Abstract
Plasma exchange is a therapeutic procedure used to treat a variety of diseases through the bulk removal of plasma. To apply this treatment to patients appropriately, it is essential to understand the methods to remove plasma, its effects on normal plasma constituents, the role of replacement fluids in the treatment, and the risks associated with the procedure. To facilitate the appropriate evidence-based use of plasma exchange and to encourage research, the American Society for Apheresis has published guidelines providing practical guidance and information to those responsible for ordering or providing this treatment.
Introduction
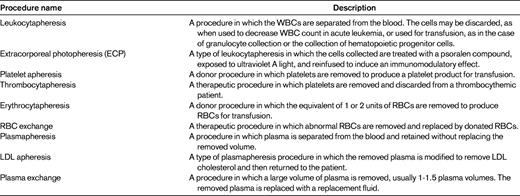
The word “apheresis” is derived from the Greek word “aphairesis,” which means “to separate,” “to take away by force,” or “to remove.” This term was originally used by Abel, Rowntree, and Turner to describe manual plasma exchange, the removal of units of whole blood anticoagulated with heparin followed by centrifugation to separate the blood into the cellular elements and plasma.1 The cellular elements were then mixed with a replacement for the discarded plasma and reinfused. Since this initial use, the term has been used more broadly to describe several procedures, all of which involve the separation of whole blood into its components with removal or modification of one or more of these components. Table 1 lists the apheresis procedures performed commonly within the United States.2
Apheresis procedures performed commonly in the United States2
LDL indicates low-density lipoprotein.
Of the procedures listed in Table 1, therapeutic plasma exchange (TPE) is the procedure that is performed most commonly. As defined in Table 1, TPE is a procedure in which a large volume of plasma is removed from a patient.2 The volume removed is such that if it were not replaced, significant hypovolemia resulting in vasomotor collapse would occur. As a result, the removed plasma must be replaced with some form of replacement fluid.2 Plasmapheresis removes a smaller amount of plasma, usually less than 15% of the patient's blood volume, and therefore does not require replacement of the removed plasma. The most common plasmapheresis procedures performed in the United States are those in which plasma is collected from healthy donors for transfusion or manufacture into products such as albumin, IVIG, factor concentrates, and laboratory reagents. In common usage, the terms plasma exchange and plasmapheresis are used interchangeably, although the 2 procedures are different. The lack of clarity in usage of these 2 terms could result in problems when searching the medical literature. Plasmapheresis and plasma exchange are 2 separate Medical Subject Headings (MeSH) in the National Library of Medicine. The incorrect usage of the terms by authors has led to incorrect categorization, meaning that literature searches should include both terms to identify all relevant literature. For the remainder of this article, only TPE will be discussed, because the devices used to perform therapeutic plasmapheresis procedures, other than the devices used to perform low-density lipoprotein apheresis, have not been approved by the Food and Drug Administration for use in the United States.
A wide variety of diseases seen by different medical specialists can be treated with TPE. The diseases/disorders treated with TPE that have been categorized by the American Society for Apheresis (ASFA) are listed in Table 2. This list does not represent all of the diseases and disorders to which TPE has been applied, only those for which sufficient published literature exists to provide guidance for the use of TPE. The treatment of specific diseases or disorders will not be discussed herein, but rather the general concepts and mechanisms of TPE, including the mechanism of action, important patient care concepts, and complications of TPE. The purpose and structure of the ASFA guidelines will also be reviewed.
