Abstract
Thrombocytopenia is a common problem among sick neonates admitted to the neonatal intensive care unit. Frequently, platelet transfusions are given to thrombocytopenic infants in an attempt to decrease the incidence or severity of hemorrhage, which is often intracranial. Whereas there is very limited evidence to guide platelet transfusion practices in this population, preterm infants in the first week of life (the highest risk period for bleeding) are nearly universally transfused at higher platelet counts than older infants or children. To a large extent, this practice has been influenced by the observation that neonatal platelets are hyporeactive in response to multiple agonists in vitro, although full-term infants exhibit normal to increased primary hemostasis. This apparently paradoxical finding is due to factors in the neonatal blood that enhance the platelet-vessel wall interaction and counteract the platelet hyporeactivity. Relatively few studies have evaluated the platelet function and primary hemostasis of preterm infants, the subset of neonates at highest risk of bleeding and those most frequently transfused. Current understanding of platelet production and function in preterm and full-term neonates, how these factors affect their response to thrombocytopenia and their primary hemostasis, and the implications of these developmental differences to transfusion medicine are reviewed herein.
Introduction
Platelets first appear in the human fetus at 5 weeks after conception and increase in number during fetal life, reaching a mean of 150 × 109/L by the end of the first trimester of pregnancy and values within the normal adult range by 22 weeks of gestation. Because 22 weeks is the lowest gestational age at which a newborn infant is potentially viable, this means that even the most immature infants cared for in neonatal intensive care units (NICUs) usually have platelet counts between 150 and 450 × 109/L, although platelet counts between 100 and 150 × 109/L are more common among otherwise “healthy” preterm infants than they are among full-term neonates, older children, or adults. For these reasons, thrombocytopenia in neonates, as in adults, has been defined traditionally as a platelet count < 150 × 109/L. This definition was challenged by a recent large population study involving 47 291 neonates treated in a multihospital system. In that study, reference ranges for platelet counts at different gestational and postconceptional ages were determined by excluding the top and lower 5th percentile of all platelet counts.1 Using this approach, the lowest limit (ie, the 5th percentile) of platelet counts for infants ≤ 32 weeks gestation was found to be 104 × 109/L, compared with 123 × 109/L for neonates > 32 weeks of age. Whereas this was the largest study of platelet counts in neonates published to date, it is important to keep in mind that ill neonates were not excluded from the study, and therefore these values should be regarded as epidemiologic “reference ranges” for neonates admitted to the NICU, rather than as “normal values” for this population.
No studies to date have applied these age-adjusted reference ranges to determine the incidence of thrombocytopenia in neonates. Using the traditional definition (ie, a platelet count < 150 × 109/L), several studies reported a much higher incidence of thrombocytopenia among sick neonates admitted to the NICU than in the general neonatal population (18%-35% vs < 1%, respectively).2,3 The highest incidence was found among the smallest and most premature infants, with approximately 70% of neonates born at a weight < 1000 g developing thrombocytopenia at some point during their hospital stay.4 Preterm infants also have the highest incidence of intracranial hemorrhages of any age group, with approximately 25% of infants born at < 1500 g experiencing an intraventricular hemorrhage (IVH), usually during the first week of life. However, the pathogenesis of IVH in this age group is complex and multifactorial and the individual contribution of thrombocytopenia has been difficult to establish, particularly because IVH frequently occurs in preterm infants with normal platelet counts. Nevertheless, the high incidence of intracranial hemorrhage in this patient population (which frequently leads to poor neurodevelopmental outcome), combined with the so-called “immaturity of the hemostatic system” in preterm neonates, has led to the widespread belief that thrombocytopenic neonates should be transfused at higher platelet counts than older children or adults. However, what these transfusion thresholds should be has not been established, and therefore there is currently extraordinary variability in platelet transfusion practices worldwide.
The developmental differences that exist between preterm neonates, full-term neonates, and adults in regard to platelet production, platelet function, and hemostasis are reviewed herein. These differences are of importance in pediatric transfusion medicine, because they not only heavily influence the transfusion decisions made by clinicians, but also affect the neonatal responses to thrombocytopenia and the risks associated with platelet transfusions.
Platelet production in neonates
In a very schematic fashion, the complex process of platelet production can be represented as consisting of 4 main steps: (1) the production of thrombopoietic factors (mainly thrombopoietin [Tpo]), (2) the proliferation of megakaryocyte (MK) progenitors, (3) the differentiation and maturation of MKs through a unique process of endomitosis and cytoplasmic changes, and (4) the production and release of platelets into the circulation.
