Abstract
Strategies to reduce blood loss and the need for transfusions in surgery include enhancement of coagulation, inhibition of fibrinolysis, and an improved decision algorithm for transfusion based on bedside monitoring of global hemostasis. The synthetic antifibrinolytic drug tranexamic acid has emerged as an effective alternative in this respect for orthopedic and cardiac surgery. Although it seems less effective than aprotinin, it has not been associated with the increased risk of mortality of the latter. Thromboelastography to monitor the global hemostatic capacity and to guide the appropriate use of blood components in cardiac surgery is also effective in reducing the need for transfusion. Patients on antithrombotic drug therapy may need reversal before surgery to avoid excessive blood loss, or intraoperatively in cases of unexpected bleeding. Available options are protamine for unfractionated or low-molecular-weight heparin, recombinant activated factor VII for fondaparinux, prothrombin complex concentrate for vitamin K antagonists and possibly for oral factor Xa inhibitors, dialysis and possibly activated prothrombin complex concentrate for oral thrombin inhibitors, desmopressin for aspirin and possibly for thienopyridines, and platelet transfusions for the latter.
Introduction
Reduction of operative blood loss is important to avoid reoperation, to avoid or minimize the need for blood transfusions, and to diminish postoperative anemia that could lead to congestive heart failure, delayed wound healing and mobilization, and other complications. Allogeneic blood transfusions have been associated with increased mortality and risk of infection,1 and major bleeding events in patients with acute coronary syndromes have been linked to worse prognoses, with a 5-fold increment of mortality within 30 days.2 Although strict selection and screening of blood donors and donated blood have drastically reduced the risk of transfusion-transmitted diseases, there is still a small residual risk. Additional concerns are febrile nonhemolytic transfusion reactions and transfusion errors.
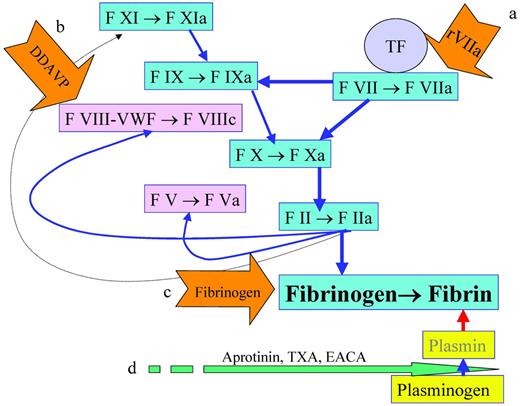
In addition to optimization of surgical techniques to reduce bleeding, the use of hemostatic agents has also been explored. There are here essentially 2 types of hematostatic agents, procoagulant and antifibrinolytic, as shown by the examples in Figure 1. The former has the intuitive peril of being prothrombotic, particularly in the state of postoperative acute-phase reaction, with increased levels of factor VIII (FVIII), VWF, and fibrinogen as well as fibrinolytic shut-down.3
Reduction of blood loss. Blood loss reduction can be achieved by the enhancement of fibrin formation with rFVIIa to increase the formation of FIXa and FXa(a), with desmopressin to stimulate the release of FVIII and VWD(b), or by providing more fibrinogen(c). Another strategy is to decrease the degradation of fibrin by inhibiting the conversion of plasminogen to plasmin with aprotinin, TXA, or EACA(d).
Reduction of blood loss. Blood loss reduction can be achieved by the enhancement of fibrin formation with rFVIIa to increase the formation of FIXa and FXa(a), with desmopressin to stimulate the release of FVIII and VWD(b), or by providing more fibrinogen(c). Another strategy is to decrease the degradation of fibrin by inhibiting the conversion of plasminogen to plasmin with aprotinin, TXA, or EACA(d).
