Abstract
The treatment of symptomatic and high-risk myelodysplastic syndrome (MDS) spans several therapeutic goals and options. Key to the successful therapy of these heterogeneous diseases is careful characterization and diagnosis, including clinical, cytogenetic, biological, and molecular evaluation of individual patients. Any novel management strategy in MDS must be based on accepted and validated prognostic scoring systems, such as the International Prognostic Scoring System (IPSS), and should take into account predictive parameters of response to the available therapeutic agents and individual comorbidities. For IPSS lower-risk MDS patients, several first-line options are available, including erythropoietic stimulating agents, lenalidomide, and immunosuppressive drugs. Sequential therapy is advisable whenever response is lost, and the activity of azacitidine and decitabine in first- or second-line therapy is relevant, especially in patients with symptomatic cytopenias and anemia. Hypomethylating agents have a central role in therapy of IPSS higher-risk MDS patients. These agents include azacitidine and decitabine, which allow treatment of very elderly and frail patients, resulting in hematological improvement and transfusion independency in roughly half, and for azacitidine a demonstrated significant prolongation of survival. Because hypomethylating agents are not curative, they are not satisfactory for younger MDS patients, for whom a transplantation strategy should be planned. Although hypomethylating agent therapy is used extensively, a growing number of MDS patients fail to respond or progress. The future challenge is not only to find treatment regimens that target the dysplastic clone(s) so that durable remissions are achieved (particularly in high-risk patients with short survival and/or increased leukemic transformation rates), but also to also identify active salvage regimens.
Introduction
Hypomethylating agents constitute an essential tool in the treatment of myelodysplastic syndrome (MDS), but are not the only available therapeutic option and their use can be further optimized and integrated in a strategy of sequential treatment. The hypomethylating agents (or DNA methyltransferase inhibitors) have allowed the treatment of higher-risk elderly and frail patients, who in the past were treated exclusively with the best supportive care.1–8 Although none of these agents achieves final cure, they do induce an improvement in hematopoiesis and, at least for azacitidine, a demonstrated prolonged overall survival1 (Table 1). For elderly patients, this is quite clearly an enormous advantage,9 whereas younger patients with longer life expectancies cannot be treated with hypomethylating agents alone and will need subsequent transplantation. The next step in the use of hypomethylating agents as treatment for MDS is to set up a strategy for increasing the number of responding patients and to further improve the durability of responses. Evidence is accumulating on the feasibility of hematopoietic stem cell transplantation (HSCT) in MDS patients who received prior azacitidine or decitabine therapy.10
Principal clinical studies with hypomethylating agents in IPSS higher-risk MDS patients
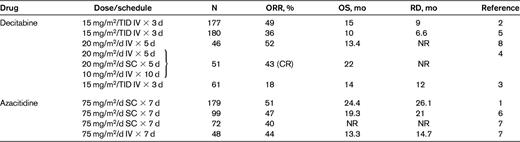
N indicates number of patients; ORR, overall response rate; OS, overall survival; RD, response duration; TID, 3 times/d; SC, subcutaneous; NR, not reported; and CR, complete response.
Several lessons have been learned through clinical experience with hypomethylating agents: (1) the beneficial effects of hypomethylating agents are noted in the majority of patients only after approximately 4 cycles1,3,5,7 ; (2) the achievement of hematological improvement per se is sufficient to ensure prolonged overall survival1 ; (3) interruption of treatment induces relapse in almost all patients1–8 ; (4) patients who are resistant or relapse after treatment with hypomethylating agents have extremely limited survival times11,12 ; and (5) patients with complex karyotypes involving monosomy 7 or 5 have a negligible survival advantage from hypomethylating agent therapy despite achievement of response.13,14
These basic concepts demand a new attitude in assessing the response to treatment and in planning long-term management and have an evident impact on health care systems. Therefore, cost-effectiveness evaluations must also be included in our integrated strategy of decision making.
