Abstract
Historically viewed in isolation as an odd, rare, and invariably fatal blood disease, aplastic anemia is now of substantial interest for its immune pathophysiology, its relationship to constitutional BM failure syndromes and leukemia, and the success of both stem cell transplantation and immunosuppressive therapies in dramatically improving survival of patients. Once relegated to a few presentations in the red cell and anemia sessions of the ASH, the Society now sponsors multiple simultaneous sessions and plenary and scientific committee presentations on these topics. This update emphasizes developments in our understanding of immune mechanisms and hematopoietic stem cell biology and new clinical approaches to stem cell stimulation as a therapy, alone and in combination with conventional suppression of the aberrant immune system.
Introduction
Advances in BM failure syndromes now are almost annual topics in the ASH Educational Program, and this review therefore emphasizes the most recent developments in our understanding of the pathophysiology and treatment of the paradigm of the disorders, acquired aplastic anemia. The reader is referred to more comprehensive publications for fuller discussions and ample bibliographies.1-3
Blood counts can be decreased in overwhelming infection (eg, bacterial sepsis) and secondary to a few diseases (eg, cirrhosis with hypersplenism, systemic lupus), but severe persistent pancytopenia has a limited differential diagnosis and almost always implies BM involvement. The “empty” BM defines aplastic anemia by a simple and uniform criterion with little pathologic variation or need for refined classification based on morphology. The BM is not truly empty but is replaced by fat cells.4 Mouse models of aplastic anemia, produced by the destruction of BM cells using radiation, cytotoxic drugs, and immune cells, have been useful in defining the hematopoietic stem cell and illustrating the potency of small numbers of lymphocytes in specifically inducing apoptosis of BM targets5 and their cytokines (eg, IFN-γ) as negative effector molecules.6 Suggestions from more recent sophisticated, but often contrived, murine models that adipocytes inhibit hematopoiesis, for influential “field effects” of stroma on stem cell function, and of an immune privileged stem cell niche require further replication in animals and validation in humans.7
Immune-mediated BM destruction
The most powerful evidence that acquired aplastic anemia in adults is secondary to immune destruction comes from the clinic. In our most recent National Institutes of Health (NIH) clinical trial of standard horse antithymocyte globulin (ATG) and cyclosporine, nearly 70% of patients had hematologic responses at 6 months.8 Of patients who fail first therapy and are then treated with either rabbit ATG or Campath, a further 30% of primary nonresponders show hematologic improvement to transfusion independence.9 Conversely, a substantial number of responding patients, approximately one-third, either relapse or need sustained cyclosporine administration to maintain their blood counts. A long taper of cyclosporine can delay relapse and patients may do well on very low doses of drug.
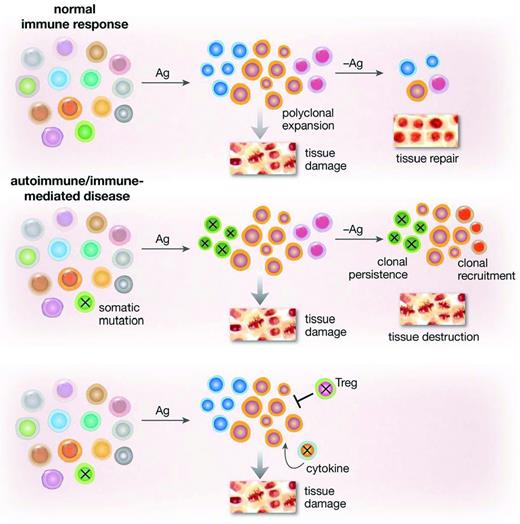
There are abundant laboratory data supporting an immune pathophysiology, but detailed mechanisms are lacking—as they are for most human autoimmune or immune-mediated diseases. A few antigens have been detected from peptide screens of sera, but their relationship to the T-cell response is unclear. Cytotoxic lymphocytes and type 1 cytokines appear to be the proximate effector cells. Why aberrant cellular immunity persists is unclear. T-regulatory cells appeared to be deficient in quantity10 and function11 and their numbers were strikingly different in the randomized trial of horse versus rabbit ATG, with recovery correlating with better response. In large granular lymphocytosis, in which a single T-cell clone dominates and suppresses BM function, acquired mutations in STAT3 are prevalent and functionally would result in constitutive activation12 (acquired STAT3 mutations have been reported in autoimmune diseases). These mutations may also be present in acquired aplastic anemia.13 Genomics applied to oligoclones in BM failure states might reveal an acquired genetic basis for abnormal persistence of an initially appropriate immune response (Figure 1).
