Abstract
Venous thromboembolism (VTE) has a variable recurrence rate after the discontinuation of anticoagulant treatment. Therefore, the duration of anticoagulation therapy after a first VTE should be tailored to the estimated risk for recurrence. Anticoagulant therapy should be discontinued after the initial 3 to 6 months in those patients who had the first episode in association with temporary risk factors. The duration of anticoagulant therapy in patients who had a first episode of cancer-associated VTE should be reassessed over time based on the persistence of cancer and anticancer therapy. After 3 to 6 months of anticoagulant treatment for VTE, patients with a first unprovoked event and an estimated low risk for bleeding complications should be evaluated for indefinite treatment on an individualized basis. New oral anticoagulants have been evaluated for the extended treatment of VTE. Large phase 3 studies have shown that dabigatran, rivaroxaban, and apixaban are effective and safe in this indication. These agents do not require monitoring for dose adjustment and could make extended treatment more feasible and acceptable to patients.
Introduction
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common disorder associated with considerable morbidity and mortality. The annual incidence of VTE is ∼ 1 to 2 cases per 1000 persons in the general population.1 The in-hospital case-fatality rate associated with PE ranges between 5% and 12%.1
VTE is regarded as a chronic disease because it tends to recur after an acute event. Observational studies have shown a recurrence rate of ∼ 30% at 10 years after the first VTE event if anticoagulation is discontinued.2,3 In a study by Prandoni et al, the cumulative incidence of recurrent VTE was 8.6% after 6 months, 17.5% after 2 years, and 24.6% after 5 years.2 In a retrospective cohort study, the hazard rate for recurrence was particularly high in the first 6 to 12 months after the VTE episode and then decreased, but never fell to zero.3 In that study, the hazard rate per 1000 patient-days for first probable/definite recurrence was 30 ± 10 per 1000 patient-days in the first 7 days, 10 ± 4 per 1000 patient-days at 6 and 12 months, and 4 ± 1 per 1000 patient-days at 5 and 10 years. The long-term case-fatality rates were 19%, 25%, and 30% at 1, 2, and 3 years after the acute event, respectively.1
Current guidelines suggest indefinite anticoagulation for patients at particularly high risk for recurrent VTE.4 Treatment with vitamin K antagonists (VKAs) reduces the rate of recurrent VTE by ∼ 80% to 90%, so the risk for recurrence while on treatment is reduced to 1% to 2% per year.5 The trade-off for this remarkable efficacy is the risk for bleeding complications and the need for laboratory monitoring and dose adjustments.6 Therefore, in clinical practice, most VTE patients are usually treated for a period of time ranging from 3 to 12 months and indefinite treatment is reserved for selected patients. Anticoagulants are discontinued when the perceived risk of anticoagulation-related bleeding and the inconvenience of remaining on treatment outweigh the risk of recurrent VTE.
New anticoagulant agents with an improved efficacy-safety profile and more predictable pharmacologic properties that overcome the limitations of VKAs have been developed recently.7 The efficacy and safety of these agents for the treatment of VTE have been evaluated in large phase 3 clinical trials.
Extended treatment of VTE
Treatment of VTE includes an initial phase (usually for 5-7 days), a so-called “long-term” phase (from 7 days to at least 3 months), and an extended phase (beyond 3 months).8 Several studies have shown that courses of anticoagulant treatment shorter than 3 months are associated with an unacceptably high rate of recurrent VTE after treatment discontinuation.9 Therefore, treatment is recommended for at least 3 months for nearly all patients. Randomized studies have shown that prolonging anticoagulant treatment beyond 3 months delays recurrences, but does not reduce the risk of recurrence after treatment withdrawal and is associated with an increased risk of major bleeding.10-12 The extended phase of treatment starts after the initial 3-month period and is usually reserved for those patients at a sufficiently high risk for recurrence to merit prolonged anticoagulation. In these patients, prolonged or indefinite anticoagulation is not intended to be life-long, but rather a treatment the duration of which will be periodically reassessed based on the evolving balance between the risks of recurrence and bleeding (eg, in case of recurrence or bleeding events).