Mechanism of action of plasma exchange
TPE, through the bulk removal and replacement of plasma, removes pathologic substances such as pathologic Abs, immune complexes, and cytokines. It has been presumed that the removal of these substances represents the major mechanism of action of TPE. However, this mechanism does not explain the length of response seen in some disorders. Additional evidence suggests that TPE may have an immunomodulatory effect beyond the removal of Ig. Reported effects of TPE on immune function include T-cell modulation with a shift from in the Th1/Th2 balance with a shift toward Th2,3 suppression of IL-2 and IFN-γ production,4,5 and in vitro cultures demonstrating an increase in concanavalin A–induced suppressor cell function.6
Mechanism of plasma removal
Devices used to perform TPE can be divided into 2 broad categories, those that separate the plasma from the cellular components based on size and those that separate components based on density.7 Devices separating based on size use filters, whereas those separating by density use centrifugation. In the former, whole blood flows over a membrane that separates the plasma from the cellular elements, which are then returned to the patient. Different configurations of filters have been used to separate plasma from cellular elements and all have similar capabilities. Filtration-based apheresis devices for performing TPE-utilizing filters are not widely used in the United States.7
The predominant method used for TPE in the United States is centrifugation. In these apheresis devices, whole blood is pumped into a rapidly rotating separation chamber. Components separate into layers based upon their density, with the most dense element, RBCs, migrating the furthest from the axis of rotation and the least dense portion, plasma, layering closest to the axis of rotation. Intermediate layers, moving from the axis of rotation outward, are platelets, lymphocytes, and granulocytes.7 In TPE, the plasma layer is removed and discarded and the remaining cellular elements are mixed with a replacement fluid and returned to the patient. It is important to realize that there is some mixing that occurs at the interface between the layers in the centrifuge. The implication of this is that some platelets may be present in the plasma layer and, depending upon several factors, there may be a resulting loss of platelets during TPE.7
The fact that a replacement fluid is necessary to perform TPE and that it is administered while the procedure is occurring has implications for the removal of substances circulating in the plasma. The removal of a substance in the plasma and limited to the intravascular space can be described by the following exponential equation: Y/Y0 = e−x, where Y is the final concentration of a substance, Y0 is the initial concentration, and X is the number of times the patient's plasma volume is exchanged.8 Because of the dilution of the plasma by the replacement fluid, the substance of interest cannot be completely removed from the circulation. For each 1-1.5 plasma volume exchanged, approximately 60%-70% of substances present in the plasma at the start of that plasma volume will be removed. As additional plasma volumes are exchanged, the absolute amount removed becomes lower, although removal of a fixed 60%-70% still occurs.8 For this reason, routine practice is to exchange only 1-1.5 plasma volumes during a TPE.2 Treating volumes beyond 1.5 plasma volumes removes smaller, less clinically important amounts of pathologic substance present in the plasma while prolonging the procedure and exposing the patient to more replacement fluid and anticoagulant. The result is an increasing risk of complications without increasing benefit to the patient. There are diminishing returns in treating beyond 1.5 plasma volumes.
The equation provided in the preceding section assumes that there is no exchange between the intravascular and extravascular compartments during the procedure. However, this assumption is not valid for all substances, so the amount removed and the concentration in the plasma at the end of the procedure may not match that predicted. For example, IgG is evenly distributed between the intravascular space and the extravascular space and can move between these compartments. During TPE, as the concentration of IgG in the intravascular space decreases, IgG within the extravascular space moves into the intravascular space. After the procedure, the plasma concentration of IgG will be greater than predicted, suggesting that the TPE was not as efficient as expected.8 This has led some to believe that the removal of such molecules is less efficient and that greater volumes should be treated, but the amount of IgG in the waste bag is actually greater than predicted, indicating that TPE was more efficient than expected due to redistribution during the procedure, with removal of IgG from both the intravascular and extravascular compartments.8
Because TPE involves the bulk removal of plasma, anything circulating in the plasma will be removed. The procedure is nonselective, removing both normal and pathologic plasma components. For example, during a 1 plasma volume exchange using albumin as the replacement fluid, coagulation factor activity decreases and coagulation tests may become abnormal. Significant declines in factor V (FV), FVII, FVIII, FIX, FX, and VWF activity occurs.9–11 Activities of FVIII, FIX, and VWF return to normal within 4 hours after TPE, whereas the remaining coagulation factors achieve pre-TPE activity levels by 24 hours.9 The exception to this is fibrinogen, which reaches 66% of pre-apheresis levels by 72 hours.10 Additional substances removed include inhibitors of coagulation such as antithrombin11,12 and the pseudocholinesterase necessary for metabolism of some drugs.13,14 Theoretically, the removal of inhibitors of coagulation could predispose patients to thrombosis, but this has not been demonstrated definitively.11,12 Reports of prolonged neuromuscular blockade due to decreased pseudocholinesterase activity have been reported.13,14 The bulk removal and replacement of plasma also has implications for laboratory testing. The removal of Abs from the patient can result in false negative tests for infectious diseases, autoantibodies, alloantibodies, and enzyme and coagulation factor activity. Samples for such testing should be collected before the initiation of TPE. Finally, in addition to the removal of these normal components of plasma, TPE may also remove medications. Although the effect of TPE on the majority of medications is unknown due to limited pharmacokinetic studies, some drugs have been reported to have significant removal.15–17 Drugs that have been reported to be removed by TPE are listed in Table 3.
