Abstract
Establishing the prognosis for patients with myelodysplastic syndromes (MDS) is a key element of their care. It helps patients understand the severity of their disease and set expectations for their future. For physicians, an accurate estimate of prognosis drives decisions about the timing and choice of therapeutic options to consider. The International Prognostic Scoring System (IPSS) has been the standard tool for MDS risk stratification since it was released in 1997. It has been used to describe patients in pivotal clinical trials and is a key element of practice guidelines. Subsequent changes to the classification scheme for MDS and an underestimation of risk in some patients from the low and intermediate-1 categories have led to the development of several newer prognostic models. The most recent is the revised IPSS (IPSS-R), which addresses several of the perceived deficiencies of its predecessor. Despite their utility, none of the available prognostic systems incorporates disease-related molecular abnormalities such as somatic mutations. These lesions are present in the nearly all cases and many have been shown to improve upon existing prognostic models. However, the interpretation of somatic mutations can be challenging and it is not yet clear how best to combine them with clinical predictors of outcome. Here I review several prognostic scoring systems developed after the IPSS and describe the emerging use of molecular markers to refine risk stratification in the MDS patient population.
Introduction
syndrome /syn·drome/ (sin′drōm): a set of symptoms occurring together; the sum of signs of any morbid state; a symptom complex.1
When a group of disorders are described as syndromes, it implies a certain degree of diagnostic uncertainty, nonuniformity in their clinical presentation, and an incomplete understanding of their etiology. Myelodysplastic syndromes (MDS) are no exception. They represent several related disorders defined by a set of common features, of which the most prominent is morphologic dysplasia associated with inefficient hematopoiesis and the development of peripheral cytopenias. These findings are manifestations of abnormalities in clonally expanded diseased cells that may be modified by extrinsic features such as interactions with the immune system or alterations in the BM microenvironment. A consequence of these abnormalities, shared by all MDS subtypes to various degrees, is an increased risk of transformation to acute myeloid leukemia (AML). Despite commonalities, the clinical presentation of MDS can be highly variable and patients with seemingly similar features often have very distinct disease courses. The ability to accurately predict outcomes for individual patients is a cornerstone of MDS treatment and will be the focus of this article examining prognostic scoring systems for MDS.
MDS subtype classification
The clinical heterogeneity of MDS has led to the development of various classification schemes designed to identify groups of patients with similar disease features, patterns of progression, molecular etiology, and likelihood of response to common therapies. The current standard is the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia last revised in 2008.2 It divides MDS into several categories based on the number of dysplastic cell lines, the proportion of blasts in the BM, and, in one case, the presence of a specific chromosomal abnormality (deletion of chromosome 5q). Patients within each WHO MDS subtype are more homogeneous and are likely to share important prognostic features, but despite its prognostic value, this system is not primarily intended to predict outcomes.
Clinical variability makes the prediction of prognosis of particular importance for MDS patients. Affected individuals will want to know how severe their disease is and how it is likely to affect their longevity. For clinicians, the decision to treat, how to treat, and when to treat patients is largely based on the predicted disease course. The calculation of potential toxicity versus expected benefit for a given therapy is made in the context of understanding the risk of not treating the patient. For those with few symptoms and a low risk of progression, the best option could be to avoid side effects and stick to careful observation. For higher-risk patients, earlier intervention with more toxic regimens capable of extending survival, such as DNA-methyltransferase inhibitors, can be justified. This basic premise has led to the development of various methods for predicting the prognosis of MDS patients. Several such tools will be discussed, along with the promise and challenges of incorporating molecular biomarkers into prognostic scoring systems.
Clinical prognostic scoring systems
It is useful to consider what an ideal system for predicting the prognosis of patients with MDS would look like. Most importantly, this system should be accurate and subdivide patients into groups with meaningful differences in predicted overall survival, yet not be so coarse that it lacks precision. This theoretical system should be widely applicable both to patients with a wide range of MDS subtypes and to patients at various stages in their disease. It should consider as much informative data as possible while still being relatively simple to apply and should not require a multitude of esoteric or expensive tests. Finally, it should be included in common clinical guidelines. In practice, however, several of these features are mutually exclusive and any prognostic scoring system will have to make compromises that limit its performance in specific situations. Therefore, each of the various models that have arisen over the past 16 years has a slightly different focus and utility.
