Abstract
Antithrombotic therapy plays an essential role in the management of some of the most common and morbid medical conditions. Triple oral antithrombotic therapy (TOAT) is defined as the administration of both therapeutic oral anticoagulation (OAC) and dual antiplatelet therapy (DAPT) to patients with indications for both treatments. The current societal guidelines regarding TOAT are derived from observational studies and some trials of the use of warfarin in addition to antiplatelet therapy in patients with atrial fibrillation and a recent acute coronary syndrome or percutaneous coronary intervention. The general apprehension to administer TOAT is due to the heightened concern for bleeding, rendering warfarin's pharmacokinetic properties concerning. Newer anticoagulant agents may serve as appealing alternatives, and further investigations are warranted. The results of the recent trials that have studied the use of these agents in atrial fibrillation and acute coronary syndrome offer some useful applications to TOAT. Ultimately, selecting the most favorable antithrombotic strategy is going to involve weighing the risks and benefits for each patient.
Introduction
Oral anticoagulation (OAC) is the cornerstone of stroke prevention in atrial fibrillation (AF), especially among patients at moderate to high risk of thromboembolic events. Dual antiplatelet therapy (DAPT) is the standard of care to reduce recurrent ischemic events after acute coronary syndrome (ACS) and to prevent stent thrombosis after percutaneous coronary intervention (PCI). Given the high prevalence of these conditions, in addition to the many other indications for both OAC and DAPT, triple oral antithrombotic therapy (TOAT), defined as OAC in addition to DAPT, is often used. Although TOAT may be necessary to prevent both ischemic and thromboembolic events in patients with both AF and coronary heart disease, the potential for increased bleeding has raised concerns regarding the overall utility of TOAT.
Antithrombotic therapy in AF
AF is a common condition, with a prevalence of approximately 1% and a lifetime risk of approximately 25% after the age of 40.1,2 The annual risk of stroke ranges from 2%-18% depending on other risk factors.3 Antithrombotic therapy reduces the risk of stroke, and warfarin has been shown to have a relative risk reduction of approximately 60% compared with control and to be significantly more effective than aspirin.3,4 Furthermore, warfarin has been shown to be superior to DAPT as an alternative antithrombotic treatment strategy.2 Therefore, OAC with warfarin has become the standard of care for stroke prevention in patients with AF (Table 1).5
Efficacy and safety of antithrombotic agents in AF derived from the ACCP antithrombotic therapy for AF guidelines
Predictions of the potential benefits/risks are based on available data from several studies and on a number of broad assumptions.
Warfarin, however, has limitations, including multiple interactions with other drugs and foods, genetic variability in metabolism, delayed onset and offset, and the need for frequent monitoring and dose adjustments. An ideal oral anticoagulant would have predictable pharmacokinetics, minimal drug and food interactions, rapid onset/offset, and an antidote.
Given the limitations of warfarin, clinicians and patients have been interested in the development of newer oral anticoagulants. Therefore, there have been studies investigating the efficacy and safety of these agents. The largest, most recent studies evaluating stroke prevention in nonvalvular AF include trials of one direct thrombin inhibitor, dabigatran (RE-LY), and 3 trials of the direct factor Xa inhibitors rivaroxaban (ROCKET-AF), apixaban (ARISTOTLE), and edoxaban (ENGAGE-TIMI 48, ongoing).6–8 The pharmacologic properties of these agents differ slightly, but all 4 come much closer to fitting the profile of a more favorable oral anticoagulant compared with warfarin. Table 2 summarizes the distinctions in the phase 3 study populations.
ROCKET AF studied a higher-risk population, with 87% of patients having a CHADS2 score greater than 2, whereas RE-LY and ARISTOTLE enrolled approximately 30% of their subjects with this degree of thromboembolic risk. The time in therapeutic range (ie, INR between 2 and 3), a metric that addresses the quality of warfarin dosing, also differed across the trials. ROCKET AF and ARISTOTLE were double-blind trials, whereas RE-LY did not conceal assignments to dabigatran or warfarin.
Based on these differences in the study populations, direct cross-trial comparisons can be problematic. Nonetheless, the initial goal of studying direct thrombin and factor Xa inhibitors was to find an oral anticoagulant with a more ideal drug profile than warfarin and a noninferior ability to prevent stroke in AF. The evidence shows that these agents provide more favorable safety profiles and are at least as efficacious as warfarin (Table 3). Although further data on cost-effectiveness will be helpful in making broad recommendations regarding the use of these agents, their role in stroke prevention in AF is promising.