To make a decision about whether to continue or stop anticoagulant treatment, the rates of recurrent VTE and major bleeding and their specific case-fatality rates could be useful. The incidence of fatal recurrences of VTE were reported to be 0.4% during the first 3 months of anticoagulation (case-fatality rate 11.3%) and 0.3% patient-year (case-fatality rate 3.6%) after discontinuation of anticoagulation.13 A meta-analysis by Douketis et al showed that the rates of fatal recurrences during anticoagulant treatment are higher in patients treated for a first PE (case-fatality rate 26.4%) compared with patients treated for a first DVT (case-fatality rate 8.8%).14 The rates for major bleeding during anticoagulant treatment for VTE were reported to be 2.06% (case-fatality rate 9.3%) during the first 3 months and 2.74% (case-fatality rate 9.1%) beyond the initial 3-month period.15 Therefore, although the incidence of recurrent VTE seems to decline over time, neither the incidence nor the case-fatality rate of major bleeding complications associated with anticoagulant treatment seems to decline.
Risk assessment for recurrent VTE
The risk for recurrent VTE depends on several factors, including patient features, nature of the index event (proximal or distal DVT or PE), and presence and number of risk factors16 (Table 1). Recurrences of VTE seem to cluster in the first 2 to 3 months after discontinuation of anticoagulant treatment and then decline, reaching a plateau of < 3% per year in patients treated for provoked VTE and 5% to 10% per year in patients treated for an unprovoked VTE.9
Patient features
The role of aging as a risk factor for recurrent VTE has been reported in several studies3,11 (Table 1). Male sex appears to confer a higher risk of recurrent VTE. A meta-analysis of 9 randomized controlled trials and 4 observational studies showed a 1.6 hazard ratio (HR) for recurrent VTE in men compared with women (95% confidence interval [CI], 1.2-2.0).17
Nature of the index event
In 646 patients, unprovoked VTE was characterized as isolated DVT, DVT + PE, or isolated PE. Patients with a first episode of unprovoked isolated DVT were found to have a 2.1 (95% CI, 1.2-3.7) higher risk of recurrent VTE than patients with a first episode of unprovoked isolated PE after anticoagulation was discontinued.18
A patient-level meta-analysis of 7 randomized trials showed that the risk for recurrent VTE after stopping anticoagulant therapy is lower after isolated distal than proximal DVT (HR = 0.49; 95% CI, 0.34-0.71).9 The incidence of recurrent VTE was similar in patients treated for a first DVT and in those treated for a first PE (HR = 1.19; 95% CI, 0.87-1.63). However, it has been shown that patients with PE recur more frequently with a PE, whereas patients with DVT recur more frequently with a DVT.10,11 Indeed, the risk of fatal PE is 2- to 3-fold higher after an episode of PE than after an episode of DVT.13,14
Risk factors
In the study by Prandoni et al, patients without an identifiable transient risk factor had a higher risk for recurrent VTE than patients with surgery or trauma-associated VTE (HR = 0.36; 95% CI, 0.21-0.62 and HR = 0.51; 95% CI, 0.32-0.87, respectively).2
In a cohort study, the cumulative recurrence rate in 570 patients was 11% during 2 years of follow-up.19 The cumulative incidence for recurrence was lowest after surgery-related VTE (86 patients; recurrence rate 0%) and highest after unprovoked VTE (193 patients; recurrence rate 19.4%; P < .001). In patients with nonsurgical temporary risk factors, the incidence of recurrence was 8.8%.
