelodysplasia in patients like this can potentially result in misdiagnosis and inappropriate management. A further example of an underlying nutrient deficiency that may be missed if not suspected is demonstrated in case 1, a patient in whom pernicious anemia was not at first suspected because of undue focus on 2 features in the young female patient's presentation (thrombocytopenia and schistocytes on the smear) that were attributed to possible TTP and thus treated with plasmapheresis, with apparent response. This misleading response was subsequently explained by the B12 contained in the multiple plasma units received by the patient during pheresis. Preconceived but erroneous notions regarding the unlikely occurrence of pernicious anemia in someone of the age and ethnicity of the patient likely played a role in this pursuit of false leads before the true, and easily remediable, nature of the cause of the problem was established. As exemplified in both case 1 and case 2, a gratifying aspect of identifying an anemia caused by a micronutrient deficiency is that, unlike many other causes of anemia, it is correctable. The “buzz about B's” and the “elementary” aspects of the scope of this presentation are illustrated by the first 2 cases.
The other facet of the subtitle, the “nonelementary” causes of puzzling anemias, is portrayed by case 3, a patient with PNH without a history of hemoglobinuria, in whom thrombocytopenia and the presence of schistocytes on the blood smear also were at first mistakenly attributed to TTP. It is noteworthy that in both case 1 and case 3, it was the conspicuous presence of schistocytes on the blood smear in conjunction with thrombocytopenia that, as a dyad, played a prominent role in diverting attention away from the true underlying cause of anemia and toward TTP. Examination of RBC morphology on a blood smear is important, but a caveat to remember is that, apart from sickle erythrocytes, RBC morphologic abnormalities are rarely pathognomonic of a particular disease entity. This maxim applies not only to schistocytes, but also to other poikilocytes, including dacryocytes, elliptocytes, and leptocytes. In case 3, BM hypoplasia with low iron stores and the identification of a dimorphic population of RBCs even before transfusion initiated a search for possible PNH and led to the correct diagnosis.
Acknowledgments
The author thanks Dr Mingyi Chen for sharing information on case 2, Dr Mrinal Dutia for sharing information on case 3, and Dr Joo Song for critical reading of the manuscript.
Disclosures
Conflict-of-interest disclosure: The author has consulted for Emisphere and Teva Pharmaceuticals. Off-label drug use: None disclosed.
Correspondence
Ralph Green, MD, PhD, FRCPath, University of California, Davis, Department of Pathology and Laboratory Medicine, 4400 “V” St, Sacramento, CA 95817; Phone: 916-734-8078; Fax: 916-734-0299; e-mail: ralph.green@ucdmc.ucdavis.edu.