Abstract
The prognosis of adult acute lymphoblastic leukemia (ALL) remains poor and novel treatment strategies are needed. Antibody-based therapies represent such an approach. ALL cells express various surface antigens that are targets for monoclonal antibodies. This review focuses on 4 major classes of antibody therapy: (1) naked antibodies, (2) T-cell-engaging bispecific single-chain antibodies, (3) immunoconjugates/immunotoxins, and (4) chimeric antigen receptors. Preclinical and clinical data are reviewed. This area of research represents an exciting new approach to help improve the outcome of this disease. Several clinical trials are currently incorporating this therapy in the treatment of newly diagnosed and relapsed adult ALL patients.
Introduction
The overall outcome of adults with acute lymphocytic leukemia (ALL) remains poor. The relapse rate remains high and, at the time of relapse, achieving a second remission is difficult, with response rates in the 20% to 30% range with standard therapy.1,2 The median overall survival for patients with relapsed ALL remains dismal (3-5 months) despite allogeneic hematopoietic cell transplantation (AHCT).1,2 Therefore, novel approaches are needed. This review focuses on antibody-based therapies in the treatment of precursor (pre-) B- and T-cell ALL with a particular emphasis on pre-B-ALL.
ALL cells express various surface antigens that are targets for monoclonal antibodies. Favorable antigenic features include a high percentage of blasts expressing the antigen, a high density of antigen expression, and a lack of expression in normal cells.3 Factors affecting the efficacy of the various agents include the efficacy of the toxins/immunoconjugates, the achievement of adequate dose levels, pharmacokinetics, and the effect of the antibodies on the immune system (Table 1).3
Eighty percent of ALLs are of the pre-B-cell immunophenotype, with more than 90% of cases expressing CD19 and more than 80% expressing CD22.4 Therefore, antibodies targeting CD19 and CD22 have been the focus of many of the treatments discussed below. Although only half of pre-B-ALLs express CD20,5 the success of the anti-CD20 monoclonal antibody rituximab in nonHodgkin lymphoma (NHL) has led to the rapid development and completion of two phase 2 trials with rituximab in combination with chemotherapy for adults with newly diagnosed CD20+ pre-B-ALL. Both trials have demonstrated an improvement of relapse-free and overall survival in patients < 60 years of age6,7 compared with historical controls, demonstrating a potential role for other antibody-based therapies.
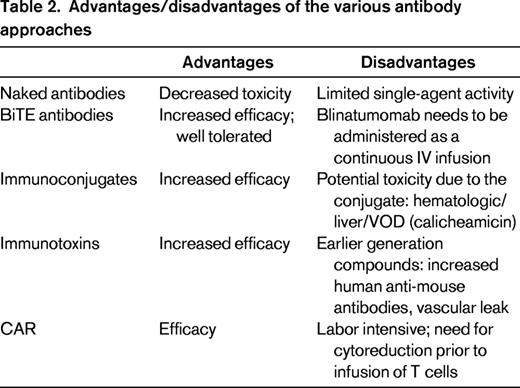
In this review, we focus on 4 major classes of antibody therapy for ALL: (1) naked antibodies, (2) T-cell-engaging bispecific single-chain (BiTE) antibodies, (3) immunoconjugates/immunotoxins, and (4) chimeric antigen receptors (Table 2). This area of research represents an exciting new approach to help improve the outcome of this disease.
Naked antibodies
When used as single agents, naked antibodies have limited activity in acute leukemia. Therefore, most clinical trials have combined antibody therapy with chemotherapy.