Hemostatic agents
rFVII
Recombinant activated FVIIa (rFVIIa) has been studied in randomized trials in cardiac surgery, spinal surgery, liver transplantation, liver resection, retropubic prostatectomy, posttraumatic reconstruction of the pelvis, and dental extractions in patients with liver cirrhosis, all of which are off-label use.4 There is indeed an increased risk for arterial thromboembolic complications with rFVIIa compared with placebo (odds ratio [OR] = 1.68; 95% confidence interval [CI], 1.20-2.36). This increased risk was observed particularly in elderly patients (OR = 3.02; 95% CI, 1.22-7.48) and the risk is distributed over all off-label indications.4 Therefore, prophylaxis with rFVIIa should not be used to reduce blood loss.
Desmopressin
Another pharmacologic pathway that increases coagulant activity is the vasopressin analog D-arginine-deamino-vasopressin, which elevates the levels of FVIII and VWF. There is also a transient increase in fibrinolytic activity by the release of tissue plasminogen activator. In 19 trials with 1387 patients, there was no reduction in the number of patients transfused with blood (relative risk [RR] = 0.96; 95% CI, 0.87-1.06).5 Desmopressin did reduce the blood loss by a weighted mean of 242 mL (95% CI, −388-−96) but not the risk for reoperation (RR = 0.69; 95% CI, 0.26-1.83). There was no evidence of harm from desmopressin, but the positive effects were not convincing. It is possible that desmopressin has a more important role in patients with known disorders of the primary hemostasis.
Fibrinogen
In a randomized pilot trial, 20 patients undergoing elective coronary artery bypass surgery with a fibrinogen level < 3.8 g/L received 2 g of fibrinogen or no infusion before surgery.6 Postoperative blood loss was 32% lower in the fibrinogen group (565 vs 830 mL, respectively; P = .01) and there was one subclinical graft occlusion in this group. Larger studies are obviously required to confirm these data.
Antifibrinolytic agents
Pharmacologic diminution of fibrinolytic activity can be achieved with the relatively specific synthetic lysine analogs tranexamic acid (TXA) and epsilon aminocaproic acid (EACA) or with a broad-spectrum serine protease inhibitor (eg, aprotinin). The lysine analogs block the lysine-binding sites on plasminogen, thereby preventing its activation to plasmin.
Aprotinin was associated with increased mortality compared with TXA in a large randomized trial in high-risk cardiac surgery (RR = 1.55; 95% CI, 0.99 - 2.42),7 which led to the withdrawal of aprotinin from the market in 2008. EACA has been associated with hypotension, cardiac arrhythmias, myopathy, and rhabdomyolysis, but is the only available antifibrinolytic agent in some places. TXA is the most promising alternative due to a favorable benefit-risk ratio, experience from several decades of use for most types of bleeding or surgery in patients with congenital or acquired bleeding disorders,8,9 and the 10 times lower dose required than with EACA. The drug has been studied mainly in orthopedic and cardiac surgery.
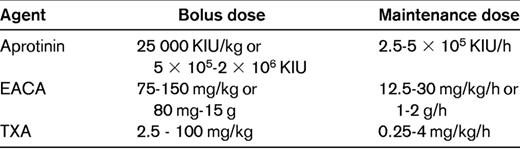
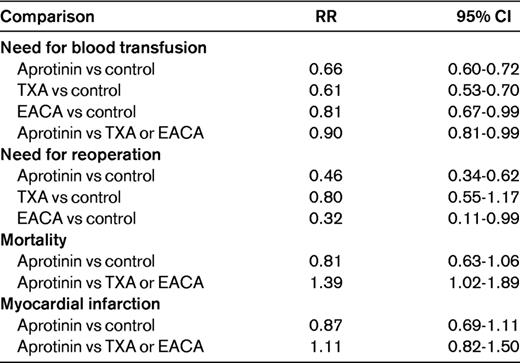
In a systematic review of randomized trials in the setting of elective surgery and with any of the 3 antifibrinolytic drugs compared with placebo or with direct comparisons between them, 252 studies with more than 25 000 patients were included.10 The main results are summarized in Table 1. The investigators pointed out the heterogeneity of the data, probably due to publication bias. There has also been a range of doses used in the trials (Table 2).
