Abstract
Interest in platelet-rich plasma (PRP) has skyrocketed over the last decade, with a growing body of research contributing to both excitement and skepticism regarding its use. Despite mixed opinions in the medical field, interest from the public has fueled increased utilization of PRP for musculoskeletal conditions, particularly those that are difficult to treat such as chronic, degenerative tendinopathy and osteoarthritis. PRP's reputation as a “natural healer” and stories in the lay press featuring the use of PRP by professional athletes and celebrities has created a lucrative market for PRP even absent insurance reimbursement, casting further doubt regarding motivation for use by some practitioners. Research of PRP is clouded by the fact that PRP is a heterogeneous term representing a variety of different platelet preparations and there are many variables in technique and postprocedure rehabilitation, all of which may have significant effects on outcome. This article discusses definitions and classification of PRP, reviews rationale and evidence for use of PRP in chronic tendon injuries and osteoarthritis, and looks at future directions.
Definition and classification of platelet-rich plasma
Blood is composed of plasma and cellular elements. Defined at its simplest, platelet-rich plasma (PRP) is an increase from baseline blood values of platelets and is liquid in form.1 Forms in which the fibrin has been activated and are solid or semisolid are not PRP and are more accurately described as platelet-rich fibrin matrix. These forms are often used in surgical applications because they can be incorporated or sutured into surgical repairs, but should not be considered PRP. PRP is made by separating elements of blood, typically by centrifuging blood and taking advantage of the differential density of the solid elements. Platelets, RBCs, WBCs all have different sizes and densities, leading to a layering effect when centrifuged. Different manufacturers have created proprietary systems using various collection systems and centrifugation protocols, which lead to distinctly different constituents in the makeup of PRP.
For simplicity, PRP can be classified primarily into 2 basic types: leukocyte-rich PRP (LR-PRP), in which the WBCs are included, and leukocyte-poor PRP (LP-PRP), in which there are minimal WBCs. In general, the systems that produce LR-PRP are systems that use faster centrifugation speeds or 2 centrifugations and the PRP is created from the buffy coat, resulting in a product that is 5 to 9 × the baseline level of platelets. Systems that produce LP-PRP often use a single, slower centrifugation, with the RBCs and WBCs layering out in the lower part of the test tube while the platelets remain suspended in the plasma. LP-PRP systems typically produce a platelet count 1.5 to 3 × the baseline level of platelets. The PRP produced will vary dependent on the baseline platelet count of the patient and the efficiency and variability of the PRP collecting device (Table 1 lists the different types of PRP). When evaluating studies using PRP, it is important to assess what type of PRP is being used. The efficacy or lack thereof in some studies could be related to the quality of the PRP. Ideally, the exact content of the PRP injected would be consistent and characterized, but this is rarely the case.
In addition to PRP, some are using other PRP-like products, such as IL receptor antagonist (IL-1RA) for musculoskeletal indications, particularly the treatment of osteoarthritis (OA). This is typically created by collecting whole blood, allowing it to clot and incubating it with glass beads for 24 hours then centrifuging the blood and filtering it. The negative charge on the glass beads will activate the platelets causes them to release their growth factors and also stimulate WBCs to produce IL-1RA. IL-1 is thought to be a major contributor to the destruction of hyaline cartilage, so a product that inhibits it is theorized to both decrease the pain and slow the progression of OA.2 This substance is often categorized with PRP, but should be differentiated from it when considering both mechanism of action and efficacy (Table 1).
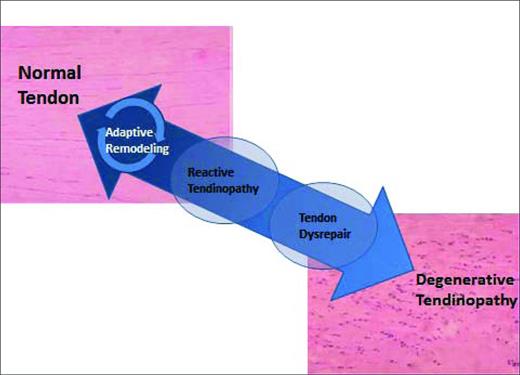
Tendinopathy continuum
Tendons undergo continuous remodeling according to the physiologic stresses put on them. Older concepts of tendon injury being an inflammatory process have been replaced by the idea that tendon pathology occurs along a continuum.3 When a tendon is excessively loaded, it will begin a process of progressive degradation, first reaching a state of reactive tendinopathy, then tendon disrepair, and finally degenerative tendinopathy. If the tendon is loaded appropriately at any point during this cycle, it has the capacity to reverse this process and repair itself3 (Figure 1).