Antithrombotic therapy after ACS
Antithrombotic therapy is a key component of management to prevent recurrent ischemic events and stent thrombosis after ACS and/or PCI, with the majority of benefit thus far being associated with the use of antiplatelet agents. Aspirin has been long known to significantly reduce cardiovascular events after ACS.9 Over the past decade, DAPT has also been studied extensively in the ACS setting and found to improve outcomes significantly compared with aspirin alone, with studies focusing on clopidogrel as the second antiplatelet agent.10 More recently, newer antiplatelet agents have been evaluated as part of DAPT in the setting of ACS. Prasugrel and ticagrelor generally achieve higher degrees of platelet inhibition than clopidogrel and do not appear to be affected by CYP2C19 polymorphisms. Both medications have been associated with reductions in cardiovascular events in patients with ACS, although with increases in the rates of spontaneous bleeding.
DAPT also reduces adverse cardiac events after elective PCI compared with aspirin.11 The current societal guidelines recommend at least 12 months of DAPT after PCI; however, this recommendation has generated discussion. Several published and ongoing trials have compared short-term (6-12 months) versus long-term (> 12 months) DAPT after PCI. Although the design and results of these individual trials can be debated, the overall results suggest a trend toward a lack of benefit of long-term DAPT, regardless of the type of stent used.12–15 Ongoing studies, such as the DAPT trial and PEGASUS-TIMI 54, will further explore the concept of extended DAPT after a cardiovascular event.
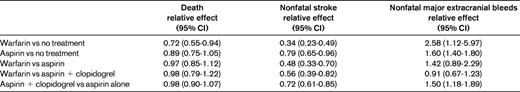
In addition to antiplatelet therapy, anticoagulation has also been evaluated as part of the long-term management after ACS. Prior trials studying unfractionated and low-molecular-weight heparins found that the benefit was restricted to the first week after ACS, with limited additional reduction in ischemic events when anticoagulation was continued for a prolonged period of weeks to months.16,17 Several trials then evaluated the efficacy of long-term warfarin on a background of single antiplatelet therapy (SAPT) after ACS and found mixed results. Two large meta-analyses analyzed these trials. The first included 10 trials comparing warfarin + aspirin versus aspirin alone, and found that warfarin decreased recurrent myocardial infarction (rate ratio = 0.56; 95% confidence interval [CI], 0.46-0.69), along with an increase in major bleeding (rate ratio = 2.5; CI, 1.7-3.7).18 Another meta-analysis evaluated 14 trials and found a similar increase in major bleeding but no significant efficacy benefit overall.19 However, when looking specifically at the trials that targeted a goal international normalized ratio (INR) of 2-3, warfarin was associated with a significant decrease in major adverse events (odds ratio, 0.73; 95% CI, 0.63-0.84; P < .0001). Given the higher risk of bleeding and difficulties associated with warfarin, this anticoagulant has not been widely used for long-term therapy after ACS.
TOAT with warfarin
TOAT refers specifically to the combined use of therapeutic OAC and DAPT. Given the high prevalence of AF and ACS/PCI, there are many patients who will have indications for both OAC and DAPT, leading clinicians to face the dilemma of whether to institute TOAT. When faced with managing these patients, clinicians would ideally have data on the efficacy and safety of TOAT versus DAPT versus OAC + SAPT in patients of various risk profiles to determine the best strategy for each individual patient. In addition, integrating information on newer antiplatelet agents such as prasugrel or ticagrelor would be of interest. However, both US Food and Drug Administration (FDA) labels make reference to concerns regarding concomitant use of medications that increase the risk of bleeding (eg, anticoagulant therapy) and, in the case of prasugrel, this information is included in a boxed warning, further tempering the enthusiasm to integrate these agents into TOAT. Ultimately, there are limited randomized controlled trials in this area; the available evidence that has influenced the current guidelines and practice is obtained primarily from observational studies and some trials.20 These studies vary with regard to patient characteristics, treatment strategies, defined outcomes, and event reporting.