As has been stated, TPE requires the replacement of the removed plasma. The composition of the replacement fluid, as alluded to in the section describing the removal of coagulation factors, influences the effects of TPE on the patient. It is important to realize that one-third of the replacement fluid administered at the beginning of the TPE will be present by the end, with the majority having been removed. Administering plasma as a replacement fluid at the beginning of a TPE results in exposure of the patient to blood products without benefit.
The most commonly used replacement fluid is 4%-5% human albumin in physiologic saline. This solution has the advantage of avoiding disease transmission and transfusion reactions (eg, transfusion-related acute lung injury), both of which can occur with plasma. The main disadvantage of albumin is its expense relative to plasma. This replacement fluid is slightly hyperoncotic compared with plasma and may therefore expand intravascular volume. This effect can be beneficial in avoiding hypovolemia. Because the albumin replacement fluid is the most expensive component of a TPE procedure18 and use of 100% albumin as a replacement does expand intravascular volume, some practitioners will use lower albumin concentrations, such as 70% albumin and 30% saline. When this is done, the albumin and saline are alternated, with the majority of the albumin being given at the end of the procedure to avoid hypovolemia from redistribution of the crystalloid. It should be noted that the use of albumin and saline has been associated with a greater frequency of hypovolemic reactions compared with using albumin alone.19 Plasma is used as a replacement fluid in a limited number of disorders, for example, to replace ADAMTS13 when treating thrombotic thrombocytopenic purpura, to treat coagulation factor deficiencies, and to prevent dilutional coagulopathy in patients with active bleeding.20
To perform TPE, it is necessary to obtain vascular access. It is frequently assumed that central venous access is required and that every patient must have a central venous line to have a successful course of TPE. However, this perception is not supported by the published literature.21–23 The Canadian Apheresis Study group found that 67% of 5234 TPE procedures could be completed successfully with peripheral venous access alone.21 In a clinical trial of the use of TPE to treat multiple sclerosis, 96% of patients considered for enrollment had adequate peripheral vascular access and, of those enrolled, only 4% could not complete the trial due to inability to obtain peripheral venous access.22 In another study of patients with neurologic disorders undergoing TPE, 50% could complete their entire course of therapy using only peripheral venous access.23 Why is it important to consider peripheral access in the performance of TPE? Studies examining the complication rates of apheresis procedures have found that the frequency of complications due to the placement of central venous catheters exceed the frequency of complications directly related to the procedure.24 In one study, all serious complications were related to central venous access, including a death due to a hemopneumothorax.24 Central venous access has also been identified as a major risk factor for complications of TPE in other studies.19,25
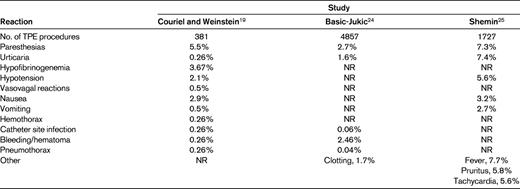
Complications of TPE
The frequency of complications associated with TPE reported in the literature is variable and is dependent upon what is or is not considered a reaction or an expected physiologic response. In an early study in which complications of central venous catheters were considered as being related to the procedure, the rate of complications was 17%, of which 6.14% were severe, requiring hospitalization or significant intervention.24 More recent studies have seen markedly divergent reaction rates ranging from 4.75%25 to 36%.19 The study demonstrating the rate of 36% did not report any severe reactions, with those that did occur being mild and easily treated.19 Table 4 summarizes the reported types of reactions and their frequencies in these series. The most common reaction seen are paresthesias related to hypocalcemia resulting from the use of citrate anticoagulant to keep blood from clotting in the apheresis device. The reactions reported are, for the most part, mild and easily treated. Risk factors for reactions include the use of plasma as a replacement fluid,19,24,25 central venous access,19,24 and the presence of neurologic disease.19
The American Society of Apheresis clinical guidelines