IPSS
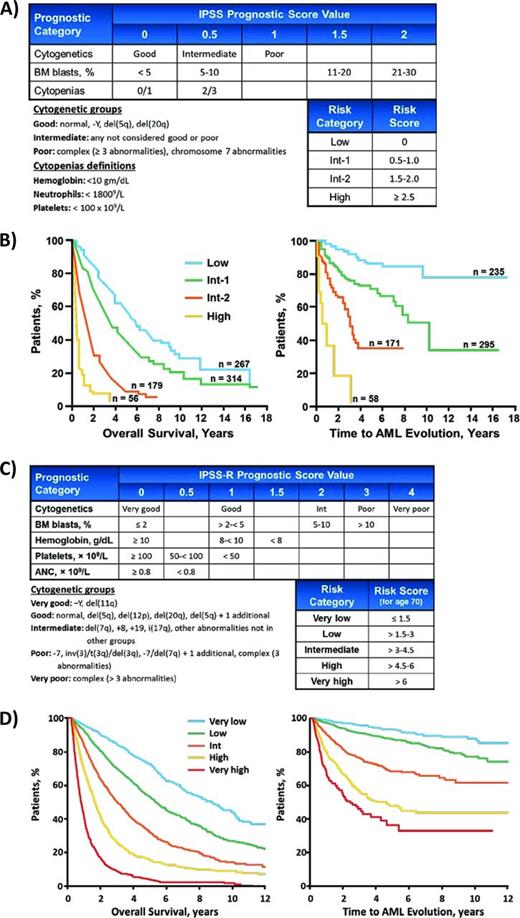
The IPSS, first published in 1997 and subsequently validated, has become the most widely adopted predictor of prognosis for patients with MDS.3 It was generated by examining the clinical characteristics of 816 patients at the time of diagnosis to determine associations with overall survival. These patients could not have therapy-related MDS, proliferative chronic myelomonocytic leukemia, or have received disease-modifying treatments such as chemotherapy or stem cell transplantation at any point in their disease course. The final model included only 3 disease variables—BM blast proportion, cytogenetic abnormalities, and the number of peripheral cytopenias—which were weighted as shown in Figure 1A. Based on the scores in these categories, patients were assigned to 1 of 4 risk groups with significant differences in overall survival and risk of transformation to AML. The strengths of the IPSS include its simplicity and that it does not require any testing beyond the routine diagnostic evaluation. More importantly, it has become the standard for describing populations of patients participating in pivotal clinical trials for MDS, including those that led to the approval of lenalidomide, azacitidine, and decitabine in most countries around the world. In addition, the IPSS is explicitly used by clinical guidelines such as those published by the National Comprehensive Cancer Network (NCCN) to help inform the choice of therapy for MDS patients.4 These features have allowed the IPSS to remain the standard of reference even as subsequent prognostic scoring systems have been developed to address some of its weaknesses.
IPSS. (A) The original IPSS. (B) Overall survival and AML transformation curves for patients in the 4 IPSS risk groups. (C) Compared with the IPSS, the IPSS-R includes additional cytogenetic risk groups and describes more chromosomal abnormalities. It decreases the relative weight of elevated BM blast percentage and it considers cytopenias individually, with additional weight given to more severe cytopenias. (D) Overall survival and AML transformation curves for patients in the 5 IPSS-R risk groups.
IPSS. (A) The original IPSS. (B) Overall survival and AML transformation curves for patients in the 4 IPSS risk groups. (C) Compared with the IPSS, the IPSS-R includes additional cytogenetic risk groups and describes more chromosomal abnormalities. It decreases the relative weight of elevated BM blast percentage and it considers cytopenias individually, with additional weight given to more severe cytopenias. (D) Overall survival and AML transformation curves for patients in the 5 IPSS-R risk groups.