Somatic mutations in lymphocytes might drive an aberrant immune response. Tissue damage is a normal component of an inflammatory response and resolves with withdrawal of the stimulating antigen. Acquired mutations, altering proliferation, susceptibility to apoptosis, or a variety of signaling pathways in an effector population or in regulatory cells would lead to persistence of a clone, tissue destruction, exposure of new antigens, and further recruitment of immune cells.
Somatic mutations in lymphocytes might drive an aberrant immune response. Tissue damage is a normal component of an inflammatory response and resolves with withdrawal of the stimulating antigen. Acquired mutations, altering proliferation, susceptibility to apoptosis, or a variety of signaling pathways in an effector population or in regulatory cells would lead to persistence of a clone, tissue destruction, exposure of new antigens, and further recruitment of immune cells.
Stem cell loss in aplastic anemia
The finding of absent hematopoietic precursors in the clinical BM specimen has been extended by functional studies of mature and primitive progenitors, which are always markedly decreased, and low numbers of CD34 cells and specific subpopulations that correlate with progenitors. Long-term initiating cells and cobblestone-forming cells are as close as we can assay the human stem cells and these too are sparse in all patients. Despite sophisticated assays, blood counts, presumably reflecting stem cell reserve, are the best predictors of outcome in nontransplantation therapy. Although the “Camitta criteria” continue to be used to define severe aplastic anemia, in the era of immunosuppression, reticulocytes and lymphocytes pretreatment14 and the robustness of the hematologic response after ATG15 are the most powerful predictors of response and thus prognosis. Likely also correlating with stem cell reserve are the better outcomes in children compared with adults and, as described in detail below, the impact of telomere length and rate of attrition on late complications after immunosuppression. That a stem cell stimulator small molecule can improve blood counts in some chronic refractory aplastic anemia patients and accelerate hematologic recovery in treatment-naive patients implies that stem cells are present in even extremely hypocellular BM and are susceptible to proliferative signals. Is the stroma responsible for failure to respond to immunosuppression? This hypothesis seems unlikely because engraftment rarely hinders stem cell transplantation in this population and there is an absence of supportive laboratory data for such a mechanism. The simpler and more likely reason for lack of response to immunosuppression is the limited number of hematopoietic stem cells that are also functionally unable to regenerate the failed hematopoietic compartment appropriately.
Genetics of BM failure in “acquired” aplastic anemia
The historically clear distinction between constitutional and acquired aplastic anemia is now blurred due to the discovery of mutations in the telomere repair complex in otherwise typical adult cases with no apparent family history and lacking classic physical anomalies. Experienced hematologists have occasionally been surprised by late presentation, well into adulthood, of Fanconi anemia, and a rare young patient may present with pancytopenia but show typical Schwachman-Diamond mutations.16 However, whereas it is a matter of debate (between pediatricians and internists?), patients with telomeropathies, especially due to TERT and TERC mutations, probably should not be classified as late dyskeratosis congenita (eg, classically in boys with X-linked mutations in DKC1) due to the highly variable penetrance of TERT and TERC, the pleomorphic effects of mutations affecting telomere repair on various organs (BM, lung, and liver), and the still uncertain prognosis of these lesions in the clinical setting.