A therapeutic strategy for MDS
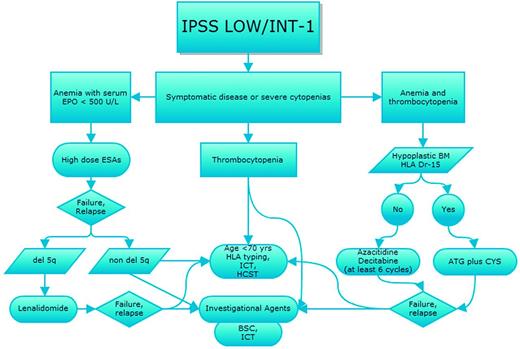
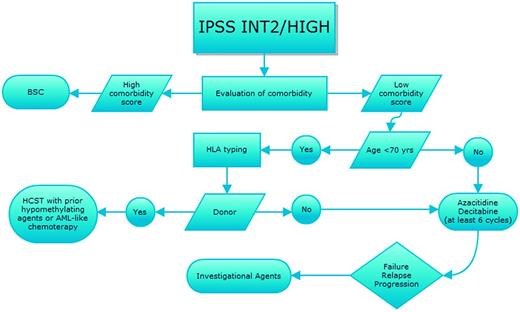
Prognostication of MDS, which is based on the International Prognostic Scoring System (IPSS),15 the Revised International Prognostic Scoring System (IPSS-R),16 or the World Health Organization (WHO) classification-based Prognostic Scoring System (WPSS-R),17 and evaluation of individual comorbidities according to one of the several scores available for hematologic patients should always drive therapeutic choices, as recently indicated by various national guidelines18,19 (Figures 1 and 2). In parallel, we should weigh the response predictive parameters developed for some of the therapeutical options.
Therapeutic algorithm for IPSS intermediate-1- and lower-risk MDS patients. Shown is the strategy for treatment choices for patients with IPSS lower-risk MDS according to type of cytopenias and with sequential treatment in case of relapse/resistance after first-line therapy. EPO indicates erythropoietin; ICT, iron chelation therapy; HSCT, hematopoietic stem cell transplant; ESA, erythropoietic stimulating agents; and BSC, best supportive care.
Therapeutic algorithm for IPSS intermediate-1- and lower-risk MDS patients. Shown is the strategy for treatment choices for patients with IPSS lower-risk MDS according to type of cytopenias and with sequential treatment in case of relapse/resistance after first-line therapy. EPO indicates erythropoietin; ICT, iron chelation therapy; HSCT, hematopoietic stem cell transplant; ESA, erythropoietic stimulating agents; and BSC, best supportive care.
Therapeutic algorithm for IPSS intermediate-2- and high-risk MDS patients. Shown is the strategy for treatment choices for patients with IPSS higher-risk MDS with sequential treatment in case of relapse/resistance after first-line therapy. BSC indicates best supportive care; and HSCT, hematopoietic stem cell transplant.
Therapeutic algorithm for IPSS intermediate-2- and high-risk MDS patients. Shown is the strategy for treatment choices for patients with IPSS higher-risk MDS with sequential treatment in case of relapse/resistance after first-line therapy. BSC indicates best supportive care; and HSCT, hematopoietic stem cell transplant.