The limitations of the IPSS are that it was not intended for use after initial diagnosis and its value in previously treated patients is less clear. It also considers patients with refractory anemia with excess blasts in transformation, a category of MDS patients with 20% to 30% BM blasts that was later redefined as AML in the WHO classification. More importantly, because the IPSS weights only the number of cytopenias present, it appears to underestimate disease risk in a subset of patients with severe cytopenias but few other risk factors.5,6 Subsequent prognostic models, including the revision to the IPSS, address several of these concerns.
WHO-based Prognostic Scoring System (WPSS)
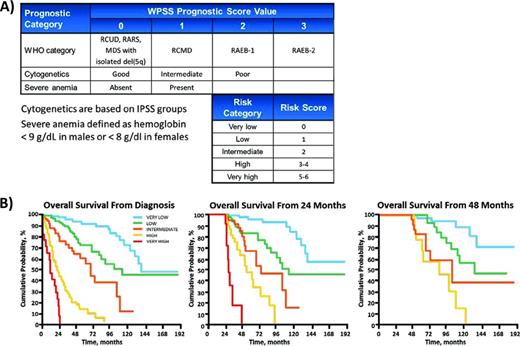
The WPSS is an MDS prognostic scoring system based on the WHO classification system (Figure 2).7 The WHO subtypes are defined largely by the number of dysplastic lineages present and on the proportion blasts in the BM, thus incorporating these features into the WPSS. The WPSS then considers the same cytogenetic risk groups as the IPSS and, in its most recent revision, adds the presence of severe anemia as an additional risk factor. These variables are used to assign patients to 1 of 5 risk groups with significant differences in overall survival. Like the IPSS, the WPSS is very simple to apply and does not require additional testing to implement. It is also included in the NCCN guidelines for the treatment of MDS.4 Another strength of the WPSS is that it has been validated at time points other than diagnosis, allowing it to be used as a dynamic scoring system.
WPSS. (A) Point values for elements of the WPSS. (B) Overall survival curves at various time points demonstrating the dynamic utility of the WPSS. Adapted with permission from Malcovati et al.7
WPSS. (A) Point values for elements of the WPSS. (B) Overall survival curves at various time points demonstrating the dynamic utility of the WPSS. Adapted with permission from Malcovati et al.7
Lower-Risk MDS Prognostic Scoring System (LR-PSS)
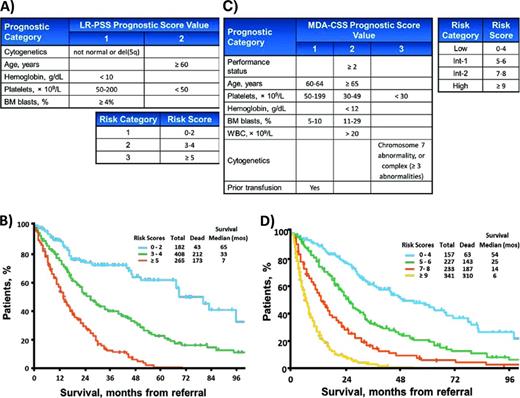
The LR-PSS from the MD Anderson Cancer Center is designed to refine risk stratification of patients considered to have low or intermediate-1 risk by the IPSS (collectively termed “lower” risk).8 It was created by examining 856 lower-risk patients for features associated with shorter overall survival and has been subsequently validated. This relatively simple model incorporates blast proportion and cytogenetics, but more heavily weights age and cytopenias, with a particular emphasis on severe thrombocytopenia (Figure 3A). The resulting scores are used to place patients into 1 of 3 risk Categories. Nearly a third of patients fall into the Category 3 risk group, which has a median overall survival comparable to that of IPSS Intermediate-2 patients. This group appears to be at greater risk than predicted by the IPSS, an important finding because treatment guidelines recommend more aggressive therapies for higher-risk MDS patients.4,9,10 Although the LR-PSS appears to improve upon the IPSS, its utility may be limited now that the IPSS has been revised and includes greater weight for severe cytopenias and easier adjustments for age.