The ASFA is a professional society composed of physicians, scientists, and allied health professionals. It was founded in 1982 when the Society of Hemapheresis Specialists, an allied health organization, and the American Society for Apheresis Symposia, a physician and scientist organization, merged. Since that time, a goal of ASFA has been to advance the “science of apheresis medicine.” A difficulty with this goal is the lack of randomized controlled clinical trials examining the use of apheresis to treat disease. Shehata et al performed a systematic review to identify all randomized controlled trials involving apheresis published between 1976 and 1999.26 A total of 592 articles were identified, of which only 85 (14%) were randomized controlled trials.26 As is demonstrated by this review, the quality of the medical literature for therapeutic apheresis is limited. Because the diseases treated with apheresis are rare, for many diseases, the published evidence may consist solely of case reports or small case series. Whereas controlled trials may exist for more common diseases, randomized trials are uncommon.26
To provide practical, evidence-based guidance to the apheresis practitioner and to encourage critical science in the field of apheresis medicine, ASFA has published guidelines on the use of therapeutic apheresis in clinical practice. The latest guidelines, published in 2010, are the 5th edition.2
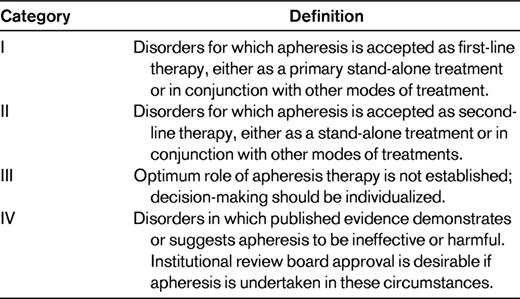
To create these guidelines, a group of 10-12 apheresis experts reviews the previous guidelines and suggestions from the ASFA membership or interested parties to determine which diseases and disorders should be evaluated. The members are then each assigned 6-10 specific diseases. They are asked to review the English language literature related to the use of therapeutic apheresis in their assigned disorders. The individual committee members draft a standardized, single-page “fact sheet.” This document includes information such as the disease name, incidence, apheresis procedure used to treat the disorder, summary of published evidence, description of the disease, brief description of nonapheresis treatments, rationale for the use of apheresis in treating the disorder including important publications, technical notes on the performance of the apheresis procedure, and guidance on the duration and discontinuation of the procedure. These “fact sheets” are then reviewed by 2 other committee members, with input being used to generate a second draft. This is then reviewed by the entire committee, with additional input creating a third draft. After this, based upon consensus of the committee, each disorder is assigned an ASFA category and recommendation grade.2 The ASFA category provides a description of the role of apheresis in the treatment of a disease. The categories are defined in Table 5. The recommendation grade is based upon the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system27 and provides the strength of recommendation and an indication of the quality of evidence supporting the use of the apheresis treatment. A grade 1 recommendation represents a strong recommendation either to perform or not perform the apheresis treatment, depending upon the ASFA category. A grade 2 recommendation represents a weak recommendation. This is further modified by an A, B, or C indicating high-quality evidence, moderate-quality evidence, or low-quality evidence, respectively.2 The finalized fact sheets are then sorted alphabetically by disease name and compiled into a document that is then published in the Journal of Clinical Apheresis. The ASFA guidelines are revised and published every 3 years, with the next publication scheduled to occur in June of 2013.
The ASFA guidelines have been recognized worldwide and have been translated into Spanish and Russian, with plans for translation into simplified Chinese. Several international apheresis societies have endorsed or adopted these guidelines. Finally, many third-party payers are basing their coverage decisions upon the information included in the guidelines.
Summary
Plasma exchange is a therapeutic procedure used to treat a wide variety of diseases through the bulk removal of plasma. Whereas the mechanism of action has been thought to be the removal of pathologic Igs, there is evidence suggesting an immunomodulatory effect. The procedure is safe, with the majority of reactions and complications being mild, easily treated, and of limited duration. Unfortunately, the published evidence supporting the use of plasma exchange is of limited quality. To assist the practitioner in the determining the appropriate use of plasma exchange and other apheresis treatments, and to promote additional studies of the role of apheresis, the ASFA has created evidence-based guidelines that have been accepted internationally as indications for the use of apheresis in clinical medicine.
Disclosures
Conflict-of-interest disclosure: The author is on the board of directors or an advisory committee for ASFA, has received research funding from Fenwal Inc and Asahi Kasei Corporation, and has received honoraria from Fenwal Inc and Terumo BCT. Off-label drug use: None disclosed.
Correspondence
Jeffrey L. Winters, MD, Division of Transfusion Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905; Phone: 507-538-1707; Fax: 507-284-1399; e-mail: winters.jeffrey@mayo.edu.