Abstract
Disseminated intravascular coagulation (DIC) is a devastating clinical condition that is characterized by the loss of normal hemostatic control in response to sustained and systemic cell injury. The inciting injury may be from infection, trauma, or malignancy, but the consequent pathophysiology is multifactorial involving intertwined feedback loops between the coagulant, immune, and inflammatory pathways. Central to this is thrombin generation, but the ubiquitous nature of its in vivo functional consequences can make it difficult to dissect away the separate but overlapping components to the clinical problem. Therefore, early recognition and resolution of the precipitating events leading to DIC remains the central tenet to clinical care. This article refreshes our conceptual understanding of DIC pathogenesis and draws in recent advances in the cycle of cell death caused by extracellular nuclear proteins. It also aims to delineate recognition of response pathways that can be predominantly procoagulant or profibrinolytic to enable a more personalized and evidence-based approach to be delivered to the patient with DIC.
Introduction
Disseminated intravascular coagulation (DIC) represents a departure from the compartmentalized hemostatic response to local injury that has been crucial for human survival. Involving coagulation factors, platelets, and the vessel wall, hemostatic plug formation is exquisitely controlled and continues as an adaptive response even as injury increases in intensity. However, this host reaction to injury may turn maladaptive depending on individual susceptibility to the increasing intensity and/or nature of the insult. It is this dysfunctional form of systemic hemostatic activation that can lead to the hemodynamic and metabolic derangements that are considered DIC. The emerging evidence points to a pathogenesis that is not just related to “coagulation gone haywire,” but fully involves all components of the inflammatory and innate immune response. Events go beyond the blood-endothelial cell interface to also involve critical subendothelial and constitutive neuronal processes. The importance of this is borne out clinically because DIC is well validated as an independent predictor of mortality. Its presence doubles the risk of mortality in patients with severe sepsis and trauma.1 DIC therefore represents hemostatic dysfunction that has net liability to the host.
Definition
DIC arises as a complication of a variety of clinical disorders and is characterized by the increasing loss of localization or compensated control in intravascular activation of coagulation. This continuum in clinicopathologic severity has definable phases that characterize patients at risk for increased mortality and, more recently, the International Society of Thrombosis and Haemostasis Scientific and Standardization Sub-Committee (ISTH-SSC) has therefore proposed working definitions to facilitate earlier detection and treatment of DIC.2 The new emphasis is on recognizing a non-overt stage of hemostatic dysfunction rather than an overt, frankly decompensated stage that is usually too late for therapeutic rescue.
Pathophysiology
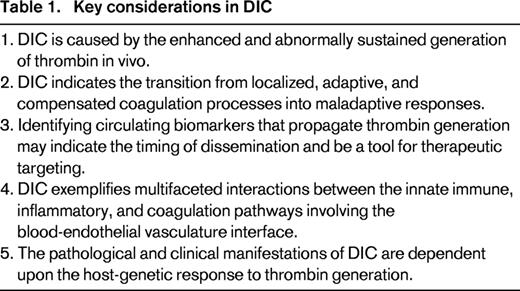
Several themes in DIC pathogenesis are important to consider in the joint clinical and laboratory approach to its diagnosis, management and relevant therapeutic strands. First is the central role of the process of thrombin generation in vivo. Second is that the mechanisms that fuel and perpetuate thrombin generation are pathogenic in their dissemination. Third is the importance of the endothelial microvasculature in this process. Fourth is the parallel and concomitant activation of the innate immune and inflammatory systems. These themes are summarized in Table 1 for consideration by the clinician approaching the patient with DIC.