MD Anderson scoring systems. (A) Point values for elements of the LR-PSS. (B) Overall survival curves for patients in the 3 LR-PSS risk categories. Adapted with permission from Garcia-Manero et al.8 (C) Point values for elements of the MDA-CSS. (D) Overall survival curves for patients in the 4 MDA-CSS risk groups. Adapted with permission from Kantarjian et al.11
MD Anderson scoring systems. (A) Point values for elements of the LR-PSS. (B) Overall survival curves for patients in the 3 LR-PSS risk categories. Adapted with permission from Garcia-Manero et al.8 (C) Point values for elements of the MDA-CSS. (D) Overall survival curves for patients in the 4 MDA-CSS risk groups. Adapted with permission from Kantarjian et al.11
MD Anderson Comprehensive Scoring System (MDA-CSS)
Researchers from MD Anderson have published another prognostic model designed to address many of the perceived shortcomings of the IPSS. The MDA-CSS was created by examining a large set of clinical variables in 1915 patients.11 These included patients with therapy-related disease, proliferative chronic myelomonocytic leukemia, and those that have been previously treated, all groups that did not form part of the IPSS. The MDA-CSS considers the same variables as the IPSS, including cytogenetics, BM blast percentage, and cytopenias; however, it treats anemia and thrombocytopenia separately, adding additional weight for severe thrombocytopenia. In addition, the MDA-CSS explicitly includes patient age, Eastern Cooperative Oncology Group performance status, and history of prior RBC transfusions (Figure 3C). This more inclusive and broad-based model appears to improve upon the IPSS, as the authors show by using the MDA-CSS to restratify patients successfully within each IPSS risk group. Like the WPSS, it does not require that patients be assessed only at the time of diagnosis. The weakness of the MDA-CSS is its relative complexity, although no additional laboratory testing is required to implement it in practice. It has been validated, but has not been formally included in consensus practice guidelines.12
Revised IPSS (IPSS-R)
A revision of the IPSS was recently released. It was created by examining 7012 MDS cases from the United States and Europe.13 The greater number of patients examined allowed the IPSS-R to address several of the limitations inherent in the original IPSS. Patients were still evaluated just at diagnosis and were included only if they never went on to receive disease-modifying treatments such as lenalidomide, hypomethylating agents, chemotherapy, or stem cell transplantation. Despite this apparent limitation, the IPSS-R has been shown to risk-stratify patients treated with azacitidine or lenalidomide and is likely to function at times other than diagnosis.14-17
The most significant update in the IPSS-R is the inclusion of many more chromosomal abnormalities, which are stratified over 5 cytogenetic risk groups compared with the 3 in the IPSS (Figure 1C).18 The weight given to elevated BM blast proportions is decreased, although lower blast percentages (> 2%) are recognized as adverse. In addition, as with many of the prognostic scoring systems that followed the IPSS, the IPSS-R considers each cytopenia individually, attributing additional risk based on severity. These variables are used to assign patients to 1 of 5 risk groups instead of the 4 in the original IPSS and uses age to adjust the cutoffs that define these groups. Online calculators are available to help apply the IPSS-R (http://www.ipss-r.com). The IPSS-R achieves more precise stratification of risk than the IPSS and does not require additional information. However, it is more complicated and has yet to be incorporated into practice guidelines or used to define patient populations in clinical trials. These factors could impair the timely adoption of the IPSS-R, particularly as we learn more about biomarkers that might improve upon all of these clinical prognostic scoring systems.
Additional markers of prognosis
Several additional variables not included in common scoring systems have been shown to have prognostic value. Serum lactate dehydrogenase, performance status, and ferritin were included in the IPSS-R as supplements to the model.13 Additional potentially prognostic features include BM fibrosis, albumin, β2-microglobulin, and flow cytometric profiles, among others.6,13,19-21 Many are surrogates or confounders for variables already considered in existing models and their independent contribution to the prediction of prognosis has not been clearly established. In the absence of a particularly strong association or incorporation into novel prognostic models, these measures requiring additional testing are less likely to penetrate clinical practice.