Rituximab
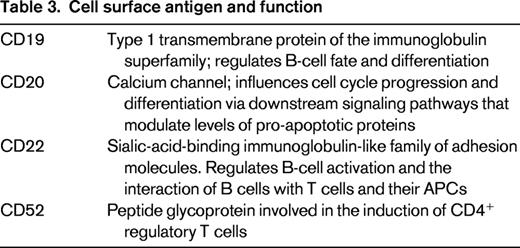
The chimeric (human/mouse) monoclonal antibody rituximab targets CD20 and kills cells by antibody-dependent cellular and complement-mediated cytotoxicity, as well as by the induction of apoptosis.8 The CD20 receptor functions as a calcium channel and influences cell cycle progression and differentiation via downstream signaling pathways that modulate levels of pro-apoptotic proteins such as Bax, Bak, NF-κB, and ERK1/ERK2 (Table 3).9,10 Therefore, increased CD20 expression may lead to dysregulation of these pathways and drug resistance, explaining the associated poor prognostic impact. Although only half of pre-B-ALL cases express CD20 on ≥ 20% lymphoblasts (the usual cutoff for considering an antigen to be positive), the presence of CD20 expression has been associated with a decreased remission duration and worse overall survival in adult ALL.11 A second trial, GRAALL-2003, found that the increased relapse rate was limited to CD20+ ALL patients with high white blood counts (WBC > 30 K/μL).12 CD20 expression is also up-regulated by treatment with chemotherapy.13 In a trial of 237 pediatric patients with pre-B-ALL receiving Berlin-Frankfurt-Münster chemotherapy, a review of sample pairs with residual leukemia demonstrated that the percentage of blasts with CD20+ expression increased from 45% to 81% by day 15.13 Levels of CD20 expression were also significantly increased. These characteristics make CD20 an attractive therapeutic target to combine with chemotherapy.
In a study by the GMALL group (7/2003),6 rituximab (375 mg/m2/dose) was added to a standard chemotherapy backbone. In adult patients 15-55 years of age (N = 133) with standard-risk CD20+ pre-B-ALL, rituximab was administered on day −1 before each induction course and before each of 6 consolidation courses for a total of 8 doses.6 Standard risk was defined as: WBC < 30 K/μL, achievement of complete remission (CR) in ≤ 4 weeks, and absence of the cytogenetic abnormalities t(4;11) and t(9;22). Minimal residual disease (MRD) was measured by clone-specific PCR of the immunoglobulin gene receptor rearrangement with complete molecular remission (CMR) defined as an MRD level < 10−4. Compared with a historical cohort, the rates of CMR (60% vs 19% at day 21; 89% vs 57% at week 16, P = not available), continuous complete remission (3 years; 64% vs 58%, P = .009), and overall survival (3 years; 75% vs 54%, P = not available) were significantly better in the rituximab-treated cohort. Similar findings were demonstrated by Thomas et al.7 In this trial, rituximab (375 mg/m2/dose) was administered on days 1 and 11 of hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone) and days 1 and 8 of methotrexate/cytarabine for 8 doses over the first 4 courses and then during months 6 and 18 of maintenance. For patients < 60 years of age (n = 97), the 3-year rate of continuous complete remission was 70% versus 38% in historic controls (P < .001) and the overall survival increased from 47% to 75% in historic controls (P = .003).7 This improvement in outcome did not extend to older patients (≥ 60 years of age; n = 28), in part related to deaths in CR.
Rituximab is currently being evaluated in a randomized study of patients with newly diagnosed CD20+ Philadelphia-chromosome-negative (Ph−) pre-B-ALL (GRAALL 2005), and the results of this trial will ultimately help to clarify prior observations.5 The development of anti-CD20 monoclonal antibodies with different properties, such as GA101 and ofatumumab, will likely lead to further trials in this arena. Although the above trials only included patients with CD20+ pre-B-ALL, it is possible that rituximab may be beneficial to a larger subgroup of pre-B-ALL patients given that CD20 expression in lymphoblasts is up-regulated upon initiation of steroids and chemotherapy. However, further study will be needed to confirm a benefit to this subset of patients.
Epratuzumab
Epratuzumab is a humanized anti-CD22 monoclonal antibody. CD22, a member of the sialic-acid-binding immunoglobulin-like family of adhesion molecules,14 regulates B-cell activation and the interaction of B cells with T-cells and APCs (Table 3).3 CD22 is rapidly internalized upon antibody or immunotoxin binding,15 making it a favorable therapeutic target for antibody–drug conjugates (ADCs) and immunotoxins. Unlike rituximab, which is directly cytotoxic, epratuzumab modulates B-cell activation and signaling.16 Epratuzumab has demonstrated modest single agent activity in NHL.17 In the initial phase 1 Children's Oncology Group (COG) trial of CD22+ pre-B-ALL,18 epratuzumab was administered twice weekly for 2 weeks then as 4 weekly doses with a standard reinduction chemotherapy platform. Fifteen patients, 11 of whom were in first relapse, were treated at a median age of 10 years. MRD was measured by flow cytometry (sensitivity 10−4) and CMR was defined as the absence of MRD. Ten patients experienced grade 1 or 2 infusion reactions. Two patients had dose-limiting toxicities (DLTs), 1 grade 4 seizure and 1 grade 3 transaminase elevation. In all but one patient, surface CD22 was not detected by flow cytometry on peripheral blood leukemic blasts within 24 hours of epratuzumab administration, indicating effective targeting of leukemic cells.18 Nine of 15 patients achieved CR and, of these, 7 of 9 achieved a CMR. Overall, this trial demonstrated acceptable toxicity, efficacy of the targeted approach, and a favorable rate of MRD negativity.