Thrombin generation in vivo
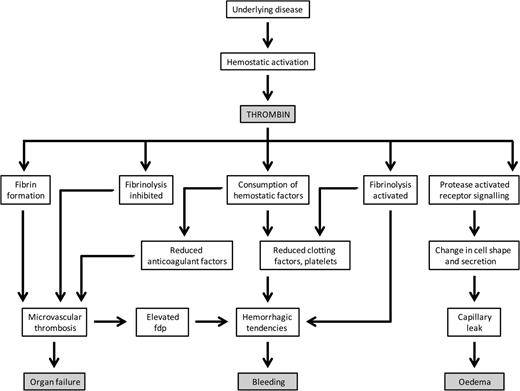
The process of generating thrombin in vivo is pivotal to hemostatic control by way of synchronizing and balancing both pro- and anticoagulant forces as well as pro- and antifibrinolytic activities. Clot formation by way of the conversion of fibrinogen to fibrin is simultaneously controlled by thrombin activation of the protein C (PC) anticoagulant regulatory pathway.3 This also serves several potential roles, including the walling off of pathogens, recruitment of cellular processes, and activation of appropriate endothelial response signals.4 Platelet activation, in addition to facilitating platelet aggregation, also leads to the release of chemokines and growth factors.5 Although fibrinolysis can be promoted directly via thrombin-induced tissue plasminogen activator release from endothelial cells to generate plasmin, the process is held in check at the site of thrombin burst through the actions of thrombin-activatable fibrinolysis inhibitor in preventing binding of plasminogen to partially digested fibrin for its conversion to the fibrinolytic enzyme, plasmin, and, at a second level, by plasminogen activator inhibitor-1 complexation of tissue plasminogen activator.6 This fine, exquisite homeostatic balance of controlled thrombin generation is lost in DIC; an appreciation of the many thrombin-mediated activities, which may either be cooperative or antagonistic to each other, is fundamental to the understanding of the different clinical manifestations and the management of this condition (Figure 1).
Mechanisms in DIC. Systemic activation of hemostatic processes leads to thrombin generation and its consequences, which include intravascular fibrin deposition, depletion of hemostatic factors, fibrinolytic pathway involvement, and PAR-mediated cell signaling responses. As a result, there may be thrombosis of small vessels contributing to organ failure, severe bleeding, capillary leakage, and edema. FDP indicates fibrin-degradatory products.
Mechanisms in DIC. Systemic activation of hemostatic processes leads to thrombin generation and its consequences, which include intravascular fibrin deposition, depletion of hemostatic factors, fibrinolytic pathway involvement, and PAR-mediated cell signaling responses. As a result, there may be thrombosis of small vessels contributing to organ failure, severe bleeding, capillary leakage, and edema. FDP indicates fibrin-degradatory products.
Mechanisms disseminating and sustaining thrombin generation
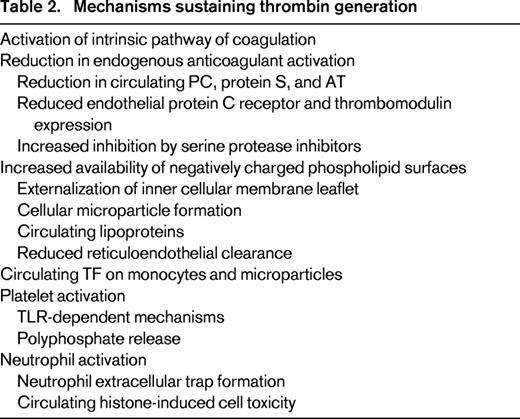
Although tissue factor (TF) is an important initiator of coagulation, it may not be as relevant in amplifying and propagating thrombin generation.7 Although new sources of TF expression may continue to sustain thrombin generation, such as via monocyte-derived microparticles, recruitment of other processes are relevant to perpetuating this process. Secondary bursts of thrombin formation can be provided by the intrinsic pathway of coagulation that can also be activated through platelet polyphosphate-dependent activation of factor XI.8,9 Increasing thrombin generation leads to the consumption and depletion of the anticoagulant regulatory proteins: PC, protein S, and antithrombin (AT). Apart from continuous consumption by ongoing protease-inhibitor complex formation, such as thrombin-antithrombin and activated PC-PC inhibitor interactions, these can be further decreased by elastase degradation and by reduced synthesis as a result of liver dysfunction. In addition to impairment in the physiological anticoagulant system, increased exposure of negatively charged phospholipid surfaces further facilitates the assembly and propagation of coagulation kinetics. In vitro, the availability of such surfaces accelerates the prothrombinase reaction dramatically by 250 000-fold.10 In vivo, the relevance of this is demonstrated by the observation that factor Xa infusions alone are not thrombogenic unless co-infused with negatively charged phospholipid surfaces.11 Such surfaces, mainly rich in phosphatidylserine, can arise from externalization of the inner leaflet of cell membranes upon activation and apoptosis. Cell damage also leads to the generation of microparticles from platelets, monocytes, and endothelial cells that increase the circulating surface area for coagulant reactions to occur. Microparticles are also able to sustain in vivo thrombin generation because they are less readily cleared by the reticuloendothelial system compared with the more rapid phagocytotic removal of apoptotic cells. These mechanisms form a spatially and temporally expansive response that is the hallmark of DIC (Table 2).