The meta-analysis of 7 randomized trials showed that the risk of recurrence in patients with an initial event that was provoked by a temporary risk factor was approximately one-half that of patients with unprovoked VTE (HR = 0.55; 95% CI, 0.41-0.74; P = .0001; Table 1).9
Several studies have been performed to assess the role of individual features as risk factors for recurrence at the end of conventional anticoagulant treatment. The main aim of this approach is to identify those patients with unprovoked VTE who could benefit from extended anticoagulant treatment.
The role of persistent residual thrombosis as a predictor of recurrence remains controversial. In a prospective cohort study, patients with persistent residual thrombosis as assessed by ultrasonography, had a 2-fold higher risk for recurrent VTE compared with patients with early vein recanalization (HR = 2.4; 95% CI, 1.3-4.4; P = .004).20 Based on these results, 538 consecutive outpatients treated with 3 months of anticoagulation for a first episode of acute proximal DVT were randomized to fixed duration anticoagulation or flexible, ultrasonography-guided duration of anticoagulation.21 Overall, 17.2% patients allocated to fixed duration anticoagulation and 11.9% patients allocated to flexible-duration anticoagulation had recurrent VTE (adjusted HR = 0.64; 95% CI, 0.39-0.99). For patients with unprovoked DVT, the adjusted HR was 0.61 (95% CI, 0.36-1.02) and the HR was 0.81 (95% CI, 0.32-2.06) for those with secondary DVT. These results were confirmed by a meta-analysis of 14 studies in which the association between residual vein obstruction and recurrent VTE was significant in the overall study population (4022 patients; odds ratio [OR] = 1.5; 95% CI, 1.1-2.0, I squared 54%), but not in the subgroup of patients who had a first unprovoked DVT (2905 patients; OR = 1.24; 95% CI, 0.9-1.7).22
After the finding that increased D-dimer levels measured 1 month after discontinuation of oral anticoagulants were associated with a significantly higher risk for VTE recurrence,23 an intervention study was performed to evaluate whether D-dimer levels could be used to tailor warfarin duration after a first episode of VTE.24 In that study, 608 patients treated for at least 3 months for an unprovoked VTE had D-dimer levels assessed at 30 days after warfarin withdrawal.19 Patients with a normal D-dimer level remained off of anticoagulant therapy and were followed clinically. Patients with an abnormal D-dimer level were randomly assigned either to remain off warfarin or to resume it for an additional 18 months. VTE recurred in 15% of patients randomized to discontinue anticoagulation compared with 2.9% of patients randomized to resume anticoagulation (adjusted HR = 4.26; 95% CI, 1.23-14.6). Among patients who discontinued anticoagulation, the adjusted HR for recurrent VTE in those with an abnormal D-dimer level, compared with those with a normal D-dimer level, was 2.27 (95% CI, 1.15-4.46; P = .02). A patient-level meta-analysis confirmed the association between increased D-dimer and recurrent VTE (OR = 2.36; 95% CI, 1.65-3.36).25 However, even though the rate of recurrence was lower in patients with lower D-dimer levels (ie, normal vs elevated), it did not seem to be sufficiently low to identify a really low-risk population. Moreover, the predictive value of D-dimer seems to vary with sex, being significantly higher in males than females (data presented at the International Society on Thrombosis and Haemostasis Congress in 2013). Whether combined D-dimer/ultrasonography strategies can be used to tailor anticoagulant therapy is currently under evaluation in randomized studies (www.clinicaltrials.gov identifier NCT00954395).
Several studies have shown that patients with cancer-associated VTE have a high risk for recurrent VTE both on treatment and after treatment withdrawal.26 In these patients, treatment with low-molecular-weight heparin (LMWH) appears to be associated with a reduced incidence of recurrent VTE compared with VKAs.27
In conclusion, the analysis of risk factors and features of the index VTE event can provide information regarding the long-term risk for recurrence. More specifically, 3 categories of VTE with different risk for recurrence have been established: (1) patients with VTE associated with temporary risk factors, (2) patients with VTE associated with persistent risk factors (mainly cancer), and (3) patients with unprovoked VTE.