COG ADVL04P2 was a phase 2 trial to determine whether the addition of epratuzumab to a backbone reinduction chemotherapy regimen increased rates of second complete remission (CR2) in patients 2-30 years of age with CD22+ ALL in first relapse.19 Two different schedules of epratuzumab were evaluated, B1 (weekly × 4 doses, n = 54) and B2 (twice weekly × 8 doses, n = 60). The CR rates in the 2 arms were comparable (65% and 66%, respectively) but not significantly higher than historical controls. However, the rate of CMR was higher (42%) than with the backbone chemotherapy regimen (25%; P = .001).19 This latter point is important because early MRD response was a strong predictor for event-free survival in the previous COG relapsed ALL trial and the kinetic pattern of MRD may also be predictive of longer-term outcomes in relapsed ALL. Therefore, longer follow-up of these patients will be important.19
An adult clinical trial, SWOG S0910, evaluated a similar approach by adding 4 weekly doses of epratuzumab to a backbone of clofarabine and cytarabine in patients with CD22+ pre-B-ALL.20 Patients with Ph+ ALL were excluded. Thirty-two evaluable and eligible patients were treated (median age of 41 years, range 20-68). The median time from diagnosis to registration was 16 months. Salvage status included: 59% first relapse, 25% second relapse, 13% refractory, and 4 patients (13%) had undergone a prior AHCT. The response rate was 45%, including 8 CRs and 5 CRs with incomplete count recovery (CRi). MRD information was present in only 5 of 13 responders—only 1 of whom achieved a CMR. No additional toxicities attributed to antibody were noted. However, the first dose of epratuzumab was given on day 7 to decrease the tumor burden and reduce the risk of infusion reactions. This CR/CRi rate was significantly higher than the 17% observed in S0530, a clinical trial of the same schedule of clofarabine/cytarabine but without epratuzumab in patients with relapsed/refractory ALL.1 Although this latter group of patients was more heavily pretreated, the significantly higher response rate suggests a potential benefit to adding epratuzumab to chemotherapy in relapsed ALL. IntReALL, an ongoing randomized study of epratuzumab in combination with chemotherapy in relapsed pediatric ALL, will hopefully better define the role of this antibody for this disease.
Alemtuzumab
The CD52 glycoprotein is expressed by 70% to 80% of both pre-T- and pre-B-ALLs,3 making it an attractive therapeutic target (Table 3). Alemtuzumab, a humanized anti-CD52 monoclonal antibody, has demonstrated significant activity in chronic lymphocytic leukemia. As a single agent, alemtuzumab has demonstrated limited activity in patients with acute myeloid leukemia and ALL (CR rate 13%).21 However, Cancer and Leukemia Group B (CALGB) 10102 evaluated alemtuzumab alone after 3 modules of intensive chemotherapy (CALGB 19802) in adult patients with newly diagnosed ALL to determine whether its addition improved rates of CR duration, overall survival, and clearance of MRD. Alemtuzumab was dose escalated to a maximum of 30 mg subcutaneously 3 times a week for a total of 4 weeks.22 DLT was defined as the inability to proceed with protocol therapy within 6 weeks after the last dose of alemtuzumab.22 Patients received the CALGB 1980223 backbone as induction/consolidation chemotherapy and maintenance. Twenty-four patients were enrolled in the phase 1 trial; their median age was 37 years, 80% had B-ALL, and 19% had T-ALL. Seventeen patients had evaluable cytogenetics: 5 favorable, 8 intermediate, and 2 poor risk by CALGB criteria. The nonhematologic toxicities were mild; however, 4 patients developed grade 3-4 myelosuppression and 4 patients had a DLT (2 CMV viremia, 1 Staphylococcus aureus empyema, 1 prolonged myelosuppression). Serial MRD measurements were performed by quantitative clone-specific PCR in 11 cases and demonstrated a median 1 log decrease in MRD in the 20 and 30 mg dosing cohorts.22 Alemtuzumab was detectable up to 10 weeks after the last dose was administered. With a median follow-up of 51 months at last presentation, the median disease-free survival was 53 months and the median overall survival was 55 months, which is impressive.22 A phase 2 trial studying 70 additional patients has completed accrual and the results are awaited to determine the efficacy and toxicity of this approach. Identification of strategies such as CMV prophylaxis to decrease toxicities will be important if this approach demonstrates promising results.