Efficacy and safety of antifibrinolytic agents in elective surgery based on a systematic review10
Orthopedic surgery
Antifibrinolytic agents in general reduce the need for blood transfusion in elective knee- or hip-replacement surgery, as demonstrated in a systematic review.11 The RR compared with control or placebo was 0.52 (95% CI, 0.42-0.64) and no increase in the RR for venous thromboembolism could be identified (RR = 0.95; 95% CI, 0.80-1.10). Each of the 3 antifibrinolytic agents was associated with a significantly smaller blood loss, which was most prominent with aprotinin. Meta-analyses have also been performed separately for various orthopedic surgeries. Thirteen studies on total hip arthroplasty were evaluated and, although there was a reduction in the need for blood transfusion when results for TXA and aprotinin were pooled, individually, this was only the case for aprotinin and then only in revision of total hip prosthesis.12 Each of the 2 agents reduced blood loss and there was no increase in thromboembolic events.
For total knee replacement, 19 studies specifically with TXA were eligible and the need for transfusion was reduced (risk ratio = 0.39; 95% CI, 0.32-0.48) and there was a mean reduction of postoperative blood loss (245 mL; 95% CI, 213-278)13 and total blood loss (591 mL; 95% CI, 536-647). These benefits were gained without any increase in the risk for deep vein thrombosis with TXA. One study had a high incidence of deep vein thrombosis (45%-48%), which was detected with a radioisotope test and no thromboprophylaxis had been given.14 The heterogeneity in the data was substantial except for the positive effect in a subset receiving high-dose (> 4 g) TXA, in whom it was consistent.
For hip fracture surgery, one randomized trial compared TXA at a dose of 15 mg/kg with placebo in 110 patients.15 The study drug was given at start of surgery and repeated 3 hours later. There was a trend to reduced need for blood transfusions with TXA versus placebo (42% and 60%, respectively; P = .06). This difference became statistically significant when the investigators pooled their results with a previous study in hip-fracture surgery (OR = 0.47; 95% CI, 0.26-0.85). The investigators also pointed out that in their study, unlike studies of TXA for elective orthopedic surgery, there was an increase in a composite of different vascular events with 16% versus 6% on placebo, but this was not statistically significant (hazard ratio = 2.96; 95% CI, 0.80-11.0).
In major spine surgery, 18 trials with 966 patients were eligible for inclusion in a meta-analysis of the effect of the 3 antifibrinolytic agents.16 There was a decrease in both the blood loss and the need for transfusions with each of the agents, and this was most prominent with EACA. No increase in the risk of venous thromboembolism was detected.
Cardiac surgery
All 3 antifibrinolytic agents were evaluated in a meta-analysis of 49 trials in cardiac surgery and indirect comparisons derived from studies against placebo or control and direct comparisons from head-to-head trials were performed.17 The need for transfusion was reduced with TXA (RR = 0.70; 95% CI, 0.61-0.80), EACA (RR = 0.75; 95% CI, 0.58-0.96), and aprotinin (RR = 0.66; 95% CI, 061-0.72), although the need for reoperation was only reduced significantly with aprotinin (RR = 0.48; 95% CI, 0.34-0.67). Although aprotinin did not increase the risk for death compared with placebo (RR = 0.93; 95% CI, 0.69-1.25), the point estimate was higher in the indirect comparison with TXA (RR = 1.69; 95% CI, 0.70-4.10), with a strong statistical trend in the direct comparison (RR = 1.43; 95% CI, 0.98-2.08) and with similar numbers in the direct comparison against EACA. There was no increase in the risk of myocardial infarction with these agents.
Dosing of TXA
A wide range of doses of TXA has been used in the trials and there is no evidence to promote a higher over a lower dose. The dose typically used for patients with congenital bleeding disorders of 10 mg/kg bolus is suggested also for bleeding prophylaxis for cardiac and major orthopedic surgery. For complex procedures taking many hours, this could be followed by a maintenance infusion of 1 mg/kg/h.