IPSS lower-risk MDS patients
The majority of de novo MDS patients are diagnosed with IPSs lower-risk disease, and although prognosis in this ample subgroup of patients is highly heterogeneous, their most frequent problem is, at least in two-thirds of the cases, confined to anemia. Therefore, first-line therapy is frequently used only to increase hemoglobin levels. Erythropoietic-stimulating agents (ESAs), including erythropoietin-alpha, erythropoietin-beta, and darbepoetin, have been found to increase hemoglobin levels and abolish transfusion dependence in 19%-68% of MDS cases. This wide range of response depends on several known biological, clinical, and drug related variables.19,20 ESAs are not approved for the treatment of MDS; however, unlike what is observed for certain solid tumors, there is no evidence of a detrimental effect of ESAs on outcomes of MDS patients. On the contrary, it has been demonstrated that MDS patients treated with ESAs (with or without G-CSF) have an advantage in terms of overall survival20 and no increase in thromboembolic events.21 When ESAs are ceased for ineffectiveness or when their activity is lost, the first goal is to ensure that patients continue to receive adequate supportive care, and there is healthy ongoing debate about whether iron chelation is necessary in this setting. Organ damage and direct DNA damage by excess iron have been reported to be noxious in MDS.22 There are reports of survival advantage in patients who received chelation treatment, but these were all retrospective, nonrandomized studies. Although the effective elimination of iron overload is feasible and intuitive in MDS patients with long life expectancies and is important for transfusion load, the real advantage remains to be demonstrated when transplantation is not an option. Recent evidence indicates a direct positive effect of chelation on hematopoiesis, which may add further support to the chelation strategy.23
Alternatively, the ESA-resistant, transfusion-dependent, lower-risk MDS patient should be treated with hypomethylating agents or recruited to an experimental trial. Clinical evidence and biological background for the use of hypomethylating agents in lower-risk MDS patients are not perfectly defined and there are few studies on their use in this population. Available evidence indicates that the overall response rates are similar to those observed in higher-risk MDS patients, and a significant proportion of patients may achieve transfusion independence (Table 2).2–8,24–27 Although hypomethylating agents should be used as second-line treatments in anemic IPSS lower-risk MDS patients, their use as first-line agents (in particular, azacitidine for the scarce myelotoxicity) may be especially considered in lower-risk MDS in whom symptomatic thrombocytopenia and/or neutropenia is dominant.18,19
Clinical studies with hypomethylating agents in IPSS lower-risk MDS patients
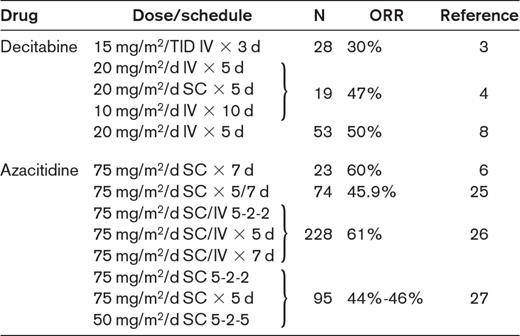
N indicates number of patients; ORR, overall response rate; TID, 3 times/d; and SC, subcutaneous.
At present, there is no direct comparison between the efficacy of hypomethylating agents and that of investigational drugs such as lenalidomide in ESA-resistant MDS patients. In ESA-refractory MDS patients without 5q alteration, the reported response to lenalidomide is 26% (transfusion independence).28 A multicenter randomized study is currently evaluating its effective activity in such a patient cohort (www.clinicaltrials.gov identifier NCT01029262). Lower-risk anemic MDS patients with del5q treated with ESAs become nonresponsive after a short remission28,29 and achieve significant erythroid response after treatment with lenalidomide, which may also induce complete cytogenetic responses and improve survival.
Based on evidence of immune-mediated pathophysiology of MDS, immunosuppressive therapy can be effective. A recent randomized phase 3 trial demonstrated that antithymocyte globulin and cyclosporine (ATG + CSA) treatment is associated with hematologic response in a subset of MDS patients, but with no apparent impact on progression of disease and overall survival.30 Because of the toxicity of such an approach, alternative treatments such as alemtuzumab have been attempted and have shown results in selected patients, but evaluation of their impact on survival is required.31
IPSS higher-risk MDS patients
The treatment of higher-risk MDS patients is more straightforward. Because the only curative option for this disease with an extremely severe prognosis is allogeneic HSCT, this should always be included in the therapeutic strategy of fit patients. At present, hypomethylating agents constitute the first choice of therapy because of their significantly lower toxicity and the achievement of hematological response1–9 and prolongation of survival in a substantial proportion of patients1 (Table 1). There is no consensus on the importance of any therapy before HSCT in MDS, and even less so for intensive chemotherapy in the pre-HSCT setting. Intensive chemotherapy with AML-like regimens has been used to reduce relapse rates, but a single nonrandomized study indicated recently that the rates of relapse were similar in patients treated with intensive chemotherapy and those receiving azacitidine, whereas the estimated overall survival was significantly higher and the mortality lower in the second treatment group.10 This approach should be considered only for high-risk MDS patients younger than 70 years with a normal karyotype. There are very few recent studies investigating the efficacy of intensive chemotherapy in MDS. One of these was performed by the Japan Adult Leukemia Study group and confirmed what had been published in the prehypomethylating agent era: that MDS patients who received idarubicin and cytosine arabinoside (randomized with an arm B receiving low-dose cytosine arabinoside and aclarubicin) had 2-year overall survival rates and disease-free survival rates of 28.1% and 26.0%, respectively.32