The effectiveness of any treatment for tendinopathy is likely to be directly correlated with the stage of tendinopathy the patient is in when treatment begins. In earlier reactive stages of tendinopathy, simple rest may be enough to initiate healing in some patients. In patients further along the continuum, eccentric exercises, extracorporeal shock wave therapy (ESWT), or physical therapy techniques involving deep mechanical pressure may be required to reverse the trend. Further still along the continuum, needling the tendon (also called tenotomy), autologous blood injection, or PRP injection may be needed. There are currently no commonly used clinical stages of tendinopathy and most studies do not account for the various stages of tendinopathy subjects may be in, leaving time before treatment as the only surrogate when evaluating studies. PRP is typical used at the far end of the continuum, in severe degenerative tendinopathy, when other treatments have failed in an attempt to restart the repair process.
PRP in tendinopathy
There are currently 9 case series4-11 and 4 randomized controlled studies12-15 in the literature on the use of PRP in tendinopathy. (Table 2). They are difficult to compare directly because they examine different tendons, use different types of PRP, include different populations (elite vs nonelite athletes), use different injections techniques, use or forgo the use of ultrasound guidance with injections, use different rehabilitation protocols, and have different outcome measures. With so many variables, it is difficult to ascertain to which variables differences in outcomes may be attributed; however, closer examination of the literature, particularly the randomized trials, can provide a better understanding of the potential benefits or lack thereof of PRP injections.
The first randomized controlled study of PRP was by DeVos in 2010.14 In this study, 54 patients with mid-portion Achilles tendinosis were randomized to receive either injection with LR-PRP or with saline. The average age of the patients was 49.5 years, with a range of 18 to 70. Subjects were required to have symptoms for only 2 months (although the average patient had symptoms for 9-10 months) and they could not have previously tried eccentric exercises (exercises in which the muscle is lengthening as it is being used). Eccentric exercises are standard first-line treatment for Achilles and other tendinopathies.16-18 The injections were performed with ultrasound guidance using a 22-ga needle with 3 separate skin punctures sites and 5 needle passes made at each site, for a total of 15 passes in each tendon. The primary outcome measure was the Victorian Institute of Sport Assessment-Achilles questionnaire (VISA-A), a functional activity and pain score ranging from 0 to 100 that is commonly used for sports-related Achilles tendon research. In the VISA-A, 0 represents no activity and maximum pain and 100 represents full activity and no pain.19 An improvement of > 12 in the VISA-A score is considered clinically significant.14,19 The initial VISA-A score in each group was 46.7 in the PRP group and 52.6 in the saline group. Both groups underwent similar rehabilitation consisting of rest for 48 hours, followed by stretching at 1 week after injection and a standard eccentric exercise program beginning at 2 weeks.14
Results of this study showed that both groups improved. The PRP group improved their VISA-A score by 21.7 and the saline group by 20.5; therefore, both groups exceeded the minimally clinically significant change of 12.14 The investigators in that study and the popular media, however, concluded that PRP was ineffective because there was no difference in outcome between the PRP group and the saline group. This conclusion is problematic for several reasons. First, all participants in the DeVos study were eccentric exercise naive. In a study by Rompe, eccentric exercises alone were successful in treating a demographically identical group in 60% of the patients, with an increase of VISA-A score of 20 points versus a “wait-and-see approach.”20 Because the DeVos participants had not previously tried and failed eccentric exercises, there was a subgroup of participants who were likely to improve no matter what treatment they were randomized to, making the study underpowered to detect a difference between treatment groups. In addition, there was no true control group. A true control group would have been a wait-and-see group as in the Rompe study; however, a wait-and-see group cannot be blinded. A true, blinded, control group in invasive tendinopathy treatment is difficult to achieve. The “control” group in the study was actually another treatment: tenotomy and saline injection. Tenotomy alone has been shown to improve chronic tendinosis 60% of the time,21-24 and injection of any fluid with subsequent disruption of the matrix of the tendon may have some effect on tendon healing. What this study did show was that in a group of eccentric exercise-naive patients, PRP injection was no more effective than saline injection. Evaluating the DeVos study14 in combination with the Rompe study,20 the recommendation that eccentric exercises should be the initial treatment for tendinopathy is strengthened.