The mortality risk for many MDS patients is not defined by their hematologic disorder alone, but by the comorbid conditions that coexist with it.22 Age and performance status are imperfect surrogates for this information and models that explicitly examine extrahematologic conditions can improve on our ability to predict outcomes in MDS patients.23,24 However, the type of information that comorbidity models add has different implications for patients and physicians. If an MDS patient has a short life expectancy driven by their comorbidities, they may be poor candidates for aggressive therapy compared with a healthier patient at high risk due to the MDS itself. With this in mind, molecular abnormalities may be better ways to refine risk prediction. Molecular genetic abnormalities represent the pathophysiologic mechanisms responsible for the development and progression of MDS and can be highly associated with disease phenotypes.25,26 Therefore, molecular genetic features may identify patients with more uniform clinical presentations and disease risk.27-31
Gene expression profiling (GEP) and chromosomal abnormalities detectable by single nucleotide polymorphism arrays (SNP-As), but not by karyotype banding, have both been associated with MDS prognosis.32-34 GEPs have the advantage of describing the functional state of diseased cells regardless of the abnormalities that led to their development. In this sense, GEPs integrate the relevant contributions of a diverse set of molecular lesions and could simplify their interpretation. SNP-A genotyping can detect cryptic deletions and amplifications as well as regions that have lost heterozygosity through copy-number-neutral mitotic recombination. In some regions, these abnormalities are surrogates for somatic alterations or recurrently mutated genes such as TET2, TP53, EZH2, and CBL.35-37 Even the presence of less-well-understood SNP-A abnormalities in patients with otherwise “normal” cytogenetics is associated with a worse prognosis.33 However, neither the GEP nor SNP-A testing is standardized and the manner in which they are analyzed could affect their prognostic significance. Currently, neither test is considered part of the standard of care for any myeloid malignancy.
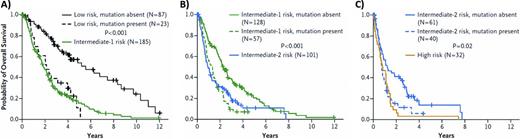
Conversely, somatic mutations are less challenging to detect and are routinely evaluated in the care of patients with AML and myeloproliferative neoplasms (MPNs).2 Recurrent mutations of more than 40 genes have been identified in MDS and the majority of patients, if not all, will carry at least one such disease-driving mutation.38 Mutations of splicing factor genes or epigenetic modifiers such as TET2 and DNMT3A are collectively present in more than 70% of cases, making these abnormalities much more prevalent than cytogenetic abnormalities.29 Somatic mutations in specific genes are associated with prognostic disease features such as complex karyotype, elevated blast proportion, and the severity of cytopenias, all factors considered by current prognostic scoring systems. Despite these associations, mutations in several genes have been shown to add prognostic information independent of models such as the IPSS and the LR-PSS. In particular, mutations of TP53, EZH2, ETV6, RUNX1, and ASXL1 were each shown to identify patients with shorter survival than predicted by the IPSS (Figure 4).28 No prognostic model yet incorporates somatic mutations explicitly. However, as has happened with AML and MPNs, our greater understanding of the genetic lesions that underlie MDS should lead to more molecularly defined disease subtypes and the use of somatic mutations to refine risk stratification.
Impact of prognostically adverse mutations on IPSS risk groups. (A-C) Overall survival of patients in the low-, intermediate-1, and intermediate-2 IPSS risk groups, respectively, stratified by the presence and absence of mutations in TP53, EZH2, ETV6, RUNX1, or ASXL1. The overall survival curve for patients in the next-highest IPSS risk group is included for comparison. Patients with one or more of these mutation have a survival risk comparable to those in the next highest IPSS risk group. Used with permission from Bejar et al.28
Impact of prognostically adverse mutations on IPSS risk groups. (A-C) Overall survival of patients in the low-, intermediate-1, and intermediate-2 IPSS risk groups, respectively, stratified by the presence and absence of mutations in TP53, EZH2, ETV6, RUNX1, or ASXL1. The overall survival curve for patients in the next-highest IPSS risk group is included for comparison. Patients with one or more of these mutation have a survival risk comparable to those in the next highest IPSS risk group. Used with permission from Bejar et al.28
Challenges facing molecular genetics in the clinical setting
The availability of clinical cancer sequencing is growing faster than our ability to interpret its results. Several commercial laboratories now offer sequencing of panels of genes and many academic cancer centers are performing their own genetic analyses of tumor material. However, mutation data are fundamentally different from many of the other tests that clinicians are used to interpreting and their complexities will have to be dealt with before mutation testing can be considered in the standard of care.