The molecular and cellular biology of telomeres and telomere repair require clarification for the practicing hematologist given the novelty of the telomere diseases, the availability of clinical assays, and easy confusion between genetic, physiologic, and pathophysiologic telomere attrition.17 Telomeres are the termini of linear chromosomes consisting of hundreds of hexamer repeats (TTAGGG) and associated shelterin proteins. The telomere structure protects the end of the chromosome from recognition by DNA excision enzymes as fragmented or foreign DNA. Nevertheless, due to the asymmetry of DNA replication, loss of telomeric DNA is inevitable with every cell division, and telomere length is the explanation for the Hayflick phenomenon (limited cell division in tissue culture) and a “mitotic clock” of an individual cell. Indeed, telomeres shorten physiologically with aging of an organism, including humans. However, telomere loss is actively compensated by a molecular machine called telomerase or the telomere repair complex. The complex consists of the TERT enzyme, a reverse transcriptase, its RNA template TERC, and stabilizing proteins including DKC1. Inherited mutations in the genes encoding components of the repair complex lead to accelerated telomere attrition. Telomerase is very tightly regulated, especially the TERT gene, and transcription is affected by multiple critical pathways, including Myc, WNT, and many other signals. Telomerase is active in embryonic tissues and in adult cells with replicative demands, including hematopoietic stem cells and lymphocytes. A high rate of telomere attrition also can be evidence of pathophysiology: telomeres are composed of DNA and can be damaged by reactive oxygen species and replicative stress can accelerate telomere loss. When telomeres in an individual cell become critically short, the result is cell senescence or apoptosis, an appropriate and harmless mechanism to protect an organ from aged cells. However, if DNA damage responses are impeded, cells with very short telomeres continue to proliferate and their chromosomes are susceptible to instability, aneuploidy and nonreciprocal translocations, and malignant transformation.
In clinical practice, telomere length is obtainable from commercial laboratories, usually measured by flow cytometry of individual cells (Flow-FISH) or gene amplification of a cell population (quantitative PCR amplification for telomere DNA of total leukocytes or lymphocytes and granulocytes), with results adjusted for patient age. Certain features of a personal or family history are highly suggestive of a telomeropathy: chronic macrocytic anemia or thrombocytopenia, frank aplastic anemia, myelodysplastic syndrome or acute myeloid leukemia, pulmonary fibrosis, and liver cirrhosis. Early graying (sometimes disguised by hair dye) is suggestive if less specific. Any of these findings should be followed by telomere length determination. Because patients with TERT and TERC mutations usually do not have any one sign of the classic mucocutaneous triad of dyskeratosis congenita and many do not have a family history, telomere length testing may be advisable in all BM failure cases. Extremely short telomeres (in the first percentile for age) establish the diagnosis of a telomeropathy. Genetic sequencing is performed in some research laboratories and gene expression and enzymatic activity can be assessed semiquantitatively.
TERT or TERC mutations are considered risk factors, not genetic determinants of BM failure. In pedigrees, individuals with mutant genes show BM hypocellularity, low CD34 cell numbers, and decreased progenitors, but they often have normal blood counts or only subtle abnormalities such as macrocytosis. Patients with mutations can respond to immunosuppressive therapy, so an immune component to the development of aplastic anemia is inferred (as are alcohol and viral infection in cirrhosis and smoking in pulmonary fibrosis factors in disease development secondary to these genetic regenerative defects). There are no systematic data regarding transplantation outcomes in patients with TERT and TERC mutations, but genetic testing of potential family members is crucial to avoid choosing a donor bearing the same mutation and a consequently inadequate stem cell reserve. Sex hormones increase telomerase activity by up-regulating the TERT gene,18 and blood count improvement can be obtained with androgen therapy in patients with mutations in telomere repair complex genes.19-21 Other chemical modulators of telomerase activity have been or could be developed.22
In acquired BM failure, limited numbers of stem cells struggle to support blood counts, and this hematopoietic stress results in accelerated telomere attrition, a mechanism that is independent of an inherited genetic mutation. Short telomeres of leukocytes at presentation increase the risk of relapse and especially of clonal evolution in aplastic anemia8 and chromosome instability can be detected in the BM of patients long before transformation to myelodysplasia or leukemia.23 Markedly accelerated telomere attrition—and neither the presence nor the accumulation of mutations in candidate genes identified in malignant myeloid disease—precedes clonal evolution in aplastic anemia.24 Genes implicated in myelodysplastic syndromes and acute myeloid leukemia appear to be infrequently mutated in patients with aplastic anemia25 (and Feng, our unpublished data).