Endothelial cell activation and dysfunction
The normal and adaptive response of endothelial surfaces is directly relevant to the regulation of coagulation and inflammation. Natural anticoagulant mechanisms at the endothelial surface dampen this inflammatory-coagulant interface, and sensory neurons, when stimulated, can increase prostacyclin production from the endothelium to reduce injury. Although receptors of the PC pathway such as the endothelial PC receptor (EPCR) and thrombomodulin (TM) have direct anticoagulant properties in facilitating activated PC (APC) generation, they also have direct anti-inflammatory effects. APC, when bound to EPCR, can directly cleave and activate protease-activated receptor 1 (PAR1) to generate anti-inflammatory, anti-apoptotic, and cytoprotective effects. These effects are directly opposite to those generated by thrombin on PAR1 and exemplify the yin-yang balance between thrombin and APC in harmonizing the processes of coagulation and inflammation to maintain vascular homoeostasis.3 As for TM, the lectin-like domain has been implicated in binding and reducing the proinflammatory effects of high-mobility group box protein 1 (HMGB1) and conferring protection from neutrophil-mediated tissue injury.12 Dysfunction and failure of this large, physiological endothelial organ beyond the adaptive nature of the host response can, however, lead to significant collateral damage in its efforts to re-equilibrate and is indicated by the development of DIC. The degree of this relates directly to the triggering disease process and the dominance of thrombotic versus bleeding sequelae or capillary leakage depends on genetic and host-related response factors.13 Due to its higher endothelial cell surface to blood volume ratios, the microvasculature is particularly afflicted in DIC.14 In this respect, lungs are particularly susceptible in the onset and progression toward multiple organ failure. Importantly, circulating mediators play a key role in distant organ damage; for example, extracellular histones cause the adult respiratory distress syndrome15 that is associated with DIC.
Links among coagulation, innate immunity, and inflammation
Homeostatic balance is usually lost when there is extensive cell death that can arise from infection, cancer, or trauma. Release of DNA, chromatin, and granular proteins can result in extracellular traps.16 Traps associated with neutrophils (abbreviated as NETS) are particularly integral to immobilizing and killing bacteria and can contain potent enzymes such as elastase or myeloperoxidase and histones, which in circulation can be extremely toxic to endothelial cells.17 NETS can activate platelets directly or indirectly via histones, leading to intense formation of the fibrin network. Indeed, from a DIC perspective, vast amounts of histones can destroy platelets, cause release of procoagulant material to enhance thrombin generation, and lead to profound thrombocytopenia.9,18 In addition to platelet and endothelial damage in amplifying coagulation activation, circulating histones trigger proinflammatory cytokine release directly (eg, IL-6 release from neutrophils) in a partially TLR4-dependent manner.15 Although cytokines and proinflammatory mediators can induce coagulation, thrombin, factor Xa, and probably the TF/VIIa complex can interact with PARs on cell surfaces to promote further activation and additional inflammation.19 The involvement of platelets is critical and mechanisms of its immune mediation and interactions with other cells have been reviewed elsewhere.4,5,20 In addition, PAR signaling up-regulates adhesion molecules and triggers the production of chemokines that activate neutrophils and monocytes, the products of which can injure tissues directly. This cycle of events typifies the pathogenic damage of ischemia-reperfusion. The concomitant subendothelial perturbation recruits macrophages that can further drive the inflammatory reaction. When this process generalizes, it escapes the well-developed local checks and balances and results in a dysregulated, undirected response that fuels the vicious cycle between inflammation and coagulation.