BiTE antibodies
BiTE antibodies are a novel class of bispecific single-chain antibodies that retarget cytotoxic T lymphocytes at preselected surface antigens on tumor cells.
Blinatumomab
CD19 is the most commonly expressed antigen in pre-B-ALL and has the highest density of expression.3 However, its internalization rate is slower compared with CD22. CD19 is a type I transmembrane protein of the immunoglobulin superfamily and regulates B-cell fate and differentiation through modulation of the B-cell receptor (Table 3).3 Blinatumomab is the first member of a novel class of BiTE antibodies and combines a CD3-binding site for T cells and a CD19-binding site for B cells. The agent engages T cells for directed lysis of CD19+ target cells.24 The activated T cells induce perforin-mediated death of the target cell.25
The first trial in ALL was in the setting of MRD and demonstrates a novel approach. In patients with MRD-positive ALL, the median time to relapse was 4-5 months.24 The only known cure is AHCT, but long-term survival is uncommon.24 Adult patients with B-lineage ALL in complete hematologic remission were eligible if they expressed the pre-B phenotype and were either molecularly refractory (ie, had never achieved MRD-negative status) or had a molecular relapse (ie, became MRD positive after having been MRD negative) with a quantifiable MRD load of ≥ 1 × 10−4 starting at any time point after consolidation I of frontline therapy within GMALL protocols.25
Blinatumomab was administered to 21 patients as a 4-week continuous IV infusion at a dose of 15 μg/m2/d. The median age was 47 years and 7 patients had poor-risk cytogenetics (5 were Ph+ and 2 were mixed-lineage leukemia positive). Eighty percent of patients achieved a CMR (measured by clone-specific PCR), most by the end of 1 cycle of treatment. The most common adverse events (AEs) were pyrexia, chills, and low immunoglobulin levels.25 One-third of patients developed grade 3 or 4 lymphopenia. Two CNS events occurred: 1 seizure and 1 episode of syncope. In follow-up, there was continued amplification of the T-cell effector memory subset.26 This drug does lead to significant B-cell depletion, and B lymphocytes typically remain undetectable for the entire treatment period.26 Conversely, T-cell counts decline and recover within a few days, and then expand with continuous infusion. Longer follow-up of this trial is now available and has demonstrated impressive results.24 With a median follow-up of 33 months, the relapse-free survival rate is 61%.24 Six of 9 patients proceeding to AHCT remain in a hematologic remission. However, even more exciting, 6 of 11 patients not proceeding to transplantation remain in remission.
A subsequent trial has evaluated blinatumomab in morphologically relapsed/refractory ALL.27 In this trial, blinatumomab was administered as a continuous infusion for 28 days, which was followed by a 14-day break. The dosing for the first week was 5 μg/m2/d because of concern for infusion reactions with active disease.27 The majority of AEs were grade 1-2 (pyrexia: 67%; headaches 33%), with 7 grade 3 or higher AEs occurring in 5 patients (infection, seizures, decreased platelets).27 Responders were allowed to receive 3 additional cycles of therapy. As of the last report, 36 patients had been enrolled and 25 were evaluable. Seventeen patients had achieved CR or CR with partial hematologic recovery and MRD response within 2 cycles of therapy. Thus far, the median response duration is 7.1 months, with a median overall survival of 9.7 months.27 Of the 6 relapses, 3 patients developed a CD19− clone. This latter mechanism of resistance is a concern with the development of antibody-targeted therapies and approaching this mechanism of resistance in future trials will be important.