Treatment
BM transplantation, both conventional and experimental, is discussed elsewhere in this publication by Professor Socié.26 Matched sibling transplantations are always preferred in children. Transplantation in older patients and, in research settings, from alternative donors have been increasingly successful.
Immunosuppressive therapy
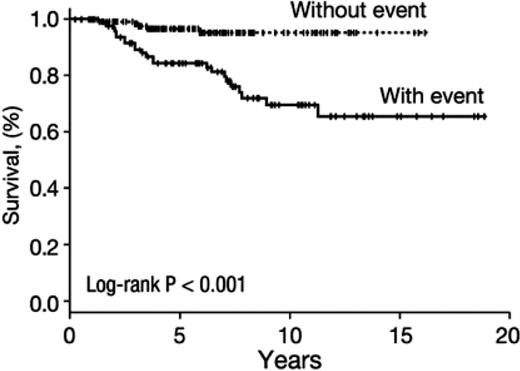
Horse ATG combined with cyclosporine remains standard as first-line immunosuppressive therapy, based on a prospective randomized comparison with rabbit ATG8 and confirmed in several retrospective studies (for review, see Scheinberg and Young1 ). Hematologic responses to transfusion independence can be expected in approximately two-thirds of patients, but they may not be durable because 30% to 40% of patients eventually either relapse (defined by the need for renewed immune therapy) or blood counts are dependent on cyclosporine administration. In our prospective trial of a long course of cyclosporine (2 years, with tapering of the dose after the conventional 6 months of full doses of drugs), relapse was delayed but not ultimately prevented. However, the kinetics of blood count changes suggested that a low dose of cyclosporine might be adequate maintenance. In general, patients who respond to standard immunosuppression do well (Figure 2).
Survival after response to immunosuppression in severe aplastic anemia. A large cohort (N = 243) of NIH patients who responded to treatment with the standard regimen of horse ATG plus cyclosporine was analyzed. Shown are long-term outcomes including the negative impact of a complicating event. Events were defined as relapse (need for further immunosuppression after protocol treatment) and clonal evolution (myelodysplasia/acute myeloid leukemia; almost always accompanied by a new cytogenetic abnormality in the BM). Approximately half of the patients did not experience a clonal event and poor survival was largely a consequence of disease progression. Data were censored for transplantation.1
Survival after response to immunosuppression in severe aplastic anemia. A large cohort (N = 243) of NIH patients who responded to treatment with the standard regimen of horse ATG plus cyclosporine was analyzed. Shown are long-term outcomes including the negative impact of a complicating event. Events were defined as relapse (need for further immunosuppression after protocol treatment) and clonal evolution (myelodysplasia/acute myeloid leukemia; almost always accompanied by a new cytogenetic abnormality in the BM). Approximately half of the patients did not experience a clonal event and poor survival was largely a consequence of disease progression. Data were censored for transplantation.1
The randomized trial, and other attempts at improving on the standard regimen, revealed deficiencies in our understanding of the mechanism of action of horse ATG. The most dramatic biologic differences detected in patients who were treated with antisera of horse and rabbit sources was the anticipated more profound lymphocyte depletion with Thymoglobulin (rabbit ATG) compared with ATGAM (horse ATG), particularly of CD4 T cells and including T-regulatory cells, but this difference represents an association, not an explanation for horse ATG's superiority in this clinical circumstance. Indeed, rabbit ATG was shown previously to be effective in rescuing a proportion of patients who had failed horse ATG therapy.9,27