Models for risk of recurrent VTE
A nomogram to calculate the cumulative probability of recurrence in an individual patient was developed in a prospective cohort study including 929 patients with a first unprovoked VTE.28 Male sex (HR = 1.90 vs female sex; 95% CI, 1.31-2.75), proximal DVT (HR = 2.08 vs distal DVT; 95% CI, 1.16-3.74), PE (HR = 2.60 vs distal thrombosis; 95% CI, 1.49-4.53), and elevated levels of D-dimer while on anticoagulant treatment (HR = 1.27 per doubling; 95% CI, 1.08-1.51) were associated with a high recurrence risk. These items were used to derive a nomogram that has to be validated in prospective cohort studies before being applied in clinical practice.
In a cohort of 646 participants with a first unprovoked major VTE, Rodger et al identified women with none or one among: hyperpigmentation, edema, or leg redness; D-dimer ≥ 250 μg/L while taking warfarin; body mass index ≥ 30 kg/m2; age ≥ 65 years as being at low risk for recurrent VTE after discontinuation of anticoagulant therapy (1.6%; 95% CI, 0.3%-4.6%).29
A further tool for risk assessment was derived from a cohort of 1818 patients with unprovoked VTE treated for at least 3 months with a VKA; D-dimer, age, sex, and hormonal therapy (DASH) were included in the tool that revealed a receiver operating characteristic area of 0.71.30 This model has not been validated in a different study population.
Similar efforts have been conducted in cancer patients and were aimed at identifying candidates for indefinite anticoagulant treatment. In a cohort study of 543 patients with cancer-associated VTE, Louzada et al identified the model with the best classification performance for recurrent VTE, including 4 independent predictors (gender, primary tumor site, stage, and prior VTE).31 The model had 100% sensitivity, a wide separation of recurrence rates, 98.1% negative predictive value, and a negative likelihood ratio of 0.16 for recurrent VTE. Patients with a score ≤ 0 had low risk (≤ 4.5%) for recurrence and patients with a score > 1 had a high risk (≥ 19%) for recurrence. Subsequently, the rule was applied and validated in an independent set of 819 patients from 2 randomized, controlled trials comparing LMWH with VKA treatment in cancer patients.
Overall, none of these models is ready for clinical use because management studies or external prospective validation are still needed (Table 2). The use of different items across different models could be due to differences among derivation samples or differences in pools of considered items. Should different items produce different results across different study populations, this could be considered as a signal for limited strength of the association with the risk of recurrent VTE.
Clinical prediction models for risk assessment of recurrent VTE
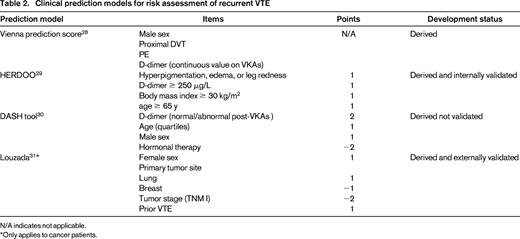
N/A indicates not applicable.
*Only applies to cancer patients.
Despite such a large amount of evidence about the risk for recurrent VTE, no clear evidence exists regarding risk factors for major bleeding apart for increasing age in patients with VTE. The knowledge of risk factors for bleeding in this clinical setting could help to drive decisions on long-term anticoagulation.
Based on this evidence, after the initial 3 months, anticoagulant treatment can be stopped in the case of VTE associated with a transient or reversible factor because the expected risk for recurrence is ∼ 3% per year. Patients with a particularly high risk of recurrent VTE, such as those with cancer, are candidates for an indefinite duration of anticoagulant treatment. In these patients, anticoagulant treatment for the first 6 months with LMWH has been shown to be more effective and safer than treatment with VKAs. In patients with no triggering risk factor (idiopathic or unprovoked VTE), anticoagulant therapy should be continued as long as the benefit-risk balance is favorable.