Of the various novel therapeutics, blinatumomab is one of the most promising. Ultimately, moving blinatumomab to the upfront setting may improve outcomes for ALL patients. The US Intergroup (E1910) is planning a trial of chemotherapy with and without blinatumomab in adults with newly diagnosed ALL to help address this question. The initiation and outcome of this trial are eagerly awaited.
Immunotoxins/immunoconjugates
Immunotoxins/immunoconjugates are composed of a monoclonal antibody or a cell-antigen-binding fragment and a toxin moiety that induces cell death.28 This linkage dramatically increases the activity of the monoclonal antibody, enabling cell kill with relatively few target sites.29 The National Cancer Institute has used a variable fragment of an antibody linked to a 38 kDa truncated derivative of Pseudomonas exotoxin A (PE38) as the toxin moiety for their immunotoxins.29 These agents bind to CD22, after which they are internalized via receptor-mediated endocytosis and processed by furin releasing the toxin portion, which is then transferred to the endoplasmic reticulum and translocated to the cytosol.30 Cytotoxicity is caused by toxin-mediated ADP ribosylation of elongation factor 2 (EF-2), leading to inhibition of protein synthesis and induction of cell death.30
BL22 and CAT-8015
CD22 represents an attractive therapeutic target for this approach because the CD22 antigen-immunotoxin is rapidly internalized.28 The first-generation immunotoxin (PE38) BL22 demonstrated cytotoxicity both in vitro and in vivo28 and has significant activity in hairy cell leukemia.15 In a phase 1 trial including ALL patients, no allergic/infusion reactions, vascular leak, or hemolytic uremic syndromes were noted. Three of 23 patients did develop neutralizing antibodies.15 Increased plasma levels were seen with higher doses of immunotoxin; however, there was rapid clearance of the drug with high disease burden.15 Only modest activity was noted in ALL and there were no CRs. Sixteen of 23 patients did have a reduction in blast count.3
A second-generation immunotoxin (PE38), CAT-8015, was subsequently developed.28 Serial modifications have reduced nonspecific toxicities, increased stability, enhanced tissue penetration, and improved targeted cellular toxicity of the immunotoxins.15 CAT-8015 has targeted mutations in the hot spot region of the complimentary determining region-3, leading to a 14-fold increased binding affinity for CD22 and improved cytotoxicity.15,28 In vitro studies demonstrated activity in B-ALL pediatric samples including newly diagnosed, relapsed, and steroid-resistant patients.29 In a clinical trial, 4 of 9 ALL patients achieved a CR.3 An ongoing trial is evaluating CAT-8015 in children and young adults with relapsed/refractory CD22+ ALL or NHL (NCT00659425). However, immunotoxin resistance has been observed in ALL cell lines due to a low level of DPH4 mRNA and protein.30 This renders EF2 refractory to the effects of CAT-8015; protein synthesis is not inhibited and cell death does not occur. Further analysis of the DPH4 gene promoter demonstrated heavy methylation in the resistant cells. This resistance could be reversed by the treatment of cells with the hypomethylating agent 5-azacitidine and suggests that such an approach may be applied clinically. Study of such mechanisms of resistance and the addition of other therapies to circumvent this will be important in the development of other antibody-based therapies.
Combotox
Combotox is a 1:1 mixture of immunotoxins prepared by coupling a deglycosylated ricin A chain to monoclonal antibody directed against CD22 and CD19.31 This therapy has an advantage by targeting the 2 different antigens. Preclinical studies demonstrated activity in cell line and patient samples. In a pediatric study, 3 of 17 ALL patients achieved a CR.31 In a phase 1 study of adults with relapsed/refractory ALL, the DLT was vascular leak syndrome. This appears to be due to a unique amino acid motif in the ricin toxin A chain that damages vascular endothelial cells. Ongoing approaches include mutating the recombinant ricin toxin A to disable this site. Another approach is to shorten the half-life of the immunotoxin in vivo, and studies with this latter approach are ongoing.32 Other AEs have included reversible grade 3 transaminase elevations31 and the development of human anti-mouse antibodies (7% of patients). Serum levels of the agent correlated with dose level and the percentage of circulating blasts. The often rapid rebound in peripheral blasts after the last dose of combotox suggests that continued dosing with a reduced dose might lead to more durable remissions.31 In addition, giving the immunotoxin with a lower tumor burden may lead to less of an “antigen sink” and to improved outcomes.31 Preclinical studies have demonstrated synergy with sequential administration of combotox with cytarabine in a murine model of advanced ALL and have led to a phase 1 clinical trial that is exploring this combination in adults with relapsed or refractory B-ALL (NCT01408160).