Treatment
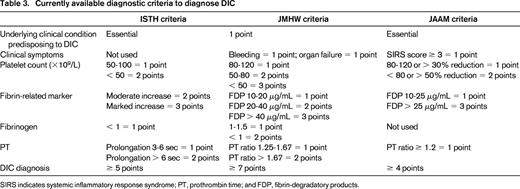
Because DIC develops secondary to other diseases, the cornerstone of therapy is the treatment of the underlying disorder. Its prompt recognition is therefore important and has been well covered elsewhere,21 with recent efforts by the ISTH-SSC to harmonize guidance from the British Committee for Standards in Haematology, the Japanese Society of Thrombosis and Hemostasis (JSTH), and the Italian Society of Thrombosis and Hemostasis on its diagnosis and management. There is no one gold standard for diagnosis, but the 3 respective national guidelines place an emphasis on simple algorithms and scoring systems based on readily available laboratory tests. Table 3 presents the scoring systems for DIC that are the most evidence based with respect to pathophysiological outcomes such as organ failure and mortality. The Japanese Association for Acute Medicine (JAAM) criteria now form the basis of the first therapeutic guidelines for DIC in Japan, but this remains a nation-specific recommendation that is not endorsed internationally.22 Scoring systems based on trend analysis from longitudinal sampling have also been proposed to capture an earlier, non-overt form of DIC, but this remains to be better validated through further prospective studies.23,24 Although many single markers, including the use of thromboelastography techniques, have been reported for DIC, there is no single marker that has been shown to be sufficiently robust. Therefore, the harmonized ISTH-SSC guidance recommends that DIC is not diagnosed with a single marker. With regard to treatment, measures are less likely to be successful if the inciting process cannot be halted. The classical example is obstetrical DIC, for which the best treatment would be placental delivery (or the fetus), which is the triggering factor. In addition, ancillary measures have to be considered for a good outcome. In broad terms, these may be divided into blood product support and therapies that modulate thrombin generation. Given the ubiquity of thrombin activity, this can be more specific in targeting procoagulant and profibrinolytic activities.
Transfusion support
Blood product support for patients with DIC includes platelets, fresh frozen plasma, and fibrinogen replacement in the form of cryoprecipitate or fibrinogen concentrates. Platelets should be considered in the bleeding patient if the platelet count is < 50 × 109/L, although this is only guidance and threshold platelet count should be based on the patients' clinical situation and the downward trend.21 Fresh frozen plasma is given if there is bleeding and prolonged prothrombin time and activated partial thromboplastin time (> 1.5 times the upper limit of normal range).21 The recommended dose is 15-20 mL/kg, although a higher dose may be necessary if volume overload is permissible in patients with severe bleeding. Prolongation of the clotting screen in this context may also be related to the interference by fibrinogen or fibrin degradation products, reaffirming the notion that plasma transfusion should be reserved for patients with bleeding. As to the use of prothrombin complex concentrates, the smaller volumes required may be attractive in this setting and are currently not licensed; they lack certain coagulation factors such as factor V and may promote thrombosis. Cryoprecipitate (2 pools) can be considered in patients with bleeding and fibrinogen levels less than 1.5 g/L. Fibrinogen concentrates may offer more standardized protein concentrations but are currently not licensed in DIC. Because fibrinogen is an acute phase reactant, levels can increase to 4 g/L or more and a falling level requires consideration of an ensuing DIC process and correction, although there is an absence of directly applicable high-quality studies in this scenario. In the context that levels of hemostatic parameters may change rapidly in tandem with continuing thrombin generation, a single set of blood results likely provides a snapshot of the situation. In addition, a prolonged prothrombin time should not be automatically attributed to DIC, but vitamin K deficiency needs to be excluded in such critically ill patients who may not have received adequate nutrition and several antibiotics, thereby affecting vitamin K homeostasis.
Modulating thrombin generation
Heparin.
Heparin has been used historically as a treatment for DIC with various outcomes in different clinical situations, but there is little evidence that heparin reverses organ dysfunction associated with DIC. In recent years, there is a trend toward using low-molecular-weight heparin (LMWH) in place of the unfractionated heparin due to its predictive pharmacokinetics and lesser bleeding risks. The Japanese Ministry of Health and Welfare (JMHW) approved the LMWH dalteparin for use in DIC after a multicenter trial showed significantly reduced organ failure and higher safety than the unfractionated version.25 In addition, LMWH prophylaxis should be strongly considered in patients with DIC in the critical care unit, where the incidence of venous thromboembolism is extremely high.26 In patients who may be deemed to have a high risk of bleeding, unfractionated heparin may still be considered due to its shorter half-life and reversibility with protamine sulfate.
Anticoagulant factor concentrates.