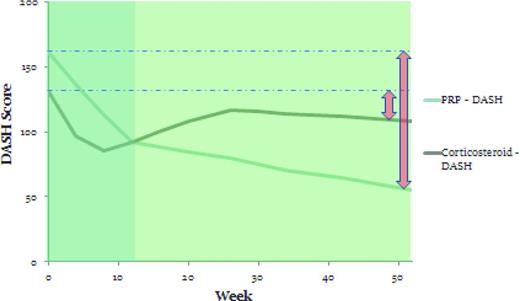
Another randomized controlled study by Peerbooms et al looked at the use of PRP in chronic lateral epicondylosis.15 Patients were randomized to receive either injections of either LR-PRP (n = 51) or corticosteroid (n = 49). Subjects were required to have had pain for at least 6 months and to have failed prior treatment. They could not have received a corticosteroid injection in the previous 6 months. The average age of the patient was 47 years. Injections were performed without ultrasound guidance at the point of maximal tenderness with 5 needle penetrations in a peppering technique. Rehabilitation for both groups was 24 hours of rest followed by 2 weeks of stretching and then an eccentric exercise program. The primary outcome measure was a 25% reduction in the Disabilities of the Shoulder, Arm, and Hand (DASH) score, a validated functional outcome score for the upper extremity. Results of this study showed that 73% of the PRP group and 49% of the corticosteroid group met the primary outcome measure at 1 year.15 More clinically relevant was the path of the DASH curves over time. The corticosteroid group had initial relief of pain and improvement of function, with better DASH and visual analog pain scores at 4 and 8 weeks; however, by 12 weeks, the scores were nearly identical, after which time the corticosteroid group returned to near baseline but the PRP group continued to improve. This cohort was followed for an additional year and there was continued improvement in the PRP group without similar improvement in the corticosteroid group (Figure 2).6 This study demonstrates that, in chronic tendinopathy, PRP begins a slow healing process with effects seen best at 6 months or greater.
DASH scores at 1 year in the PRP and corticosteroid groups in the Peerbooms et al study.15
DASH scores at 1 year in the PRP and corticosteroid groups in the Peerbooms et al study.15
The third randomized controlled trial was by Krogh et al comparing PRP (n = 20), saline (n = 20), and corticosteroid (n = 20).13 The average age of the patients was 45 years, and patients had symptoms for 25 months; however, the PRP and saline group had only had symptoms for 17 months whereas the corticosteroid group had symptoms for 36 months. The primary outcome measure was the Patient Rated Tennis Elbow Evaluation (PRTEE) score, a validated pain and function score for the elbow. This study was initially designed to look at outcomes at 6 months and 1 year; however, more than half of the study participants in all groups had dropped out by 6 months due to unsatisfactory effects so the results at 3 months were analyzed. At 1 month, the corticosteroid group had better PRTEE scores than the other groups; however, at 3 months, all groups had statistically equivalent scores.13 This result, as well as the trend lines, are consistent with the Peerbooms et al study. At the time the study was ended, the corticosteroid group was returning toward its baseline and the PRP group was trending toward improvement. It is unfortunate that 6- and 12-month data were not available as in other studies6,12,14,25 because PRP study end points of at least 6 months in chronic tendinopathies are required to adequately assess response to treatment.
The fourth randomized controlled study examined PRP versus ESWT for chronic patellar tendinopathy.12 ESWT is commonly used for tendinopathy in Europe and Canada. The average age in this study was 26 years, with an average duration of symptoms of 18 months. A 22-ga needle was used to inject LR-PRP with ultrasound guidance without local anesthetic and without any additional needle passes (no tenotomy). The ESWT group had 3 sessions 48 to 72 hours apart. One week after the last treatment session, a standard stretching and strengthening protocol was started, with a return to activity at 4 weeks as tolerated. The primary outcome measure was the percentage of patients who rated their treatment as “good or excellent” as measured by the modified Blazina scale and an improvement on the Victorian Institute Sport Assessment-Patella (VISA-P), a validated pain and functional activity scale for patellar tendon injury analogous to that used for the Achilles.26 The PRP group had significantly better VISA-P scores at 6 and 12 months than the ECWT group, and 91% felt they had “good” or “excellent” results at 1 year compared with 60% in the ECWT group. There was no control group in this study.
In summary, all of the case control trials show that PRP is effective (Table 2); however, this level of evidence is low. There are 4 randomized controlled studies but their numbers are small. Three of these studies show benefit from PRP and one did not have an end point long enough to assess whether PRP was effective. Conclusions that can be drawn at this point are that PRP should be considered after other conservative treatments have failed, in particular eccentric exercises. In addition, if effective, PRP takes 3 to 6 months to show benefit, but healing can continue out to 2 years. PRP is a reasonable option to consider before surgery or permanent activity modification, which may adversely affect both physical and mental health. More research is needed, such as studies that include wait-and-see as a control group.