Choosing a therapeutic strategy
Prediction of response to ESAs
Notwithstanding the availability of ESAs for more than 2 decades, their common use in MDS is far from being optimal. It would be simple to examine a few clinical and biological parameters to avoid unnecessary treatment and contain expenses. The variables allowing the selection of patients potentially responsive to ESAs are: endogenous erythropoietin levels < 500 U/L, BM blasts < 10%, IPSS low to intermediate-1, diagnosis of refractory anemia, normal karyotype, transfusion independence, and short duration of the disease.20 There are also more refined biological parameters: flow cytometric analysis of MDS myeloblast phenotype according to the European Leukemia Network (ELN) method identified ESA-resistant MDS patients beyond the clinical Nordic predictive model.33 Among IPSS low-risk MDS patients, 3 distinct subgroups with a 94%, 17%, and 11% probability of responding to ESA treatment have been defined.34 EPO-dependent ERK1/235 and Stat-536 activation have been shown to be significantly lower in BM CD45−/CD71+/GPA− cells from ESA nonresponders compared with ESA responders and could predict clinical response to ESAs.
Prediction of response to lenalidomide therapy
The presence of del5q is the strongest predictive factor of response to lenalidomide, but a molecular response signature consisting of a cohesive set of erythroid-specific genes with decreased expression in responders has been identified.37,38 Lower-risk anemic, transfusion-dependent MDS patients with del5q develop an erythroid response and may achieve a cytogenetic response after treatment with lenalidomide (10 mg) daily.37 Lenalidomide is approved by the US Food and Drug Administration (FDA) in this setting, but has not yet received approval from the European Medicines Agency (EMA) because of concerns about an increased progression to acute myeloid leukemia (AML) shown in treated patients during an international, company-sponsored trial. More than 50% of patients with IPSS intermediate-1 to low-risk MDS who were treated with lenalidomide achieved transfusion independency and cytogenetic response.38 In these patients, reductions in the relative risks of death and AML progression were significant, although in that study, crossover to lenalidomide from best supportive care makes the assessment somewhat difficult.39 It seems clear that additional cytogenetic abnormalities, and in particular p53 mutations, are associated with an increased risk of progression to AML.40
The use of lenalidomide in IPSS higher-risk del5q MDS patients has been reported,41 but the hematological responses are quite scarce and therefore this treatment is discouraged in this subset of patients. Preliminary but extremely encouraging results have been obtained by adding lenalidomide to azacitidine, and the responses are not confined to del5q patients.42,43
Prediction of response to immunosuppressive therapy
Evidence regarding the outcome of MDS patients treated with immunosuppressive therapy is far from abundant. Nevertheless, we may extract sufficient information regarding the clinical and biological characteristics of the responsive patients. Because of the intrinsic toxicity and the prolonged immunosuppression, elderly (> 60 years) patients should not receive ATG + CSA. Recently diagnosed (< 1 year) IPSS lower-risk MDS patients with hypoplastic BM, < 5% blasts, and normal karyotype are more likely to respond. In addition, the occurrence of HLA-DR15 positivity is also correlated with hematological improvement after immunosuppression.19
Prediction of response to hypomethylating agents: molecular and cytogenetic parameters
Hypomethylating agents induce overall responses in approximately 40%-60% of patients1,9 with both IPSS higher- and lower-risk MDS (Tables 1 and 2). How can we determine which patients are prone to respond to facilitate the choice of treatment? Both hypomethylating agents inhibit DNA methyltransferase activity and methylation of new DNA strands. In MDS, the frequency of hypermethylated genes is higher than that of mutated genes, indicating that epigenetic alterations are an important pathogenic event in such diseases.44 Many recently identified MDS molecular aberrations involve DNA methylation and epigenetic processes; moreover, hypermethylation of CpG islands and levels of gene promoter methylation are correlated with disease severity and survival.44,45 This could explain why MDS, in particular in the advanced stages, is most responsive to agents interfering with epigenetic alterations. Although a correlation between TET2 mutations and response to azacitidine has been shown, it has no impact on final outcome in terms of overall survival.46 Preliminary results indicate that the presence of EZH2 mutations is not only predictive of response to azacitidine, but also of prolonged survival.47 It has to be stressed that these molecular alterations are not yet assessed routinely, but their prognostic value should be validated in a larger series of MDS patients.