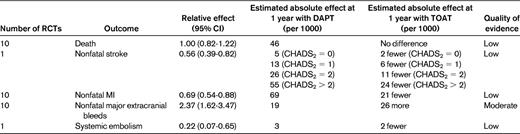
Several of these studies looked specifically at TOAT versus DAPT (predominantly aspirin and clopidogrel), mostly in patients with AF who underwent PCI.5,21–26 Table 4 provides information based on the American College of Chest Physicians (ACCP) antithrombotic therapy for AF guidelines, which extrapolates from multiple studies and makes several broad assumptions in an effort to better quantify the risks and benefits of TOAT versus DAPT. It is useful to note the decreased stroke and myocardial infarction risk with TOAT at the cost of an approximately 2- to 3-fold increased bleeding risk. Although patients receiving TOAT experienced a higher risk of major bleeding than did patients receiving DAPT, the shorter versus longer durations of TOAT have been associated with a 2-fold lower risk of major bleeding.20 Therefore, especially among patients at high thrombotic risk, TOAT in the appropriate settings should be actively considered.
Newer oral anticoagulant agents in TOAT
Some of the newer oral anticoagulant agents have been studied recently as part of the long-term management in patients after ACS. These studies investigated the effects of adding OAC to DAPT. The results are not directly applicable to the TOAT discussion, specifically altered by the fact that the patients in these studies did not have AF and the comparator groups were placebo, not warfarin. Nevertheless, these trials provide some data that have the potential to help inform the management of patients requiring TOAT.
APPRAISE-1 was a phase 2, dose-ranging study that randomized 1715 patients with recent ACS to receive either placebo or apixaban at 1 of 4 doses: 2.5 mg twice daily (BID), 10 mg QD, 10 mg BID, or 20 mg QD.27 Nearly all patients received aspirin and 76% received DAPT. The primary outcome was major or clinically relevant bleeding. The 2 higher-dose arms were discontinued early because of a relative excess in bleeding. The remaining results showed a dose-dependent increase in bleeding risk (hazard ratio [HR] = 1.78; 95% CI, 0.91-3.48; P = .09 for 2.5 mg BID; HR = 2.45; 95% CI, 1.31-4.61; P = .005 for 10 mg QD) and a trend toward a reduction in ischemic events (HR = 0.73; 95% CI, 0.44-1.19; P = .21 for 2.5 mg BID; HR = 0.61; 95% CI, 0.35-1.04; P = .07 for 10 mg QD).
APPRAISE-2 was a phase 3 trial that randomized 7392 patients after ACS to receive either apixaban 5 mg BID or placebo with a primary outcome of cardiovascular death, myocardial infarction, or ischemic stroke.28 Ninety-seven percent of patients received aspirin and 81% received DAPT. The study was terminated early due to an increase in major bleeding (HR = 2.59; 95% CI, 1.50-4.46; P = .001) without a significant improvement in efficacy (HR = 0.95; 95% CI, 0.80-1.11; P = .51) after a median follow-up of 241 days.
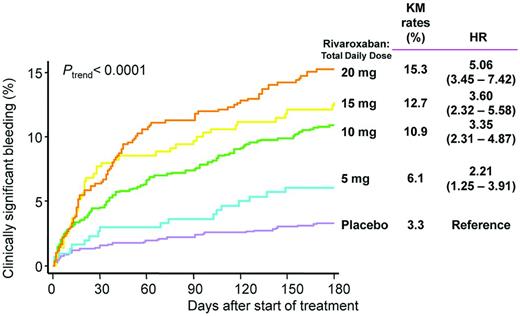
ATLAS ACS-TIMI 46 was a phase 2 trial that randomized 3491 patients after ACS to receive either placebo or rivaroxaban at total daily doses of 5, 10, 15, or 20 mg, administered either QD or BID.29 Approximately 21% of patients received only aspirin, whereas the remainder received DAPT. The results showed a dose-dependent increase in clinically significant bleeding using a broad definition that included thrombolysis in myocardial infarction (TIMI) major bleeding, TIMI minor bleeding, and bleeding requiring medical attention (Figure 1). In terms of efficacy, there was an overall significant reduction in death, myocardial infarction, or stroke with rivaroxaban (HR = 0.69; 95% CI, 0.50-0.96; P = .027), with the numerically lowest HRs seen with the lowest BID doses.
Rates of clinically significant bleeding in ATLAS ACS-TIMI 46. Shown is a composite of TIMI major bleeding, TIMI minor bleeding, and bleeding requiring medical attention.
Rates of clinically significant bleeding in ATLAS ACS-TIMI 46. Shown is a composite of TIMI major bleeding, TIMI minor bleeding, and bleeding requiring medical attention.