Whereas these 4 steps are essentially the same in neonates and adults, a mounting body of evidence over the past 2 decades has supported the existence of substantial biologic differences between fetal/neonatal and adult thrombopoiesis (Table 1). In regard to the thrombopoietic stimulus, Tpo concentrations in healthy full-term and preterm neonates are higher than in healthy adults, although thrombocytopenic neonates tend to have lower Tpo concentrations than adults with similar mechanisms of thrombocytopenia. Several studies have demonstrated that fetal and neonatal MK progenitors proliferate at a faster rate than adult progenitors. In colony assays, neonatal progenitors give rise to significantly larger MK colonies than adult progenitors. In liquid culture systems, hematopoietic progenitors (CD34+ cells) derived from cord blood (CB) generate approximately 10-fold more MKs than CD34+ cells derived from adult peripheral blood (PB).5 The MKs generated by fetal and neonatal progenitors, however, are significantly smaller and of lower ploidy than adult MKs, both in vitro and in vivo.6 Likely as a reflection of their small size, CB-derived MKs also produce fewer platelets per cell than PB-derived MKs.7 Based on these observations, MKs from neonates have been widely considered to be immature compared with MKs from adults.
Challenging this paradigm, it was reported recently that human neonatal MKs cultured for 14 days in the presence of Tpo indeed had lower ploidy levels, but were surprisingly more cytoplasmically mature than adult PB-derived MKs. Specifically, 2N and 4N CB-derived MKs had higher levels of CD42b expression (a marker of mature MKs) and ultrastructurally were more mature than adult PB-derived MKs of equal ploidy.5 These observations not only dispelled the notion that neonatal MKs are immature, but also revealed a developmentally unique pattern of fetal/neonatal megakaryopoiesis characterized by rapid proliferation followed by full cytoplasmic maturation without polyploidization. The net result of this pattern is the production of large numbers of highly cytoplasmically mature, low-ploidy MKs. From an ontogenetic perspective, this mechanism may allow fetuses and neonates to populate their rapidly expanding BM space and blood volume in this period of very rapid growth while maintaining normal platelet counts.
Recent studies have begun to elucidate the molecular mechanisms responsible for these differences, and so far have revealed a complex network of developmentally regulated pathways and transcription factors that simultaneously promote MK proliferation and cytoplasmic maturation. Among those pathways that promote MK proliferation, Klusmann et al found that murine fetal MK progenitors (but not adult progenitors) were highly dependent on insulin-like growth factor (IGF) signaling for their proliferation and exhibited an up-regulated IGF/IGF1R/mammalian target of rapamycin (mTOR) pathway, which led to increased proliferation through the transcriptional activation of E2F target genes.8 Liu et al reported that human CB-derived MKs were more sensitive to Tpo than were PB-derived MKs, because they exhibited substantially stronger phosphorylation of signaling molecules downstream of the Tpo receptor (including JAK2 and 2 molecules downstream of mTOR) in response to Tpo stimulation.5 Finally, GATA-binding protein 1 (GATA-1), a transcription factor critical for MK maturation, was found to be present at 3-fold higher levels in CB- compared with PB-derived MKs. These high GATA-1 levels likely contribute to the rapid cytoplasmic maturation of neonatal MKs, because overexpression of GATA-1 in murine adult MKs has been shown to accelerate and enhance MK maturation.9 However, GATA-1 overexpression also promotes polyploidization in murine adult MKs, an effect not seen in human neonatal MKs. Therefore, the molecular mechanisms underlying the low ploidy levels of neonatal MKs remain an unsolved mystery.
Response of fetal/neonatal MKs to thrombocytopenia
Studies in thrombocytopenic patients and in animal models have shown that, under normal conditions, the adult BM responds to increased platelet demand by first increasing the MK size and ploidy and then increasing the MK number.10 These changes ultimately lead to a 2- to 8-fold increase in MK mass. BM studies in thrombocytopenic and nonthrombocytopenic neonates and adults suggested that thrombocytopenic neonates can increase the number, but not the size, of their MKs.11 These findings were consistent with those in a murine model of fetal immune thrombocytopenia.12 In that study, adult mice treated with an antiplatelet Ab (MWReg30) showed an increase in the number and size of their MKs, whereas newborn mice exposed in utero to the same Ab did not. These findings support the notion that the small neonatal MKs have developmental limitations in their ability to increase their size in response to increased platelet demand and thus to mount an appropriate response to thrombocytopenia.