SAR3419
SAR3419 is an anti-CD19- humanized monoclonal antibody attached to a highly potent tubulin inhibitor, maytansinoid DM4, which works through antibody-dependent cytotoxicity.33 The ADC is extremely potent, with an EC50 in the subnanomolar range. SAR3419 binds to CD19 and is subsequently internalized via endocytosis and then routed to lysosomes, where it is degraded to yield the active drug.33 Phase 1 dose escalation studies have been conducted in NHL.33 The DLT was reversible corneal toxicity. No other grade 3 or 4 toxicities exceeded 10% and there were no clinically significant hematologic toxicities. SAR3419 seems to have a large therapeutic window with minimal toxicity.33 The half-life of the ADC was 4-6 days and high rates of objective responses were observed in lymphoma.33 A phase 2 study of SAR3419 is ongoing in adults with relapsed/refractory ALL. Xenograft studies demonstrated that a 3-week course of SAR3419 after 3-drug induction therapy significantly extended the length of remission, and a protracted course of SAR3419 after induction prevented relapse of leukemia into hematolymphoid tissues and peripheral organs; therefore, this will be a future avenue of study.34
Inotuzumab ozogamicin
In addition to blinatumomab, inotuzumab ozogamicin is one of the best studied and most promising new agents in relapsed/refractory ALL. The drug conjugate consists of a monoclonal antibody against CD22 bound to calicheamicin.4 Calicheamicin is a potent cytotoxic agent that binds the minor DNA groove and causes breaks in double-stranded DNA in a sequence-specific manner, leading to cellular apoptosis.4 The ADC is rapidly internalized and delivers calicheamicin intracellularly. Initial phase 1/2 studies in lymphoma demonstrated encouraging response rates and established a recommended phase 2 dose of 1.8 mg/m2 every 3-4 weeks; thrombocytopenia is the DLT.35 Mild to moderate elevations in transaminases were also noted. A phase 1/2 trial was conducted in adults with relapsed/refractory CD22+ ALL (n = 49).4 The starting dose of 1.3 mg/m2 was subsequently increased to 1.8 mg/m2. The median age was 36 years (range 16-80). All patients had ≥ 50% CD22+ lymphoblasts and the majority were heavily pretreated: 27% salvage 1, 49% salvage 2, 25% ≥ salvage 3, and 7 patients (14%) had undergone prior AHCT. In addition, almost half had poor-risk cytogenetics (14% Ph+, 10% mixed-lineage leukemia positive, 18% complex cytogenetics).4 The response rate was 57%, including 18% CRs and 39% CRi's.4 Sixty-three percent of patients achieving CR/CRi also achieved a CMR. Inotuzumab was well tolerated. Grade 3-4 AEs included drug-related fever (n = 9), hypotension related to drug (n = 1), hyperbilirubinemia (n = 2), transaminase elevations (n = 1), and high lipase levels (n = 1). Grade 3-4 myelosuppression was observed (both neutropenia and thrombocytopenia). Grade 1-2 elevations in transaminases and bilirubin were common (occurring in 24% and 55% of patients, respectively). Fevers/infections were also common, including bacterial infection (n = 8), viral (n = 2), fungal (n = 1), pneumonia (n = 9), and fever of unknown origin.4 AEs were reversible in all but 2 patients. Almost half of the patients (22/49) were able to proceed to AHCT. Most responses were short lived without proceeding to transplantation. This is not surprising given the heavily pretreated nature of these patients. Given the previous experience with gemtuzumab ozogamicin (the anti-CD33/calicheamicin conjugate), the rates of venoocclusive disease (VOD) were also evaluated in patients proceeding to transplantation. Five of 22 patients proceeding to transplant developed VOD. However, 4 of 5 of these patients received a preparative regimen of clofarabine/thiotepa. Subsequent to this, the transplantation conditioning regimen has been changed and the risk of VOD has decreased (Hagop Kantarjian, MD Anderson Cancer Center, verbal communication).