Reversal of antithrombotic agents
Antithrombotic drug use is becoming more prevalent due to the aging population with its higher prevalence of atrial fibrillation, and to increasing awareness of the need for prophylaxis against ischemic stroke in this condition. Antiplatelet agents, often in combinations, are used long-term after insertion of drug-eluting stents. Ideally, a planned interruption, possibly with the use of a bridging agent with short half-life, has been arranged for surgery or other invasive procedures. This is discussed in the article by Ortel.18 For emergency surgery in a patient known to be treated with an antithrombotic agent and elective surgery with planned continuation of the antithrombotic drug(s) but with unexpected bleeding tendency, there is a need for identification of the most responsible drug, but in the second scenario, it is also important to determine whether it is the drug, the surgical condition, or an underlying bleeding disorder that is the main cause.
Intraoperative assessment of cause of bleeding
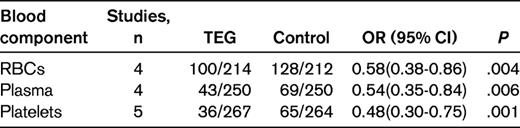
It should be relatively easy for the surgeon to exclude local etiology, such as abnormal blood vessels, tumor, infection or other conditions causing technical difficulties, from the cause of bleeing. The anesthesiologist should explore if any medical or laboratory information that would indicate the type of systemic congenital or acquired bleeding tendency has been missed. A blood sample for emergent screening for disseminated intravascular coagulation should be sent off, but the lag time until results are obtained is often problematic. Point-of-care instruments for either single tests (eg, activated clotting time or prothrombin time) or for global coagulation analysis such as thromboelastography are then very helpful. The objective is to obtain information on what part of the hemostatic system is failing. Thromboelastography to guide the transfusion of blood components has been compared with standard management, (ie, sending samples to the laboratory), in 5 randomized controlled studies.19–23 Simple pooling of the results regarding the proportion of patients transfused with RBCs, plasma, or platelets shows a benefit for point-of-care monitoring (Table 3). The mean postoperative or total blood loss was also numerically lower in this group in all studies. Although evidence is so far lacking for improved clinical outcomes, conservation of blood products is also of importance and therefore employment of intraoperative coagulation assessment with thromboelastography is recommended.
Heparins
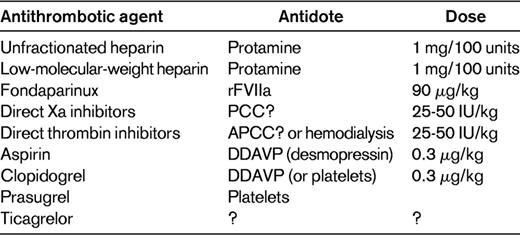
Unfractionated heparin is completely reversed by protamine sulfate, with which it forms a stable salt complex. Protamine is given at a dose of 1 mg/100 units of heparin and the circulating amount of the latter should be estimated based on last dose and half-life. The maximum infusion rate is 50 mg/10 min and the effect of reversal should be monitored with bedside activated clotting time. Overdose of protamine can cause hypotension, bronchoconstriction, platelet aggregation, and consumption with bleeding.24 Low-molecular-weight heparin is only partly reversed by protamine, but for practical purposes, this method is still useful.24
The pentasaccharide fondaparinux and heparinoids (danaparoid) are not reversed by protamine. Fondaparinux has a half-life of 18-20 hours, which becomes much longer in severe renal failure. Studies in human volunteers and in single-patient cases have demonstrated the beneficial reversing effect of rFVIIa at a dose of 90 μg/kg.25,26
Vitamin K antagonists
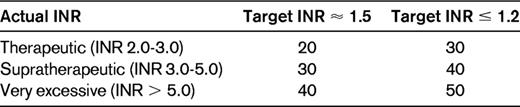
Vitamin K antagonists should in this emergency setting be reversed with prothrombin complex concentrate (PCC), because vitamin K takes far too long (6-12 hours) to take effect and the volume of plasma needed (2.3 L) to reverse other than slight prolongations of the prothrombin time will cause volume overload.27 Sufficient hemostasis is for most surgeries achieved with a prothrombin time corresponding to an international normalized ratio (INR) of 1.5. The dose of PCC is based on actual INR and body weight (Table 4). When completely normal hemostatic function (INR ≤ 1.2) is of essence, as in neurosurgery, it is advisable to increase the dose of PCC by another 10 IU/kg. In a study of 160 patients treated emergently with PCC, 44 had emergency surgery as indications; all of these patients had good hemostatic effect without any thromboembolic complications in this subset.28 In a meta-analysis of 27 studies on PCC, there was a weighted mean of thromboembolic complications of 1.4% (95% CI, 0.8-2.1).29 The population was a mixture of patients having had emergency surgery and those with active bleeding.