ATLAS ACS 2-TIMI 51 was a phase 3 trial that randomized 15 526 patients with recent ACS to receive BID dosing of either placebo, rivaroxaban 2.5 mg, or rivaroxaban 5 mg.30 Ninety-three percent of patients received DAPT. Over a mean follow-up period of 13.1 months, rivaroxaban was found to reduce cardiovascular death, myocardial infarction, or stroke (HR = 0.84; 95% CI, 0.74-0.96; P = .008), with significant reductions versus placebo for both 2.5 mg BID and 5 mg BID. The 2.5 mg dose group had a significant reduction in all-cause mortality (HR = 0.68; 95% CI, 0.53-0.87; P = .002) compared with placebo. Rivaroxaban was associated with an overall increase in major bleeding (HR = 3.96; 95% CI, 2.46-6.38; P < .001) without a significant increase in fatal bleeding (HR = 1.19; 95% CI, 0.54-2.59; P = .66); there was a significantly decreased rate of fatal bleeding in the 2.5 mg dose group compared with the 5 mg dose group (P = .04).
The results of APPRAISE-1, ATLAS ACS-TIMI 46, and ATLAS ACS 2-TIMI 51 are somewhat divergent from APPRAISE-2. This observation may be related to the baseline risk of the patients, considering that APPRAISE-2 enrolled older patients with more comorbid conditions compared with the other 3 trials. Therefore, these patients may have experienced competing risks and diseases not necessarily modified by anticoagulant therapy. In addition, APPRAISE-2 included patients with a prior stroke or transient ischemic attack, and recent studies have illustrated that this group of patients does not appear to benefit from further intensification of antithrombotic therapies.
Another difference between these trials is anticoagulant dosing. APPRAISE-2 studied apixaban at 5 mg BID, the same dose studied in AF patients in ARISTOTLE. However, with rivaroxaban, ROCKET AF studied 20 mg QD, whereas ATLAS ACS 2-TIMI 51 studied 2 lower doses, 5 mg BID and 2.5 mg BID. The observation that 2.5 mg BID reduced the primary end point in ATLAS ACS 2-TIMI 51 supports the hypothesis that even modest factor Xa inhibition can reduce recurrent ischemic events after ACS.
Ultimately, APPRAISE-2 was a trial that evaluated full-dose TOAT, DAPT + OAC, at doses studied for stroke prevention in AF and found an increase in bleeding without a significant efficacy benefit. The efficacy results are difficult to extend, because AF was an exclusion criteria, so these patients did not have the opportunity to benefit from the risk reduction of thromboembolic events secondary to AF. The bleeding results confirm the expected result that was seen in the studies with warfarin: TOAT with full-dose OAC yields an increase in bleeding.
Unlike APPRAISE-2, ATLAS ACS 2-TIMI 51 did not study full-dose TOAT because rivaroxaban was given at doses lower than that used in ROCKET AF to reduce thromboembolic events in AF. This very low-dose TOAT regimen likely yielded a significant overall survival benefit by improving efficacy without increasing fatal bleeding. As in APPRAISE-2, patients with AF were excluded, so the efficacy results are also difficult to extrapolate to the general TOAT discussion; however, in this case, it is for the inverse reason. It is unclear if the low-dose TOAT regimen in ATLAS ACS 2-TIMI 51 would adequately reduce the risk of thromboembolic stroke in patients who had underlying AF and how this strategy would compare with warfarin. A trial randomizing patients with previous AF and new ACS (treated with DAPT) to either low-dose TOAT with a newer agent, full-dose TOAT with a newer agent, or full-dose TOAT with warfarin would be informative.
Integrating TOAT
A few societies and collaborations have suggested guidelines for the management of TOAT in patients with AF who have ACS and/or undergo PCI. For example, the European Society of Cardiology (ECS) Working Group on Thrombosis provided guidance that takes into account the baseline hemorrhagic risk, clinical setting (eg, elective PCI and ACS), and the type of stent used (Figure 2).31 Additional evidence will help to refine these recommendations.32
Summary of ESC Working Group recommendations for antithrombotic therapy in patients with AF and moderate to high thromboembolic risk who undergo PCI.
Summary of ESC Working Group recommendations for antithrombotic therapy in patients with AF and moderate to high thromboembolic risk who undergo PCI.