AT is one of the natural anticoagulants that is depleted early during the excessive thrombin generation observed in DIC; it has been shown to be a predictor of clinical outcome in sepsis patients and an independent predictor of 28-day mortality in DIC.27 In the first randomized study of this agent in 51 patients with shock and DIC, AT and/or heparin was administered. The duration of symptoms of DIC was considerably shorter in the AT-substituted groups than with heparin alone.28 However, post hoc analysis of the KyberSept trial of high-dose AT in severe sepsis, which did not show any survival advantage over placebo, suggested that the negative results were mainly due to coadministered heparin, which increased bleeding in treated patients.29 This finding requires prospective validation and, although tailored, supplemented AT dosing has gained approval in Japan, AT treatment for sepsis or DIC per se is not a licensed indication worldwide.30,31
The clinical efficacy of APC in severe sepsis was demonstrated in a large randomized controlled trial (the Protein C Worldwide Evaluation in Severe Sepsis [PROWESS] trial), in which its use was associated with a reduced mortality (24.7%) compared with that of the placebo (30.8%) with highest benefit for those who had DIC.32 Further studies showed normalization of coagulation activation during severe sepsis with this agent strengthening its role as a treatment for severe sepsis and DIC.33 However, conflicting reports of its efficacy in treated patients with septic shock prompted another study, the PROWESS-SHOCK study.34 This study showed a 28-day all-cause mortality rate of 26.4% in APC-treated patients compared with 24.2% in the placebo arm, with no reduction of mortality even in those with severe PC deficiency. After this, APC was withdrawn from the market.
More recently, a recombinant, soluble form of TM has been studied, which has theoretically less bleeding risk compared with other anticoagulants such as APC because its anticoagulant properties are dependent on the amount of thrombin generated. Studies are ongoing, including a multicenter, randomized, double-blind clinical trial of patients with DIC secondary to cancer and infection that was conducted to compare the effects of recombinant soluble TM and heparin.35 The DIC resolution rates were 66.1% for the former group versus 49.9% for the heparin group, whereas improvement in bleeding symptoms were 35.2% and 20.9%, respectively. The mortality rates of DIC patients associated with infection was significantly decreased by TM (28.0% vs 34.6%), with favorable changes in laboratory markers and bleeding-related adverse events. A recent study showed significantly lower 28-day mortality in sepsis-related DIC with recombinant TM with a rapid decrease in the Sequential Organ Failure Assessment score on day 1, indicating an organ protective effect for this compound in the setting of sepsis.36
Modulating profibrinolytic activity
Because excess fibrinolysis is characteristic of DIC, antifibrinolytic agents may be considered useful adjuncts in its management. However, hyperfibrinolysis in DIC is secondary to excess thrombin generation and is a natural reactionary process to deal with the uncontrolled thrombin generation.37 For this reason, inhibiting excessive fibrinolysis with antifibrinolytic agents can prove deleterious in the setting of DIC. In the acute coagulopathy of trauma, in which hyperfibrinolysis predominates, antifibrinolytic agents are beneficial for those with persistent bleeding after adequate replacement of fresh frozen plasma.38 In this context, early administration of tranexamic acid (loading dose 1 g over 10 minutes followed by an 8-hour infusion of 1 g) was found to significantly reduce risk of hemorrhage-related death in trauma patients while not increasing the incident of vascular occlusive events.38 Another clinical situation is massive postpartum hemorrhage, for which fibrinolysis is a prime pathophysiological factor in the coagulopathy and tranexamic acid has been demonstrated to be beneficial.39
Summary
DIC should be approached and considered from the perspective of response to significant and systemic injury with excessive thrombin generation in vivo. It can be seen as an indicator that the response has become maladaptive with potentially lethal consequences, especially in relation to microvascular thrombotic events. Histones and NETs have emerged as increasingly relevant players in the critical cross-talk among coagulant, inflammatory, and innate immune processes. Treatment strategies are likely to require multimodality approaches. Because the clinical manifestations can vary depending on the cause of the DIC, a given therapeutic framework may only be suitable for one etiology and not the other. Even in the same clinical situation, the management strategy should be personalized depending on the clinical picture of thrombosis or hemorrhage. The difficulty occurs when there is a varying spectrum of both, especially at different vascular sites. Therefore, the challenge is in amalgamating what evidence base there is with sound clinical and laboratory judgement for the individual patient with DIC. Because the grade of evidence is generally low, further well-defined research is needed to improve the quality of recommendations in DIC.
Disclosure
Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Professor Cheng Hock Toh, Institute of Infection and Global Health, University of Liverpool, Liverpool, L69 3BX, United Kingdom; Phone: 151-7064344; Fax: 151-7065810; e-mail: toh@liv.ac.uk.