Platelet function and primary hemostasis in neonates
Although platelet transfusions are routinely provided to neonates when their platelets decrease below a certain threshold, it is known that not only the platelet count but also the gestational and postconceptional age, the disease process, and the platelet function and hemostatic balance at that time influence an infant's risk of bleeding significantly. Emphasizing this point, a recent study demonstrated that nearly 90% of clinically significant hemorrhages among neonates with severe thrombocytopenia occurred in infants with a gestational age < 28 weeks and during the first 2 weeks of life.13 Although preterm infants are those at the highest risk of bleeding, the majority of studies on neonatal platelet function have been conducted on full-term neonates and most have used CB samples due to the availability of larger amounts of blood.
Platelet function in healthy, full-term neonates
The first evidence of developmental differences between neonates and adults came from platelet aggregation studies performed using platelet-rich plasma obtained from full-term CB. These initial studies demonstrated that platelets from neonatal CB were less responsive than adult platelets to agonists such as ADP, epinephrine, collagen, thrombin, and thromboxane analogs (eg, U46619).14 Similar results were obtained in flow cytometric platelet activation studies, which showed decreased expression of surface activation markers in neonatal platelets stimulated with thrombin, ADP, and epinephrine.14,15 Different mechanisms are responsible for the hyporeactivity of neonatal platelets to various agents. The hyporesponsiveness to epinephrine is probably due to the presence of fewer α2-adrenergic receptors, which are binding sites for epinephrine,16 the reduced response to collagen likely reflects the impairment of calcium mobilization,17 and the decreased response to thromboxane may result from differences in signaling downstream from the receptor in neonatal platelets.18
Surprisingly, whereas the hypofunctional platelet phenotype in vitro would predict a bleeding tendency, full-term infants display no clinical evidence of abnormal hemostasis. Consistent with the clinical observations, several studies found that, despite their platelet hyporeactivity, healthy full-term neonates had enhanced primary hemostasis compared with older children or adults. Bleeding times performed on healthy full-term neonates were shorter than bleeding times in adults.19 Similarly, studies using a platelet function analyzer (PFA-100, an in vitro test of primary hemostasis) found that CB samples from full-term neonates exhibited shorter closure times than samples from older children or adults.20,21 Overall, these studies suggested an enhanced platelet/vessel wall interaction in neonates that was likely related to the higher hematocrits, higher mean corpuscular volumes, higher VWF concentrations,22 and the predominance of longer VWF polymers in the blood of neonates, all of which enhance primary hemostasis and counteract the hyporeactivity of neonatal platelets. Using a cone and platelet analyzer, which examines whole-blood platelet adhesion and aggregation on an extracellular matrix–coated plate under physiologic arterial flow conditions, healthy full-term neonatal platelets also demonstrated more extensive adhesion properties than adult platelets, a finding mediated by the higher amounts and enhanced adhesive activity of VWF in neonatal plasma.23 The available evidence strongly suggests that the in vitro platelet hyporeactivity of healthy full-term infants should be perceived as an integral part of a carefully balanced and well-functioning neonatal hemostatic system, rather than a developmental deficiency. Little is known about the activation potential of neonatal platelets in vivo, but it was reported recently that platelets from young infants (ie, < 10 days old) are activated as much as platelets from older children during cardiopulmonary bypass,24 although this finding contradicted a prior study.25
Platelet function in preterm neonates
Because the neonates with both the highest incidence of thrombocytopenia and the highest risk of bleeding are those born preterm, particularly at less than 30 weeks gestation, the study of platelet function and hemostatic balance in this subset of infants is of particular clinical importance. Several aggregometry and flow cytometry studies confirmed that platelets of preterm infants were also hyporeactive at birth compared with adult platelets.15,26 In general, the in vitro platelet hyporeactivity was more pronounced in preterm infants compared with full-term infants. These differences were most evident among infants born at the lowest gestational ages (ie, < 30 weeks), suggesting a correlation between platelet reactivity and gestational age.15,27 Two studies using a cone and platelet analyzer also found that platelets from healthy preterm infants exhibited decreased platelet adhesion compared with platelets from full-term infants (although it was still better than in healthy adults),28,29 and that adherence was correlated with gestational age in the first 48 hours of life. These differences in platelet adhesion were not related to lower levels of VWF antigen or ristocetin cofactor activity in preterm compared with full-term infants, suggesting that they were due to intrinsic platelet function developmental differences.28
Consistent with these findings, bleeding times performed on the first day of life were shown to be longer in preterm compared with full-term infants, with neonates born at < 33 weeks gestation exhibiting the longest bleeding times (approximately twice as long as those from full-term neonates).30 Closure times measured using the PFA-100 in response to ADP (but not epinephrine) were also inversely correlated with gestational age when CB or neonatal blood obtained on the first day of life were tested.31 These differences likely reflect the more pronounced platelet hyporeactivity and the lower hematocrits found in healthy preterm compared with full-term neonates. For unclear reasons, PFA-100 closure times in neonatal blood (even in the first 2 days of life) were significantly longer than closure times in CB, although at all gestational ages they remained shorter or similar to adult closure times, suggesting that preterm neonates also have adequate primary hemostasis.31,32 This result is an important consideration if the PFA-100 is used in the clinical setting to evaluate newborn infants for platelet dysfunction.