New anticoagulants: main features
In the past decade, 2 classes of oral anticoagulants have been developed for the treatment of VTE: direct inhibitors of thrombin (dabigatran etexilate) and oral direct factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban).
Among the new oral agents, dabigatran is administered as a pro-drug that is rapidly converted by circulating esterases to the active form and is 80% eliminated by the kidney.32 The bioavailability is ∼ 6.5%. None of the factor Xa inhibitors is a pro-drug and all have high oral bioavailability (> 50%).33,34 All of these agents, dabigatran etexilate and the factor Xa inhibitors, have predictable dose-proportional pharmacokinetics and pharmacodynamics. Time to peak effect and half-life times are similar, the former ranging from 1 to 4 hours and the latter ranging from a minimum of 6 to a maximum of 17 hours. Half-lives can be influenced by reduced renal clearance, this being particularly the case for dabigatran. Rivaroxaban is excreted one-third as active drug by the kidneys, whereas two-thirds are metabolized by the liver to an inactive form, one-half of which is eliminated in the urine and one-half via the hepatobiliary route.33 Apixaban is eliminated via the fecal (∼ 75%) and renal (∼ 25%) routes.34 In the case of edoxaban, approximately one-third of the drug is eliminated via renal excretion.35 However, care should be taken when using all of these new agents in patients with impaired renal function.36 Reduced doses of rivaroxaban and apixaban have been tested in patients with impaired renal function and atrial fibrillation.
New oral anticoagulants for extended treatment of VTE
Two double-blind trials have been conducted with dabigatran in the extended treatment of VTE, one versus warfarin and the other versus placebo (Table 3). In the randomized noninferiority RE-MEDY study, 2866 patients who had completed 3 to 6 months of anticoagulant therapy for a first VTE were randomized to receive dabigatran 150 mg twice daily or warfarin (international normalized ratio 2-3).37 Study treatment duration was initially planned for 18 months and was then extended to 36 months. Dabigatran was shown to be noninferior to standard extended treatment with VKAs for the prevention of recurrent symptomatic objectively confirmed VTE and VTE-related deaths (event rate 1.8% with dabigatran vs 1.3% with warfarin; HR = 1.47; 95% CI, 0.80-2.68). Major bleeding occurred in 0.9% of dabigatran-treated (n = 13) and 1.8% of warfarin-treated (n = 25) patients (HR = 0.52; 95% CI, 0.27-1.02).
Clinical trials of new oral anticoagulants and aspirin for extended treatment of venous thromboembolism.

BID indicates twice daily; INR, international normalized ratio; and CRNM, clinically relevant nonmajor.
*Incidence per patient-year.
In the RE-SONATE study, 1353 patients who completed 6 to 18 months of treatment for a first VTE were randomized to dabigatran 150 mg twice daily or placebo for an additional period of 6 months.30 A 92% relative risk reduction for symptomatic recurrent VTE was shown in favor of dabigatran (0.4% vs 5.6% in the dabigatran and placebo groups, respectively; HR = 0.08; 95% CI, 0.02-0.26); this corresponds to the need to treat 38 patients for 1 year to prevent one recurrent episode of VTE. A 0.3% rate of major bleeding was observed in the dabigatran group (2 patients) versus 0 in placebo group; clinically relevant nonmajor bleeding occurred in 5.3% of dabigatran patients and in 1.8% of placebo patients.