Azacitidine and decitabine have been shown to induce in vitro and in vivo hypomethylation, with subsequent reexpression of pathologically silenced genes. Nevertheless, researchers have failed to demonstrate a clear correlation between baseline DNA hypermethylation and response to hypomethylating agents. We do not have a validated marker predictive of clinical response, although some suggestions come from the demonstration of demethylation of phosphoinositide-phospholipase C during successful azacitidine treatment48 and from the observation of more profound global hypomethylation in MDS patients responding with complete and partial remissions to decitabine.49
Response to hypomethylating agents is also obtainable in MDS patients presenting with unfavorable cytogenetic characteristics. Treatment with azacitidine and decitabine has been demonstrated to be superior to chemotherapy and to induce complete responses in patients with chromosome 5 and 7 abnormalities, although these responses are not durable.13,49,50 IPSS higher-risk MDS patients with a complex karyotype including chromosome 5 and 7 abnormalities continue to have a grim prognosis despite the use of hypomethylating agents. There is at present an urgent unmet need for treatment options in this patient subgroup.
Prediction of response to hypomethylating agents: clinical characteristics
Age per se should not be a limiting factor when we are inclined to opt for azacitidine or decitabine treatment. In particular for azacitidine, a substudy of an international phase 3 trial has shown optimal hematological improvement and prolonged survival in MDS patients older than 75 years,9 and other studies have indicated that the efficacy and low toxicity of this agent allows the treatment of patients > 80 years of age, who have the same outcome as younger patients. We should reflect upon elderly patient frailty and carefully take into consideration existing comorbidities. The impact of comorbidities on survival of MDS patients is enormous and has been systematically analyzed in a study evaluating a large number of patients.51 Patients with a severe comorbidity had a 50% decrease in survival that was independent of age and IPSS risk group. Irrespective of treatment and IPSS, but according to the Adult Comorbidity Evaluation 27 (ACE-27) categories, median survival ranged from 31.8-9.7 months for MDS patients with none, mild, moderate, and severe comorbidities, respectively (P < .001). The interesting result is that a final prognostic score could be developed including age, IPSS, and comorbidity.
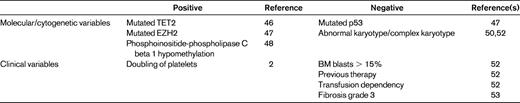
So far, the best predictive model for response to hypomethylating agents is the one developed by the Groupe Francophone des Myelodysplasies (GFM), which is based on the clinical and biological characteristics of a large number of MDS patients treated with azacitidine.52 Prognostic factors were analyzed in terms of prediction of response, duration of response, and overall survival. In multivariate analysis, previous treatment with low-dose cytosine arabinoside, BM blasts > 15%, and abnormal karyotype were associated with a significantly lower overall response rate, whereas only complex karyotype was predictive of shorter response duration. Regarding prediction of overall survival, one of the significant prognostic variables is performance status, reiterating the importance of the general health condition of the MDS patients in addition to the characteristics of the dysplastic disease. In addition, intermediate and unfavorable IPSS cytogenetic risk, presence of circulating blasts, and transfusion dependency had strong adverse prognostic values. A score was then built on the latter 4 variables, attributing 1 point to each, with the exception of poor-risk cytogenetics, which scored 2 points. How much will the attribution of MDS patients to these categories drive our decisions? Several other still unexamined factors may influence outcome after hypomethylating agents, such as the presence of BM fibrosis, which has been demonstrated to negatively influence response53 (Table 3).