An ongoing study, B1931002, is evaluating inotuzumab in ALL patients in salvage 1 or salvage 2 versus the standard of care chemotherapy: fludarabine high-dose cytarabine G-CSF (FLAG), high-dose cytarabine, or high-dose cytarabine/mitoxantrone. The hope is that such a study will lead to Food and Drug Administration approval of inotuzumab for relapsed ALL if a benefit is shown with respect to CR rate and overall survival. Two different schedules of inotuzumab have been evaluated previously: weekly and monthly dosing. The efficacy is equivalent with the 2 schedules; however, the tolerability of the weekly schedule appears better36 and the ongoing phase 3 trial is using the weekly dosing.
Chimeric antigen receptors
Most of the work with chimeric antigen receptors (CARs) has been performed in chronic lymphocytic leukemia; however, recent experience in ALL is generating great excitement. CARs are composed of a single-chain variable-fragment antibody specific to tumor antigen, fused to a transmembrane domain and a T-cell-signaling moiety, most commonly either the CD3-ζ or Fc receptor-γ cytoplasmic signaling domains.37 The resulting receptor, when expressed on the surface of a T cell, mediates binding of the target tumor antigen and activates a signal to the T cell, inducing target cell lysis. Second- and third-generation CARs have included modifications such as inclusion of the signaling domain of the T-cell-costimulatory receptors (ie, CD28, 4-1 BB, OX40) or tandem cytoplasmic signaling domains from 2 costimulatory receptors (ie, CD28-4-1 BB or CD28-OX40, respectively).37 Although the majority of clinical experience using CD19-targeted CARs has been in the setting of indolent B-cell malignancies, 8 of the 14 reported clinical trials included patients with ALL. Preliminary results of this approach in 2 children with relapsed/refractory ALL were recently published by Grupp et al.38 Both patients achieved CR, with 1 remission ongoing at 11 months. However, the other patient had a relapse with CD19− blasts. Few other results in ALL have been reported at this time. Memorial Sloan-Kettering has an open phase 1 dose-escalation clinical trial wherein patients with relapsed or MRD+ ALL will be treated with donor-derived EBV-specific CAR-modified T cells (NCT01430390). The completion and results of this trial are eagerly awaited.
T-cell disease
Fewer studies have taken place using antibodies in T-ALL, largely due to the decreased incidence of T-ALL compared with B-ALL and lymphomas. However, immunotoxins against CD25, CD7, CD5, and CD3 are being evaluated in T-cell lymphomas. Alemtuzumab is also being investigated, as discussed above.
Conclusions
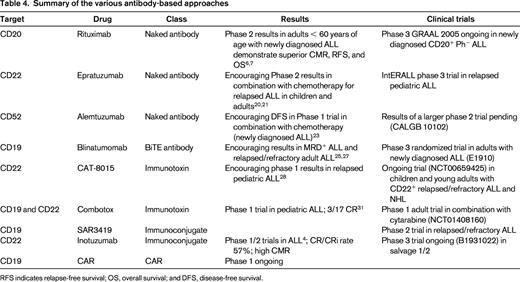
Several novel antibody-based therapies are demonstrating encouraging results in ALL (Table 4), and some of these drugs will likely be approved soon for the treatment of relapsed ALL. However, the emergence of resistance in clones lacking the respective target indicates a need to target other molecules in patients with ALL and to consider evaluation of these antibody-based therapies in the upfront setting. Rituximab is currently standard of care at many institutions, including ours, in the backbone chemotherapy regimen for patients with newly diagnosed CD20+ pre-B-ALL who are < 60 years of age. The incorporation of other strategies, such as blinatumomab or other antibodies may ultimately improve outcomes and change the paradigm regarding which patients should receive AHCT in first remission.
Disclosures
Conflict-of-interest disclosure: The author has received research funding from Immunomedics; has consulted for Talon, EUSA, and Pfizer; has received honoraria from EUSA, Sigma Tau, and Pfizer; and has been affiliated with the speakers' bureau for Sigma Tau. Off-label drug use: Rituximab for the treatment of ALL.