The randomized, double blind EINSTEIN Extension study was designed to assess the efficacy and safety of rivaroxaban for extended treatment of VTE if there was equipoise with respect to the need for continued anticoagulation.38 An additional 6 or 12 months of rivaroxaban (20 mg once daily) was compared with placebo in patients who had completed 6 to 12 months of anticoagulant treatment for a first VTE, some of whom had participated in the EINSTEIN-DVT and the EINSTEIN-PE studies. The study, that included 602 patients in the rivaroxaban group and 594 in the placebo group, showed that rivaroxaban had superior efficacy in preventing recurrent VTE compared with placebo (1.3% vs 7.1%; HR = 0.18; 95% CI, 0.09-0.39; P < .001). During the 6 or 12 months of treatment, nonfatal major bleeding occurred in 0.7% of patients in the rivaroxaban group (4 patients) and in none of the patients in the placebo group. The authors reported 34 recurrences were prevented by extended treatment with rivaroxaban at a cost of 4 major bleeds. The incidence of clinically relevant nonmajor bleeding was 5.4% in the rivaroxaban group and 1.2% in the placebo group. These bleeding events were predominantly mucosal and 81% of patients resumed or continued the study therapy, suggesting an acceptable benefit to risk profile. Total mortality and rates of cardiovascular events were low and did not differ significantly between the 2 treatment groups.
The AMPLIFY Extension study was a double blind trial in which patients with VTE were randomized to receive 2 different doses of apixaban (2.5 mg or 5 mg twice daily) or placebo after 6 to 12 months of anticoagulant treatment.39 Patients who had completed treatment with apixaban or enoxaparin and warfarin as participants in the AMPLIFY trial could be included in this study; patients were eligible for inclusion in the AMPLIFY Extension study in case there was clinical equipoise regarding the continuation or cessation of anticoagulation therapy. Experimental treatment was given for a 12-month period. A total of 2486 patients were randomized. Symptomatic recurrent VTE or death from VTE occurred in 8.8% of patients who were receiving placebo, compared with 1.7% of patients who were receiving apixaban 2.5 mg (a difference of 7.2 percentage points; 95% CI, 5.0-9.3) and 1.7% who were receiving apixaban 5 mg (a difference of 7.0 percentage points; 95% CI, 4.9-9.1; P < .001 for both comparisons). The rates of major bleeding during the 12 month treatment period were 0.5% in the placebo group (4 patients), 0.2% in the 2.5-mg apixaban group (2 patients), and 0.1% in the 5-mg apixaban group (1 patient). The rate of death from any cause was 1.7% in the placebo group, compared with 0.8% in the 2.5-mg apixaban group and 0.5% in the 5-mg apixaban group. The study showed that, compared with placebo, both the 2.5 mg dose and the 5 mg dose of apixaban reduced the risk of recurrent VTE without increasing the rate of major bleeding. Clinically relevant nonmajor bleeding occurred in 2.3% of patients receiving placebo, compared with 3.0% of patients receiving 2.5 mg of apixaban (a difference of −0.7 percentage points; 95% CI, −2.2-0.9) and 4.2% of patients receiving 5 mg of apixaban (a difference of −1.9 percentage points; 95% CI, −3.6 to −0.2). Given these results, it is estimated that the number needed to treat for 1 year to prevent one episode of recurrent VTE (fatal or nonfatal) is 14. The results of this study could provide a rationale for continuing anticoagulant therapy for an additional 12 months in patients with VTE for whom there is an uncertainty about the benefits and risks of continued anticoagulant therapy.
Overall, these placebo-controlled studies were not designed to identify the optimal duration of anticoagulation after initial VTE treatment. These studies were performed in patients considered in equipoise with respect to continuing or discontinuing anticoagulant treatment; the trials showed that the new agents are effective and safe when used for the extended treatment of VTE.