Failure or relapse: do we see a new horizon beyond hypomethylating agents?
The increasing use of azacitidine and decitabine in the treatment of MDS has improved the outcome of patients who used to have very poor survival, but still roughly half of MDS patients will not respond, will progress while on hypomethylating therapy, or will relapse at variable times after response. The same is true for lower-risk MDS patients who become nonresponsive to ESAs, lenalidomide, or ATG + CSA and need treatment with hypomethylating agents. There are no therapies approved for these patients, and available therapeutic options are almost all investigational. For lower-risk patients, the urgency is less compelling and the objective is to avoid transfusions and improve quality of life. For higher risk MDS patients, the quest for an alternative treatment that is able to rapidly block progression to AML or alleviate the severity of the disease and prolong survival is of supreme priority.11,12 Notwithstanding the growing number of patients who lose response or progress while on hypomethylating agent therapy and the awareness that the prognosis is extremely poor, there are only 2 reports of outcomes of such cases. In one study of 87 MDS patients treated with decitabine, 43% showed primary resistance, whereas 57% experienced a relapse during therapy.11 Cumulative data on all IPSS risk groups showed that the median survival after decitabine failure was 4.3 months and the estimated 12-month survival rate was 28%. A large proportion of these patients were followed up with only supportive care, but approximately one-third received experimental treatments, such as clofarabine, cloretazine, and sapacitabine; a minority of patients underwent HSCT and 10 received intensive chemotherapy regimen with anthracyclines. Outcome of the different treatment options after decitabine failure is difficult to analyze due to the small number of patients studied, but it can be affirmed that previous response to decitabine had no impact on survival or progression to AML.11
Similar results were presented in the study of outcome of 435 MDS patients after azacitidine failure. In this cohort, the median overall survival was 5.6 months and probability of survival at 24 months was 15%.12 An analysis of response to salvage treatment is possible in this study and indicates that younger MDS patients who could undergo HSCT had the best survival (median, 19.5 months), whereas investigational therapies could induce a survival of 13.2 months. Intensive chemotherapy failed to induce a substantial increase in overall survival. All salvage treatments improved outcome compared with supportive care only (4.1 months). The present challenge is therefore to design therapeutic schemes with a combination of agents or with investigational drugs for selected subpopulations (IPSS lower-risk, IPSS higher-risk, AML post-MDS, and hypoplastic blastic MDS patients) after hypomethylating agent failure.
Biological data suggest that as epigenetic drugs, histone deacetylase inhibitor inhibitors might synergize with hypomethylating agents. Pivotal studies have been conducted and azacitidine and decitabine administered in combination with phenylbutyrate, valproic acid (plus or minus all-trans retinoic acid), vorinostat, or entinostat, with results not fulfilling expectations (Table 4).54 These studies were conducted in de novo MDS patients and in patients who had previously received hypomethylating agents and failed. Although the preclinical background of these associations seemed quite robust, the synergy of these 2 types of epigenetic drugs in the clinical setting appears to produce at most an acceleration in the induction of hematological improvement. Therefore, the most promising association seems to be with vorinostat, but results of the clinical trial of vorinostat plus azacitidine have so far been published only in abstract form.54
Combination therapies with hypomethylating agents in patients with MDS
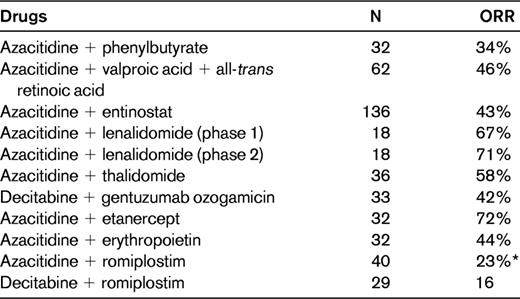
Combination studies were reviewed in Ornstein and Sekeres.54
N indicates number of patients; and ORR, overall response rate.