Rabbit ATG at standard dosage is a more potent immunosuppressant than is horse ATG, but does not improve the rate of hematologic recovery in aplastic anemia. Other attempts to increase response rates by intensifying immunosuppression have failed, including the addition of high-dose corticosteroids, of mycophenolate mofetil, or of rapamycin. High-dose cyclophosphamide (50 mg/kg/d × 4 days) is used at a few institutions but has not been broadly accepted. The low cost of the drug is offset by the great expense associated with prolonged neutropenia, and early mortality due to infection is high compared with ATG. A Chinese trial of “moderate” doses of cyclophosphamide (30 mg/kg/d × 4 d) appeared attractive when presented at a specialty conference several years ago and only recently published,28 with an abbreviated period of neutropenia, little morbidity and mortality, and a response rate comparable to rabbit ATG. However, we have not been able to reproduce these outcomes and, as with the original high-dose regimen, our patients experienced prolonged neutropenia, a high rate of fungal infection, requirement for granulocyte transfusions, often extremely prolonged hospitalizations, and unexpected mortality, including seemingly irreversible absolute neutropenia in patients with previously adequate numbers of WBCs.29 In our experience, neither regimen prevents relapse or clonal evolution. Analogous to the lower-dose attempt with cyclophosphamide, an “optimized” regimen of rabbit ATG (reduction of the dose from 3.55 to 1.97 mg/kg/d for 5 days in sequential cohorts) has been promulgated in a single-center study from Tianjin, with large differences in survival, response rates, and infection despite very similar levels of lymphocyte depletion.30 These results require replication, preferably in prospectively randomized and multicenter protocols.
Even more difficult to assess are provocative clinical (presumably research) data published in unusual formats: 100% (!) hematologic recovery in small numbers of patients with severe aplastic anemia treated with arsenic trioxide presented in correspondence31 and of traditional Chinese medicine added to cyclosporine, which appeared in an open access journal.32 Our current era of publication encompasses limited acceptance rates in first-line journals and facilitated articles in commercial online venues. Irreproducible results, concerning enough for basic laboratory and animal experiments, have obvious and potentially dire consequences for patients and their treating physicians.
Stem cell stimulation therapy
Growth factors have had limited utility in aplastic anemia, as summarized separately in this publication by Professor Marsh.33 Anemia does not respond to erythropoietin nor neutropenia to G-CSF, which is unsurprising because levels of the endogenous growth factors are very elevated in patients' blood. Therefore, improvement with eltrombopag of patients with refractory severe aplastic anemia was entirely unexpected.34 Eltrombopag is a thrombopoietin mimetic, a small molecule designed to trigger the mpl surface receptor and signal transduction pathway, and developed to avoid the problem of (neutralizing!) antibody formation to intact thrombopoietin protein and iatrogenic idiopathic thrombocytopenia. Eltrombopag is approved for use in idiopathic thrombocytopenic purpura and has an excellent toxicity profile; in addition, it is administered orally. Eltrombopag should not be effective in aplastic anemia because in this disease, in contrast to idiopathic thrombocytopenia, blood levels of thrombopoietin are extremely high.35 Nevertheless, in our pilot trial, among 2 dozen patients with refractory aplastic anemia, more than 40% responded according to protocol criteria. Responses were striking in several respects: most were bilineage or trilineage, were not restricted to platelets, and were robust, resulting in transfusion independence and often near normal hemoglobin levels with an average increase of ∼ 4 g/dL; BM at 9 to 12 months often showed normal cellularity, which is unusual after even successful immunosuppressive interventions. These features suggest activity of the thrombopoietin axis at the level of the hematopoietic stem cell, which in retrospect is consistent with the cell and molecular biology of thrombopoietin, “knockout” animal models, and the hematology of humans with genetic defects in mpl signaling.