PRP in arthritis
OA is a degenerative disease of the joints that affects the articular cartilage, synovium, and subchondral bone.27 The pathogenesis is not clearly defined, but appears to be related to injury of the chondrocyte causing an imbalance between anabolic and catabolic mechanisms and inflammatory mediators. The complex balance of growth factors and cytokines involved in the regulation of cartilage degradation and repair is not fully elucidated, but PRP has been proposed as a physiologic combination of growth factors that can favorably affect the joint milieu in osteoarthritic joints, thus halting or reversing the process.28 Patients with few options for the treatment of OA that has a significant impact on quality of life have sought out alternative and emerging treatments and have embraced the concept of a biologic treatment for this disease.
PRP and other biologics have long been used in race horses with OA, and there are basic science studies suggesting why it use may be efficacious; however, there are currently only 8 studies on the clinical use of PRP in OA in humans28-35 (Table 3) and all these studies have examined the effect of PRP in the osteoarthritic knee. None has reported any major adverse effect with the use of PRP and all have reported modest benefit on validated functional outcome scales. It is difficult to compare studies because they use different injection protocols and different types of PRP; however, Patel et al recently published a randomized, double-blind, placebo-controlled study supporting the use of PRP in OA of the knee.35 In this study, patients with moderate bilateral knee arthritis were randomized to receive either 1 or 2 injections of LP-PRP or 1 injection of saline. The 1 injection of LP-PRP was a higher platelet concentration, 10 × baseline, than the 2 injection protocol, which was 4 × baseline. Patients were followed at 6 weeks after the last injection at 3 and 6 months. There was a significant improvement in all subscales of the Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores, as well as significant improvements in the visual analog pain score. The scores were best at 3 months and regressed slightly at 6 months, but were still well above baseline compared with the saline group, which worsened over the 6 months from the initial baseline scores.35 This study supports the use of PRP in OA and supports a 1-injection protocol with a higher platelet concentration.
Although the literature on the use of PRP is limited, initial clinical studies suggest a benefit in knee OA. Its efficacy in other joints is unknown. Its mechanism of action is also unknown, that is, whether it is purely pain relieving or if it stops or slows progression of further cartilage degradation or could reverse damage. More studies are needed in this area, including quantitative measurements of pre- and posttreatment articular cartilage on MRI and measurements of synovial and joint fluid growth factors and cytokines. The possibilities of new treatments for this common and debilitating disease are intriguing.
IL-1RA in OA
Whereas it is not truly PRP, treatments with IL-1RA are being sought out for OA. IL-1 is thought to be a primary mediator of the breakdown of articular cartilage in degenerative joints. IL-1RA is theorized to mitigate this damage by blocking its actions. IL-1RA is produced by incubating whole blood for 24 hours with glass beads and then centrifuging it. This is considered to be “more than minimally manipulated” by the Food and Drug Administration and is therefore subject to different regulatory laws than PRP and is generally not available for human use in the United States. The typical protocol for this treatment is 6 injections several days apart. There are 2 randomized controlled trials on this treatment, both in knee OA.36,37 The first, in 176 knees, did not find IL-1RA to be any better than placebo saline injection as determined by WOMAC scores. The second, in 376 knees, found that IL-1RA was a more effective treatment than either hyaluronic acid or saline based on WOMAC scores. The reasons for these contradictory results are unclear and more research is needed.
Summary
The use of PRP and other biologics is an exciting new development with potential to treat injuries such as chronic tendinopathy and OA. PRP is a heterogeneous term and should be further characterized as far as platelet concentration and other blood elements such as leukocytes that may also be present. There is currently no clear evidence that one type of PRP is superior to another. There is reasonable evidence that PRP may be effective in chronic degenerative tendinopathy when other conservative treatments have been tried and have failed. However, PRP should not be considered first-line treatment. It takes 4 to 6 months to synthesize new tendon, so in sports settings, PRP should not to be considered an “in-season” treatment for chronic tendinopathy. Initial studies in the use of PRP in OA are positive, but further study should be done before its use can be recommended. Although public enthusiasm for PRP and other biologics at this point has outpaced the science behind the practice, there is reason to hope.
Disclosures
Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Kimberly Harmon, MD, University of Washington, 4060 E Stevens Circle, Seattle, WA 98116; Phone: 206-616-2495; Fax: 206-598-3140; e-mail: kharmon@uw.edu.