The availability of whole-blood tests of platelet function that require small amounts of blood made it possible to evaluate postnatal changes in platelet function over time. Studies using flow cytometry or whole-blood platelet aggregation demonstrated conclusively that the platelet hyporeactivity was still present 3-4 days after birth in both full-term and preterm infants.26 After that, the results of available studies have been somewhat inconsistent, with some reporting prolonged platelet hyporeactivity15,33 and others observing significant improvement to full normalization in the platelet function of full-term and preterm infants by 10-14 days of life.15,26,27 The latter were consistent with the findings of a recent study evaluating bleeding times in 240 neonates of different gestational ages. In that study, by day of life 10, all infants had shorter bleeding times than at birth and gestational age-dependent differences present on the first day had disappeared. Little or no further shortening occurred between days 10 and 30.30 The gestational age-dependent differences in platelet adhesion did not resolve within that time period, however, and have been found to persist for at least 10 weeks.28
Recent studies have evaluated the effects of disease processes and of common neonatal medications on neonatal primary hemostasis. Anemia (hematocrit < 28%) was shown to prolong the bleeding time in preterm infants in the first week of life.34 Using the cone and platelet analyzer, preterm infants with sepsis displayed lower platelet adherence than healthy preterm infants, suggesting a mechanism for the bleeding tendencies in this population.35 Similarly, full-term neonates born to mothers with pregnancy-induced hypertension and gestational diabetes displayed poorer platelet adhesion compared with healthy full-term neonates.29,36 Medications commonly used in the NICU can also affect neonatal closure and bleeding times. Indomethacin, which is frequently used to induce closure of a patent ductus arteriosus in preterm neonates, was associated with a doubling of the bleeding time in 25 preterm infants.37 Ibuprofen, used for the same purpose, did not affect the neonatal bleeding times, but did slightly prolong the PFA-100 closure times in response to epinephrine.32 Ampicillin, the antibiotic most commonly used to treat neonates with suspected early-onset sepsis, also prolonged the bleeding time of neonates after 3-4 doses, but did not affect neonatal closure times significantly.38 Among very-low-birth-weight infants (< 1500 g), a long course of ampicillin (> 5 days) was associated with a prolongation of the bleeding time by an average of 2 minutes in the first week of life.39 The clinical significance of these findings is very unclear, however, given the significant limitations and shortcomings of the bleeding time test and the PFA-100, particularly the multiple variables that can affect their results and the poor correlation that exists between the different tests and between test results and a person's actual bleeding risk.
In summary, whereas relatively few studies have been carried out in preterm neonates, the available evidence suggests that the platelet hyporeactivity of neonates is more pronounced (and might be less well compensated for by other factors, such as high hematocrit) in preterm compared with full-term neonates. This platelet hyporeactivity is most evident among the more premature infants (born at < 30 weeks of gestation) during the first week of life, and might be further accentuated by medical conditions and medications commonly present in the NICU. Whereas the results of PFA-100 and platelet adhesion studies suggest that, despite their platelet hyporeactivity, preterm infants do have adequate primary hemostasis (ie, their closure times are still shorter or similar to those of adults), the fact that the neonates at highest risk of bleeding are precisely those born at < 30 weeks of gestation during the first 10-14 days of life raises the obvious question of whether the platelet dysfunction present during that period contributes to this risk and, if so, whether and how it should affect platelet transfusion decisions in that population. The answers to those questions are unknown and there is currently extraordinary variability with regard to neonatal platelet transfusion thresholds. Nevertheless, it is clear from recent surveys that preterm neonates in the first week of life are generally transfused at higher platelet counts than older children or adults.40,41 Whereas the “best” platelet transfusion thresholds in this population remain to be determined, the only previous randomized trial showed that platelet transfusions in the setting of mild to moderate thrombocytopenia (platelet counts 50-150 × 109/L) did not decrease the incidence or severity of IVH in preterm infants, suggesting that factors other than the platelet count account for the high incidence of IVH in this population.42