Multiple trials of thrombopoietin mimetics, eltrombopag, and romiplostim are in progress. At our institution, single-agent eltrombopag is being examined in moderate aplastic anemia and low-risk myelodysplastic syndrome, and eltrombopag is being combined with standard horse ATG and cyclosporine in treatment-naive severe aplastic anemia (Figure 3). In these previously untreated patients, preliminary results are promising in that the response rate appears to be at least as high as with immunosuppression alone and blood counts increase rapidly (D. M. Townsley, unpublished data).
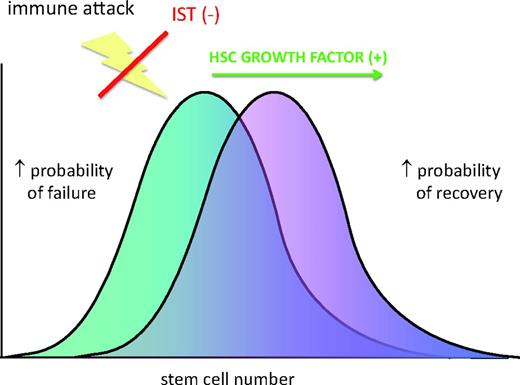
Stem cells as limiting in the response to immunosuppressive therapy. Combining immunosuppressive therapy with a factor that increases stem cell proliferation and/or self-renewal might overcome this limit.
Stem cells as limiting in the response to immunosuppressive therapy. Combining immunosuppressive therapy with a factor that increases stem cell proliferation and/or self-renewal might overcome this limit.
A small synthetic molecule has pharmacodynamics in the blood and in tissue that are very different from a large natural protein; mimetics may flood the stem cell niche at high concentration and have biologic activities different and more pronounced than normal concentrations of the endogenous hormone. Such activity carries risk, especially in patients with a proclivity to malignant transformation. Indeed, some refractory patients have progressed to myelodysplastic syndrome during or after eltrombopag treatment. Susceptibility to the emergence of cytogenetically abnormal clones may be linked to chronicity of disease and residual stem cell number, as reflected in telomere length of leukocytes. Because of this uncertainty concerning toxicity, thrombopoietin mimetics at present should not be used in BM failure diseases except in clinical research protocols.
Supportive care
Fundamentals will be discussed elsewhere in this publication by Professor Marsh.33 Even with the concentration of patients for initial therapy at experienced tertiary centers, preferably in research protocols, long-term care is managed by local physicians. We observe common errors of omission and commission: inadequate blood transfusion schedules that leave patients unnecessarily symptomatic; excessive prophylactic platelet transfusion, disregarding the current guideline of a lower threshold level of 10 000/μL; and corticosteroids, often at high doses and for long periods, resulting only in invasive fungal infections and iatrogenic Cushing syndrome (corticosteroids are used during the course of ATG to prevent and ameliorate serum sickness). The management of infection in the neutropenic patient has improved greatly through physician education and the introduction of effective and easily administered antifungal drugs, likely the explanation for the marked improvement in survival of aplastic anemia patients in general.36 Granulocyte transfusions appear to be effective and may be lifesaving under specific circumstances,37 but they are available at only a few institutions and they are expensive. There are other costs that may be paid for broad medical improvements of benefit to individual patients, including the terrifying emergence of antibiotic-resistant organisms and late effects of repeated exposures to diagnostic irradiation.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: Cyclosporine for aplastic anemia, eltrombopag for aplastic anemia.
Correspondence
Neal S. Young, Hematology Branch, National Heart, Lung and Blood Institute, National Institutes of Health, CRC-Building 10, Room 3E-5142, 10 Center Drive, Bethesda, MD 20892; Phone: 301-496-5093; Fax: 301-496-8396; e-mail: youngns@nih.mail.gov.