Two recent randomized double blind studies assessed the efficacy of aspirin (100 mg once daily) compared with placebo for the extended prevention of recurrent VTE.40,41 The main differences between these studies and the trials with the new oral anticoagulants relate to study design. Treatment duration was longer and the anticipated risk reduction was lower with aspirin. The WARFASA study showed a significant reduction of ∼ 40% in the incidence of recurrent VTE both during the study period (median 25 months) and during the study treatment (median 24 months) with an incidence of major or clinically relevant non major bleeding that was ∼ 1% per patient-year in both groups.41 The ASPIRE study, including a study population ∼ 2-fold that of the WARFASA study, showed a not significant risk reduction of ∼ 24% during the study period (37 months) and a significant 35% reduction during study treatment (27 months).41 The incidence of bleeding complications was similar to that of the WARFASA study. Taken together these studies show that a drug with a well-known safety profile, a low cost, and worldwide availability can be used to reduce the rate of recurrent VTE. The associated risk reduction may be lower than that with the oral anticoagulants but possibly associated with a lower risk for bleeding complications. The option of having aspirin in the armamentarium of drugs for secondary VTE prevention allows clinicians to tailor extended treatment on an individual patient basis (risk for recurrence, risk for bleeding, comorbidities).
Given the above data, rivaroxaban and dabigatran were included among the recommended therapies in the ninth ACCP guidelines for the treatment of VTE.4 However, waiting for more extensive evidence on the efficacy and safety of these new agents outside the clinical trial setting, these guidelines expressed a preference for VKAs and LMWHs over dabigatran and rivaroxaban. In addition, the proportion of cancer patients was < 10% in the phase 3 studies of the new oral anticoagulants and no direct comparison is currently available versus long-term LMWH treatment; the American College of Chest Physicians (ACCP) guidelines therefore recommend LMWH over dabigatran or rivaroxaban in active cancer. No recommendations have been made in favor of one of the new agents over the other because no head-to-head comparison has been performed.4
Our choice for the extended treatment of VTE
According to currently available evidence, only a minority of patients should be candidates for indefinite anticoagulation. These should be patients with unprovoked VTE and an estimated low risk for bleeding complications. An attempt to discontinue anticoagulant treatment after the initial 3 to 6 months should be done in young patients, particularly those with an active lifestyle who potentially would be on an anticoagulant for ∼ 50 to 70 years. The risk-benefit profile of continued anticoagulation should be carefully discussed with the patient (Figure 1).
Anticoagulant therapy should be discontinued after the initial 3 to 6 months in most patients who had the first episode associated with temporary risk factors. The duration of anticoagulant therapy in patients who had a first episode of cancer-associated VTE should be reassessed over time based on the persistence of cancer and continued anticancer therapy. Anticoagulation could be discontinued when the cancer has been completely cured.
The main achievement of recent trials for the extended treatment of VTE with new or alternative agents is the widening of the armamentarium of antithrombotic agents for this indication. The availability of agents with improved safety profiles and different pharmacokinetic profiles and dose regimens will allow clinicians to optimize extended treatment based on an individual's risk profile, comorbidities (ie, renal failure, gastric intolerance), and expected adherence. However, the availability of new drugs should not encourage extended use of anticoagulation in patients without a clear clinical indication for such treatment.
The case of aspirin for extended treatment of VTE is intriguing because it offers a drug that is well known in terms of safety and side effects; the lower effectiveness in terms of risk reduction was expected and should be taken into account in decision making.
Conclusions
Should clinical experience with the new agents confirm the promising results of the phase 3 clinical trials, it is likely that these agents will become the anticoagulants of choice for the treatment of VTE. Because these agents avoid the need for laboratory monitoring and dose adjustment, treatment of VTE will become easier for both patients and clinicians. Clinicians should take time to educate patients on treatment adherence and the potential side effects of these new agents.
Disclosures
Conflict-of-interest disclosure: G.A. has received honoraria from Bayer HealthCare, Boehringer Ingelheim, Sanofi, and Daiichi-Dankio. C.B. has been affiliated with the speakers' bureau for Bayer HealthCare, Pfizer, and Boehringer Ingelheim. Off-label drug use: None disclosed.
Correspondence
Giancarlo Agnelli, MD, Internal and Cardiovascular Medicine, Stroke Unit, University of Perugia, Italy; Phone: 39-075-5786424; Fax: 39-075-5782436; e-mail: agnellig@unipg.it.