Problem 1: somatic mutations are not binary
Somatic mutations are not present or absent in the manner that we consider germline genetic variants to be. Tumors evolve over time, generating subclones defined by mutations that are not present in the bulk of the tumor.38 The presence of a mutation in a dominant clone may have a different implication than one found only in a small subclone. Prognostically adverse mutations may be relevant even when present at very low levels and will require sensitive techniques to detect.39,40 Conversely, mutations that predict a favorable response to a given therapy may not do so if they are present only in a subset of tumor cells. To interpret its potential significance accurately, sequencing results will need to report not only if a gene is mutated, but at what frequency it can be found.
Problem 2: all mutations in the same gene may not have equal consequences
Genes can be mutated in a variety of ways. Tumor-suppressor genes can be deleted outright; truncated by premature stop codons, splice site alterations, or frameshift mutations, or suffer missense mutations that change only a single amino acid. Some genes, such as TET2 and TP53, can have one or both alleles affected. Certain lesions could be more clinically important than others and it will require very large studies to capture these nuances. The same is true for different activating lesions in oncogenes. At the moment, we largely ignore these differences when interpreting mutations in hematologic malignancies. For example, in FLT3 mutant AML, we do not consider the nature of tandem duplication present or whether both alleles are affected, even though we suspect that these could have clinical relevance.41
Problem 3: mutations in different genes often co-occur
Unlike MPNs, which tend to have a smaller number of subtype-defining mutations, genetic lesions in MDS are numerous and can occur in a wide variety of combinations.28,29,38 This diversity likely explains much of the clinical heterogeneity that is characteristic of MDS, but can also complicate the interpretation of mutational data. For example, gene mutations associated with a favorable prognosis may be “trumped” by less favorable coexisting mutations. Conversely, a prognostically adverse mutation may become irrelevant if it occurs alongside a mutation that predicts a high likelihood of response to a given therapy.
Problem 4: the relative importance of molecular versus clinical features
An argument can be made for considering only clinical findings such as blast count and cytopenias in the prediction of prognosis because these represent the final common manifestation of an otherwise complex and diverse set of molecular abnormalities. Clinical measures can also reflect cell-extrinsic features such as normal hematopoietic stem cell reserve and microenvironmental dysfunction that cell-intrinsic mutation patterns do not capture. However, we know that certain molecular abnormalities, such as TP53 mutations, are strongly associated with outcomes even after taking clinical features into account.28,42 Future prognostic models will likely incorporate both clinical and genetic features weighted in a manner that reflects their independent association to disease risk just as the IPSS and IPSS-R do with clinical and cytogenetic information. Getting that weighting right will require the analysis of very large cohorts of patients and may need to be repeated regularly as novel therapies alter the clinical impact of predictive mutations.
Summary
The widespread adoption of the IPSS helped to improve the clinical care of patients with MDS and spurred the development of newer prognostic models to refine risk stratification. Each has slight differences that may favor their use in certain situations, but none has yet reached the level of adoption enjoyed by the IPSS. However, all of these models may be headed toward obsolescence as we learn more about the molecular mechanisms that give rise to MDS. These abnormalities have prognostic value that is often independent of prognostic scoring systems and are likely to be incorporated into newer prognostic models that replace the old. Mutation testing is becoming more widely available and may eventually help with diagnosis, subtype classification, and even the prediction of therapeutic response the way it has in AML and MPNs.
Acknowledgments
The author thanks Stacey Rose and Jennifer Leslie from Meditech Media for their adaptation of the prognostic models and survival curves and Ben Ebert and David Steensma for their ongoing mentorship and advice.
Disclosures
Conflict-of-interest disclosure: The author is on the board of directors or an advisory committee for Genoptix and Celgene, has consulted for Genoptix and Celgene, and has received honoraria from Genoptix. Off-label drug use: None disclosed.
Correspondence
Rafael Bejar, Moores Cancer Center, Division of Hematology and Oncology, University of California, San Diego, 3855 Health Sciences Drive, MC 0820, La Jolla, CA 92093-0820; Phone: 858-534-5204; Fax: 858-534-5620; e-mail: rabejar@ucsd.edu.