New oral anticoagulants
For the new oral anticoagulants, there are so far no approved or clinically validated antidotes. The oral thrombin inhibitor dabigatran can be eliminated by hemodialysis,30 which has been applied successfully in at least one case with massive bleeding immediately after open-heart surgery.31 Activated PCC, which in an animal model was effective at counteracting bleeding induced with another oral thrombin inhibitor, Melagatran, should be evaluated in clinical situations.32
The oral anti-FXa inhibitor rivaroxaban has also shown good response in terms of laboratory parameters to PCC,33 but clinical validation is required. Most of the drugs in this class have high plasma protein binding and are unlikely to be removed with dialysis.
Reversal of platelet inhibitors
For the majority of surgical procedures except neurosurgery and prostate surgery, concomitant medication with aspirin is not a significant problem. There can be a modest but manageable increase in blood loss. If aspirin was not interrupted and there is intra- or postoperative bleeding that is difficult to manage, the vasopressin analog desmopressin can improve hemostasis.34 Desmopressin is given at a dose of 0.3 μg/kg slowly either IV or subcutaneously and is preferably combined with 10 mg/kg of TXA. Desmopressin should be avoided in patients with active coronary artery disease and TXA is contraindicated in renal or ureteral procedures due to the risk for clot formation and hydronephrosis. Desmopressin might also be effective for patients treated with the thienopyridines clopidogrel35 or prasugrel, but platelet transfusion is another alternative. Although the inhibiting effect of aspirin and thienopyridines on platelet function lasts for approximately 1 week, this pertains only to platelets that were in circulation when the last dose was given and to which the drug has bound irreversibly. Within a few hours after that, there will only be a minimal concentration of the active metabolite and new platelets will not be inhibited.
The newest antiplatelet agent, ticagrelor, provides a dilemma. The fact that it is “reversible” pertains only to its binding to the P2Y12 receptor on the platelets.36 Ticagrelor is active and remains so, with a half-life of approximately 8 hours, and during this time also inhibits transfused platelets; there is so far no antidote. In case of bleeding, the only option is supportive therapy, including blood transfusions, and to await the natural elimination of the drug, which is not dialyzable.
Conclusion
A large number of studies have demonstrated strategies to reduce blood loss and the need for transfusions in major surgery. There is now a need for knowledge translation to clinical practice so that these methods can be implemented widely. For the established anticoagulants, there are effective and generally available antidotes (Table 5). The new antithrombotic agents will pose some difficulties in case of need for urgent surgery. However, the half-life of most of these new agents is relatively short and there is also active research to identify suitable antidotes for them.
Disclosures
Conflict-of-interest disclosure: The author has received honoraria from Boehringer Ingelheim and Bayer Healthcare. Off-label drug use: PCC and activated PCC for the reversal of new oral anticoagulants.
Correspondence
Sam Schulman, Thrombosis Service, HHS-General Hospital, 237 Barton St E, Hamilton, ON, L8L 2X2, Canada; Phone: 905-527-0271, ext 44479; Fax: 905-521-1551; e-mail: schulms@mcmaster.ca.