*Decrease in hemorrhagic events.
The addition of lenalidomide to azacitidine has proven to be more effective than monotherapy,42,43 but its effect on inducing responses in patients relapsing after hypomethylating agents is at present unclear and needs to be assessed in prospective trials. In fact, a recent study suggested that the addition of lenalidomide could resensitize patients who had previously relapsed on azacitidine.43
The kinase inhibitor rigosertib and the nucleoside analogs sapacitabine and clofarabine show the highest potential among the vast series of novel investigational agents for the treatment of MDS patients failing hypomethylating agents (Table 5).
Investigational agents for MDS patients after hypomethylating therapy failure

N indicates number of patients; ORR, overall response rate; BID, 2 times/d; PO, per os; and NR, not reported.
Rigosertib (previously ON 01910.Na) is an IV styryl-sulfone multikinase inhibitor that was demonstrated to have good tolerance, decrease BM blast percentage in higher-risk MDS patients after hypomethylating agent failure, and induce complete remission and stabilization of disease.55,56 Rigosertib is under evaluation in a randomized international study, the primary objective of which is to compare overall survival in MDS patients with excess blasts (5%-30% BM blasts) who have failed azacitidine or decitabine treatment and are receiving the drug plus best supportive care with that of patients receiving best supportive care only (www.clinicaltrials.gov identifier NCT01241500). There is evidence of a selective activity on MDS with trisomy chromosome 8, the mode of action being cytotoxicity and induction of chromosome catastrophe.56
Sapacitabine is an oral deoxycytidine nucleoside analog that has shown activity in second-line treatment of higher-risk MDS. It induces single-strand breaks that are converted into double-strand breaks when cells go through a second S-phase. In the phase 1 study, 28% of patients responded.57 Early reports of phase 2 studies indicate an overall response rate of 24% (range, 38%-8%) for MDS patients who had received hypomethylating agents, with best results obtained for the uninterrupted 7-day regimens at the dose of 300 mg twice daily.58
Clofarabine in both oral and IV formulations has been used in investigational studies for higher-risk refractory/relapsed MDS (60% of the studied population had been treated with hypomethylating agents).59,60 In this group of MDS patients, the overall response rate to IV clofarabine was 30%-36% at the 2 doses of 15 and 30 mg/m2 for 5 days, but myelosuppression was quite frequently severe.59 However, the oral formulation is better tolerated and also leads to responses in one-third of hypomethylating agent–refractory/relapsed patients.60
For some of these agents, alternate schedules of sequential administration have been proposed (eg, decitabine-sapecitabine and clofarabine-cytosine arabinoside).
Two thrombopoietin-mimetic agents, eltrombopag and romiplostim, are available and approved for treatment of immune thrombocytopenic purpura. Eltrombopag is under evaluation in ongoing clinical trials in lower-risk MDS patients and in 2 other studies (www.clinicaltrials.gov identifier NCT00903422 is ongoing; another trial has just completed [NCT01113502]) in the palliative setting for treatment of thrombocytopenia in patients with advanced MDS or secondary AML/MDS after hypomethylating agent failure. Romiplostim has been shown to have activity in MDS, inducing significant platelet increases even during the first cycle of hypomethylating agent treatment.61,62 The larger international trial of romiplostim in lower-risk, severely thrombocytopenic MDS patients has been interrupted because of the frequent observation of increases in BM blasts.
Numerous more nonconventional agents and combinations are being actively tested in hypomethylating therapy failure. In a recent study, a combination of gemtuzumab ozogamicin (an anti-CD33 mAb conjugated to calicheamicin) with arsenic trioxide yielded a response in 2 of 7 patients with higher-risk MDS who had received prior azacitidine.63
Disclosures
Conflict-of-interest disclosure: The author has received honoraria from Celgene, Janssen, and Novartis. Off-label drug use: None disclosed.
Correspondence
Valeria Santini, Department of Hematology, University of Florence, AOU Careggi, Largo Brambilla 3, 50134, Florence, Italy; Phone: +39-055-7947296; Fax: +39-055-7947343; e-mail: santini@unifi.it.