Effects of platelet transfusions on neonatal primary hemostasis
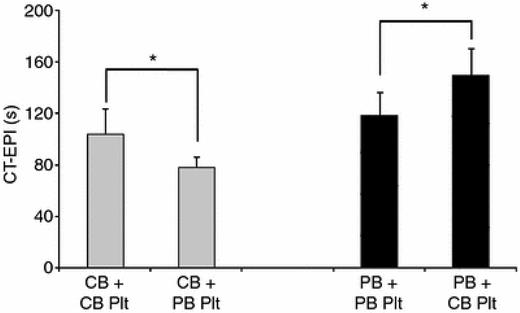
A single prior study evaluated the effects of platelet transfusions on neonatal hemostasis.43 This study was based on the premise that the platelet hyporeactivity of full-term neonates is part of a delicately balanced hemostatic system, because it is counteracted by higher hematocrits, higher VWF concentrations, and a predominance of longer VWF polymers in neonatal blood. Given that all clinical transfusions are derived from adult donors, we evaluated whether transfusing adult (comparatively hyperreactive) platelets into neonatal blood would lead to a hypercoagulable profile. To test this hypothesis, we mixed neonatal or adult platelets with neonatal and adult thrombocytopenic blood and then evaluated platelet aggregation and whole-blood hemostasis with the PFA-100 and thromboelastography. As hypothesized, this study showed that closure times after stimulation with collagen and epinephrine were significantly shorter in neonatal blood “transfused” in vitro with adult platelets compared with blood “transfused” with neonatal platelets. The opposite also held true: adult blood “transfused” in vitro with neonatal platelets had longer closure times after stimulation with collagen and epinephrine than blood “transfused” with adult platelets (Figure 1). Whereas these findings cannot be extrapolated directly to clinical scenarios, this study highlighted the importance of the differences between the neonatal and adult hemostatic systems and raised the possibility of a “developmental mismatch” associated with platelet transfusions. The potential for developing a hypercoagulable profile in transfused neonates is particularly important in neonates who are transfused for mild/moderate thrombocytopenia. Interestingly, neonates have the highest incidence of thrombosis among all pediatric age groups and most of the risk factors for neonatal thrombosis are also causes of thrombocytopenia. It is important to remember, however, that this study was conducted using full-term rather than preterm blood and most transfusions are given to preterm infants.
PFA-100 closure times after stimulation with CT-EPI. Shown are PFA-100 closure times after stimulation with closure time– epinephrine (CT-EPI) measured in thrombocytopenic CB (gray bars) and adult PB (black bars) samples transfused in vitro with neonatal (CB) or adult (PB) platelets (Plt). CB samples generated shorter CT-EPI times than PB samples. Within each whole-blood source, transfusion with adult platelets resulted in significantly shorter CT-EPI times compared with neonatal platelets. Thrombocytopenic CB samples mixed with adult platelets generated the shortest CT-EPI times.*P < .05. Used with permission from Ferrer-Marin et al.43
PFA-100 closure times after stimulation with CT-EPI. Shown are PFA-100 closure times after stimulation with closure time– epinephrine (CT-EPI) measured in thrombocytopenic CB (gray bars) and adult PB (black bars) samples transfused in vitro with neonatal (CB) or adult (PB) platelets (Plt). CB samples generated shorter CT-EPI times than PB samples. Within each whole-blood source, transfusion with adult platelets resulted in significantly shorter CT-EPI times compared with neonatal platelets. Thrombocytopenic CB samples mixed with adult platelets generated the shortest CT-EPI times.*P < .05. Used with permission from Ferrer-Marin et al.43
At this point, high-quality larger studies are needed to better characterize the platelet function and the hemostatic profile of the most premature infants in vivo, as well as their changes over time and in response to illness (eg, sepsis). These studies will be critical to establish whether the unique characteristics of the preterm hemostatic system contribute in any way to their high incidence of bleeding and, if so, what the appropriate interventions should be. For the management of thrombocytopenic infants, well-designed, multicenter, randomized controlled trials are urgently needed to establish adequate platelet transfusion thresholds in preterm infants and to determine the risks of platelet transfusions in this unique population. Finally, additional preclinical studies need to be conducted to characterize the effects of newly available Tpo mimetics on neonatal thrombopoiesis and to identify subsets of thrombocytopenic neonates with prolonged and severe thrombocytopenia who might benefit from this therapy.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Martha Sola-Visner, MD, Children's Hospital Boston, Division of Newborn Medicine, 300 Longwood Ave, Enders Research Bldg, Rm 961, Boston, MA 02115; Phone: 617-919-4845; Fax: 617-730-0260; e-mail: Martha.Sola-Visner@childrens.harvard.edu.