Likewise, the ACCP has published clinical practice guidelines for the management of antithrombotic therapy in patients with AF, including those with AF and coronary artery disease.5 For patients with AF and a CHADS2 score of greater than 1 who undergo intracoronary stenting (with or without a recent ACS), the recommendation is TOAT for the first month after PCI (if bare metal stent) or 3-6 months (if drug-eluting stent), followed by OAC + SAPT until 12 months. For patients with AF and a CHADS2 score of 0 or 1, the recommendation is DAPT rather than TOAT for the first 12 months after PCI. In the setting of AF and an ACS without stenting, during the first 12 months, the suggestion is OAC + SAPT for patients with a CHADS2 score of greater than 0 and DAPT for patients with a CHADS2 score of 0. After these initial periods, OAC alone is a consideration for patients with AF and stable coronary artery disease. However, antiplatelet therapy will be continued in other patients based on their initial ACS presentation and stenting status.
In general, the 2 guidelines share similar themes, and it is most prudent to start by integrating the baseline thrombotic and bleeding risks. From there, one can focus on steps to reduce the duration of TOAT and ways to mitigate bleeding risks. For example, bare metal stents should be used if possible. From a procedural standpoint, radial access as opposed to femoral access provides additional reductions in bleeding risk. In addition, keeping the INR range for patients receiving TOAT between 2.0 and 2.5 is desirable. The current TOAT guidelines, however, focus on vitamin K antagonists when discussing OAC recommendations, and the newer anticoagulant agents have not been specifically studied in situations in which TOAT would be indicated for concomitant AF and ACS.
Nonetheless, the available data from the AF trials (with either no background antiplatelet therapy or SAPT) suggests that the newer anticoagulants compared with warfarin provide a more favorable safety profile, especially in terms of a reduction in hemorrhagic stroke. Therefore, one could speculate that these newer agents as part of a TOAT regimen may be beneficial compared with warfarin. Although the newer anticoagulants currently lack a specific reversal agent, they have a relatively rapid onset and termination of anticoagulant effect. Therefore, in the case of an emergency, recommended measures include the discontinuation of the anticoagulant, evaluation for concomitant medications that influence hemostasis, and initiation of timely clinical support. Data on the use of newer anticoagulants as part of TOAT are limited, though, and the use of such agents in this setting should be undertaken with care. In addition, the most favorable dose for each anticoagulant within the framework of TOAT remains debatable. For example, there is a clear dose-dependent increase in bleeding with factor IIa and Xa inhibitors in the setting of DAPT (Figure 1); however, it is important to keep in mind that TOAT with warfarin could lead to equal or higher rates of bleeding in patients with AF after an ACS. Therefore, dosing of anticoagulants will continue to be an active area of discussion and a topic that future guideline committees will explore.
Conclusions
Antithrombotic therapy plays an essential role in the management of AF and ACS with or without PCI. OAC is the cornerstone of stroke prevention in AF, whereas DAPT significantly reduces recurrent ischemic events and, in some cases, mortality after ACS and PCI. When patients need both OAC and DAPT, clinicians face the dilemma of whether to initiate TOAT and, if so, for how long. There is general agreement that, when possible, one should use maneuvers to shorten the duration of DAPT and, when using warfarin, target a tighter INR range. Further studies addressing the novel anticoagulants with more favorable pharmacokinetic profiles compared with warfarin will be of increasing importance in the setting of TOAT.
The issue of TOAT extends beyond AF and ACS, and other conditions that necessitate combinations of antithrombotic therapy, such as venous thromboembolism, valvular disease, and left ventricular thrombus, will be equally relevant. Choosing the right antithrombotic strategy is ultimately an individualized decision that involves weighing the risks and benefits for each specific patient. With newer antiplatelet and anticoagulants added to the selection of antithrombotic therapies, clinicians may one day be better equipped to meet the needs of some of the most challenging patients.
Disclosures
Conflict-of-interest disclosure: J.M. has received research funding from Eli Lilly, Daiichi Sankyo, Sanofi, BMS, Bayer, and Johnson & Johnson and honoraria from Merck and Janssen. E.T.C. declares no competing financial interests. Off-label drug use: newer anticoagulants in AF, ACS, and venous thromboembolism.
Correspondence
Jessica Mega, Brigham and Women's Hospital, 75 Francis St, Boston, MA 02115; Phone: 617-278-0145; Fax: 617-734-7329; e-mail: jmega@partners.org.